Short abstract
Neuregulin1 (NRG1) is a growth factor playing a pivotal role in peripheral nerve development through the activation of the transmembrane co-receptors ErbB2–ErbB3. Soluble NRG1 isoforms, mainly secreted by Schwann cells, are strongly and transiently up-regulated after acute peripheral nerve injury, thus suggesting that they play a crucial role also in the response to nerve damage. Here we show that in the rat experimental model of the peripheral demyelinating neuropathy Charcot-Marie-Tooth 1A (CMT1A) the expression of the different NRG1 isoforms (soluble, type α and β, type a and b) is strongly up-regulated, as well as the expression of NRG1 co-receptors ErbB2–ErbB3, thus showing that CMT1A nerves have a gene expression pattern highly reminiscent of injured nerves. Because it has been shown that high concentrations of soluble NRG1 negatively affect myelination, we suggest that soluble NRG1 over-expression might play a negative role in the pathogenesis of CMT1A disease, and that a therapeutic approach, aimed to interfere with NRG1 activity, might be beneficial for CMT1A patients. Further studies will be necessary to test this hypothesis in animal models and to evaluate NRG1 expression in human patients.
Impact statement
Charcot-Marie-Tooth1A (CMT1A) is one of the most frequent inherited neurological diseases, characterized by chronic demyelination of peripheral nerves, for which effective therapies are not yet available. It has been recently proposed that the treatment with soluble Neuregulin1 (NRG1), a growth factor released by Schwann cells immediately after acute nerve injury, might be effective in CMT1A treatment. However, the expression of the different isoforms of endogenous NRG1 in CMT1A nerves has not been yet investigated. In this preliminary study, we demonstrate that different isoforms of soluble NRG1 are strongly over-expressed in CMT1A nerves, thus suggesting that a therapeutic approach based on NRG1 treatment should be carefully reconsidered. If soluble NRG1 is over-expressed also in human CMT1A nerves, a therapeutic approach aimed to inhibit (instead of stimulate) the signal transduction pathways driven by NRG1 might be fruitfully developed. Further studies will be necessary to test these hypotheses.
Keywords: Charcot-Marie-Tooth 1A (CMT1A), ErbB, Neuregulin1 (NRG1), neuropathy, peripheral nerve, Schwann cell
Introduction
Charcot-Marie-Tooth (CMT) is one of the most frequent inherited neurological diseases, affecting both motor and sensory nerves; it is clinically characterized by weakness and muscle wasting of distal limb, skeletal foot deformities, distal sensory loss, and problems with hand function.1 CMT is caused by mutations in several genes encoding proteins involved in the physiological function of the peripheral nerve axon or in the myelin sheath formation.2 CMT1A, the most common form of CMT, is an autosomal dominant disease that derives from peripheral myelin protein 22 (PMP22) gene duplication.3
Schwann cell activity is regulated by several factors, including soluble and transmembrane isoforms of the ligand NRG1, which play different roles both in myelination during development and in remyelination following nerve injury.4 Soluble NRG1 isoforms, mainly secreted by Schwann cells, play their role following nerve injury: their absence or over-expression do not affect myelination, while their down-regulation negatively affects re-myelination. Transmembrane NRG1 isoforms, mainly expressed by axons, are involved in both development and nerve repair: their down-regulation impairs—and their over-expression promotes—both myelination and re-myelination. NRG1 activates tyrosine-kinase receptors belonging to the ErbB family.5 ErbB2–ErbB3 is the NRG1 heterodimer receptor expressed by Schwann cells.
