Short abstract
In this study, we aimed to investigate the expression of miR-145 before and after hASCs osteogenic differentiation. We also intended to explore the influence of the target relationship between miR-145 and FoxO1 on osteogenic differentiation. Dual-luciferase