To the best of our knowledge, no data about NRG1 isoform expression in CMT1A nerves are available. In this study the expression of different NRG1 isoforms and receptors was analyzed during different stages of the disease in an animal model of CMT1A represented by a transgenic rat over-expressing Pmp22.6
Methods
Sciatic nerves were obtained from both females and males P3, P16, P28, P56 transgenic Sprague-Dawley rats over-expressing Pmp22 (CMT1A rats).6 Animals were bred at the IRCCS-AOU San Martino IST Animal Facility of Genova, Italy. Heterozygous transgenic animals (CMT1A) from three independent litters were compared to their age-matched non-transgenic (wildtype [WT]) littermates following Italian Health Ministry guidelines. Total RNA and proteins were extracted by TRIzol Reagent (Invitrogen) following manufacturer’s instructions, and were analyzed by quantitative real time PCR (qRT-PCR) and Western blot analysis as previously described.7 Densitometric analysis was performed by Quantity One Software (Bio-Rad).
Results
mRNA and protein expression level of different NRG1 isoforms and ErbB2-ErbB3 receptors was evaluated in sciatic nerves obtained from CMT1A or WT rats at different development stages.
NRG1 isoforms can be classified as transmembrane or soluble according to their structure, and further subdivided into α and β isoforms according to the epidermal growth factor (EGF)-like domain, and into type a, b and c isoforms, according to the C-terminal domain.8 Different primer pairs7 allowed us to discriminate among the different isoforms.
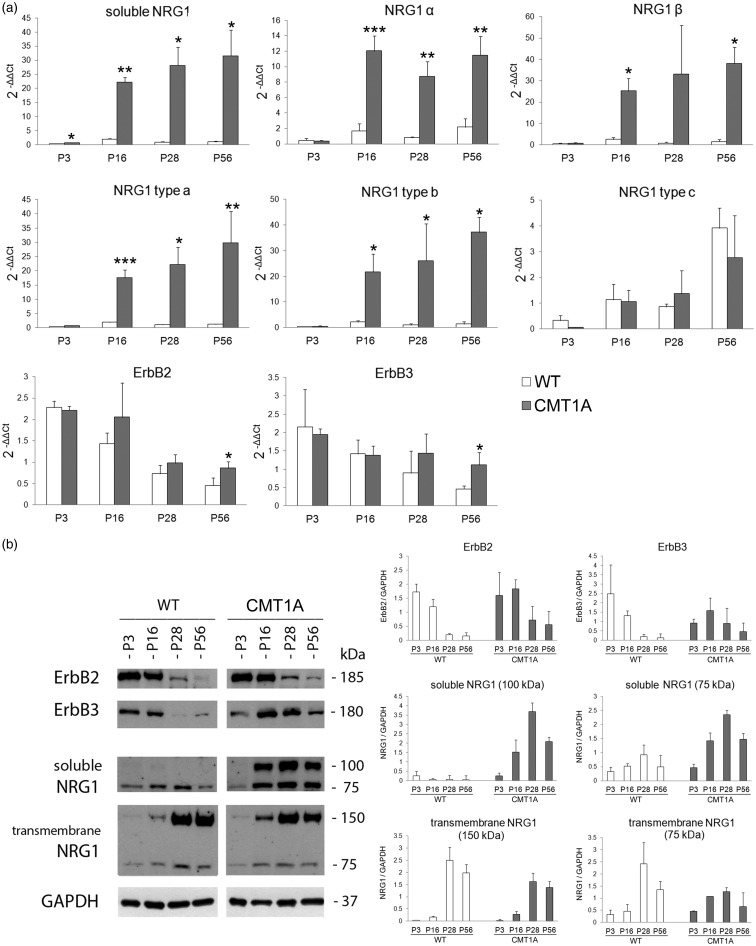
Data show (Figure 1(a)) that soluble NRG1 mRNA is strongly up-regulated in CMT1A nerves from P3. A significant up-regulation is appreciable also for NRG1 isoforms α and β and for type a and b isoforms from P16. Statistical analysis with two-way ANOVA using phenotype and age as factors revealed significant effects and significant interaction between factors for soluble, α, β, type a, and type b NRG1 isoforms, while NRG1 type c shows significant effects only for age.
Figure 1.
Soluble NRG1 is strongly up-regulated in CMT1A nerves. (a) The relative quantification (2−ΔΔCt) of different isoforms of NRG1, ErbB2 and ErbB3 receptors was obtained by qRT-PCR carried out on RNA obtained from sciatic nerves withdrawn at different time points from WT and CMT1A rats (n = 3 for each time point). For each gene, the ΔCT average of P3 WT nerves was used as calibrator. Data were normalized to the geometric mean of two endogenous housekeeping genes (ANKRD27 and RICTOR).22 Values in the graphics are expressed as mean + standard deviation (SD). Statistical analysis (Student's t-test) was performed to compare the differences between WT (white bars) and CMT1A (black bars) animals at the different time points (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001). (b) Western blot analysis of proteins extracted from WT and CMT1A sciatic nerves probed with antibodies for ErbB2 (#sc-284, Santa Cruz), ErbB3 (#sc-285, Santa Cruz), soluble NRG1 type a (#sc-348, Santa Cruz), transmembrane NRG1 (#AB5551, Chemicon), GAPDH (#4300, Ambion). A representative image of two analyses carried out on the biological duplicate and the corresponding densitometric analyses are shown. CMT1A: Charcot-Marie-Tooth 1A; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; NRG1: Neuregulin1; WT: wildtype.
At the protein level (Figure 1(b)), soluble NRG1 in CMT1A nerves shows an up-regulation of the 75-kDa band, corresponding to the C-terminus fragment of NRG1 deriving from precursor protein proteolytic cleavage, a necessary step for soluble ligand release.5,9 Moreover, a 100-kDa band, corresponding to the precursor of the soluble NRG1, is strongly expressed starting from P16, suggesting that NRG1 over-expression saturates the protease machinery responsible of protein cleavage and soluble ligand release.
The axonal transmembrane NRG1 precursor (150-kDa) is expressed at similar levels in WT and CMT1A animals until P16, while at P28 its level slightly decreases in transgenic rats. A 75-kDa band corresponding to the N-terminus fragment of transmembrane NRG1 following proteolytic cleavage,5 seems to be slightly more highly expressed in WT nerves from P28, coherently with CMT1A demyelinating phenotype.
At mRNA level (Figure 1(a)), ErbB2 and ErbB3 show a small decrease during development both in WT and in transgenic animals; at P56 a higher expression of both ErbB2 and ErbB3 is detected in CMT1A animals in comparison to WT. Statistical analysis with two-way ANOVA using phenotype and age as factors, revealed a significant effect of both for ErbB2 and ErbB3. At the protein level (Figure 1(b)), both ErbB2 and ErbB3 are more highly expressed in transgenic animals starting from P28.
Discussion
CMT1A nerves are characterized by Schwann cell de-differentiation and immaturity marker up-regulation.10 Most of these genes are up-regulated also following acute nerve injury, thus suggesting that CMT1A nerves show a gene expression pattern highly reminiscent of Wallerian degeneration.11 Indeed, we and others previously demonstrated that soluble NRG1 isoforms are strongly and transiently up-regulated after acute nerve injury7,12 and here we show that soluble NRG1 isoforms are strongly up-regulated in CMT1A nerves from P3. ErbB receptors are also up-regulated, as shown in human samples too,13 and this up-regulation may be the result of a positive feedback loop or dysregulated ErbB trafficking.14
The strong NRG1 over-expression found in CMT1A nerves raises the question on the function of NRG1 in chronic nerve injuries: does NRG1 counteract this disease, attenuating its symptoms and promoting nerve repair, or does it worsen it?
Recent results10 demonstrated that the crossbred of CMT1A mice with transgenic mice over-expressing soluble NRG1 led to a phenotype rescue. Furthermore, systemic injection from P6 to P18 of recombinant soluble NRG1 in CMT1A rat pups lead to similar results, thus demonstrating that, when provided in early development stages, soluble NRG1 ameliorates CMT1A pathogenesis, while no improvements were detected when the treatment started later.
Nevertheless, it has been also demonstrated by others that soluble NRG1 has bifunctional concentration-dependent effects on myelination: low concentration promotes, high concentration inhibits myelination.15–17 Therefore, we hypothesize that, although early acute treatment with recombinant NRG1 seemed to play a positive role10 (maybe due to the low expression of endogenous NRG1 protein at early development stages), over-expression of endogenous NRG1 in the following stages might play a negative role, worsening CMT1A pathogenesis. If our hypothesis is true, a possible therapeutic approach might consist in inactivation of the signal transduction pathways activated by NRG1, through the use of drugs targeting ErbB receptors.18
In support to this therapeutic proposal, it has been shown that treatment with ErbB2 inhibitor, Herceptin, promotes axonal outgrowth after acute nerve transection and repair.19,20 Herceptin treatment might promote regeneration by limiting the excess of soluble NRG1 activity in CMT1A chronic injury.
This therapy might bypass the limits of the early phase NRG1 treatment10—that might have a neoplastic potential21—and might have a broader use, because it would not be restricted to those patients who receive an early diagnosis of the disease.
Soluble NRG1 over-expression observed in CMT1A animal model needs to be confirmed also in human patients, but this analysis requires accurate human sample collection and further studies. If soluble NRG1 is over-expressed also in human CMT1A nerves, a therapeutic approach aimed to inhibit the signal transduction pathways driven by NRG1 might be carefully developed.
Author contributions
AS, GG, GR, SG designed the study; LN, AS supplied CMT1A animals; DV, GC managed the animals and collected the tissues; BEF, DP, GG, GR extracted and analyzed RNA and proteins; BEF, DP analyzed data and performed statistical analysis; BEF, GG, GR, LN, SG interpreted data; BEF, GG prepared the figures; GG, GR drafted the manuscript; IP, AS, SG revised and edited the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This study was supported by “Federazione Italiana Sclerosi Multipla” (FISM) # 2015/R/17 to LN, and “Fondo per la Ricerca Locale” (University of Torino) to GG.
ORCID iD
Giovanna Gambarotta http://orcid.org/0000-0003-2791-7743.
References
- 1.Schenone A, Nobbio L, Monti Bragadin M, Ursino G, Grandis M. Inherited neuropathies. Curr Treat Options Neurol 2011; 13:160–79 [DOI] [PubMed] [Google Scholar]
- 2.Baets J, De Jonghe P, Timmerman V. Recent advances in Charcot-Marie-Tooth disease. Curr Opin Neurol 2014; 27:532–40 [DOI] [PubMed] [Google Scholar]
- 3.Patel PI, Roa BB, Welcher AA, Schoener-Scott R, Trask BJ, Pentao L, Snipes GJ, Garcia CA, Francke U, Shooter EM, Lupski JR, Suter U. The gene for the peripheral myelin protein PMP-22 is a candidate for Charcot-Marie-Tooth disease type 1A. Nat Genet 1992; 1:159–65 [DOI] [PubMed] [Google Scholar]
- 4.Gambarotta G, Fregnan F, Gnavi S, Perroteau I. Neuregulin 1 role in Schwann cell regulation and potential applications to promote peripheral nerve regeneration. Int Rev Neurobiol 2013; 108:223–56 [DOI] [PubMed] [Google Scholar]
- 5.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci 2008; 9:437–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sereda M, Griffiths I, Puhlhofer A, Stewart H, Rossner MJ, Zimmerman F, Magyar JP, Schneider A, Hund E, Meinck HM, Suter U, Nave KA. A transgenic rat model of Charcot-Marie-Tooth disease. Neuron 1996; 16:1049–60 [DOI] [PubMed] [Google Scholar]
- 7.Ronchi G, Haastert-Talini K, Fornasari BE, Perroteau I, Geuna S, Gambarotta G. The Neuregulin1/ErbB system is selectively regulated during peripheral nerve degeneration and regeneration. Eur J Neurosci 2016; 43:351–64 [DOI] [PubMed] [Google Scholar]
- 8.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res 2003; 284:14–30 [DOI] [PubMed] [Google Scholar]
- 9.Fleck D, Voss M, Brankatschk B, Giudici C, Hampel H, Schwenk B, Edbauer D, Fukumori A, Steiner H, Kremmer E, Haug-Kroper M, Rossner MJ, Fluhrer R, Willem M, Haass C. Proteolytic processing of Neuregulin 1 type III by three intramembrane-cleaving proteases. J Biol Chem 2016; 291:318–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fledrich R, Stassart RM, Klink A, Rasch LM, Prukop T, Haag L, Czesnik D, Kungl T, Abdelaal TA, Keric N, Stadelmann C, Bruck W, Nave KA, Sereda MW. Soluble neuregulin-1 modulates disease pathogenesis in rodent models of Charcot-Marie-Tooth disease 1A. Nat Med 2014; 20:1055–61 [DOI] [PubMed] [Google Scholar]
- 11.Martini R, Klein D, Groh J. Similarities between inherited demyelinating neuropathies and Wallerian degeneration: an old repair program may cause myelin and axon perturbation under nonlesion conditions. Am J Pathol 2013; 183:655–60 [DOI] [PubMed] [Google Scholar]
- 12.Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci 2013; 16:48–54 [DOI] [PubMed] [Google Scholar]
- 13.Massa R, Palumbo C, Cavallaro T, Panico MB, Bei R, Terracciano C, Rizzuto N, Bernardi G, Modesti A. Overexpression of ErbB2 and ErbB3 receptors in Schwann cells of patients with Charcot-Marie-tooth disease type 1A. Muscle Nerve 2006; 33:342–9 [DOI] [PubMed] [Google Scholar]
- 14.Lee SM, Chin LS, Li L. Dysregulation of ErbB receptor trafficking and signaling in demyelinating Charcot-Marie-Tooth disease. Mol Neurobiol 2017; 54:87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syed N, Reddy K, Yang DP, Taveggia C, Salzer JL, Maurel P, Kim HA. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J Neurosci 2010; 30:6122–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanazzi G, Einheber S, Westreich R, Hannocks MJ, Bedell-Hogan D, Marchionni MA, Salzer JL. Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J Cell Biol 2001; 152:1289–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eshed-Eisenbach Y, Gordon A, Sukhanov N, Peles E. Specific inhibition of secreted NRG1 types I-II by heparin enhances Schwann Cell myelination. Glia 2016; 64:1227–34 [DOI] [PubMed] [Google Scholar]
- 18.Awada G, Gombos A, Aftimos P, Awada A. Emerging drugs targeting human epidermal growth factor receptor 2 (HER2) in the treatment of breast cancer. Expert Opin Emerg Drugs 2016; 21:91–101 [DOI] [PubMed] [Google Scholar]
- 19.Placheta E, Hendry JM, Wood MD, Lafontaine CW, Liu EH, Cecilia Alvarez Veronesi M, Frey M, Gordon T, Borschel GH. The ErbB2 inhibitor Herceptin (Trastuzumab) promotes axonal outgrowth four weeks after acute nerve transection and repair. Neurosci Lett 2014; 582:81–6 [DOI] [PubMed] [Google Scholar]
- 20.Hendry JM, Alvarez-Veronesi MC, Placheta E, Zhang JJ, Gordon T, Borschel GH. ErbB2 blockade with Herceptin (trastuzumab) enhances peripheral nerve regeneration after repair of acute or chronic peripheral nerve injury. Ann Neurol 2016; 80:112–26 [DOI] [PubMed] [Google Scholar]
- 21.Martini R. Neuregulin-1 alleviates Charcot-Marie-Tooth disease in rats. Nat Med 2014; 20:984–5 [DOI] [PubMed] [Google Scholar]
- 22.Gambarotta G, Ronchi G, Friard O, Galletta P, Perroteau I, Geuna S. Identification and validation of suitable housekeeping genes for normalizing quantitative real-time PCR assays in injured peripheral nerves. PLoS One 2014; 9:e105601. [DOI] [PMC free article] [PubMed] [Google Scholar]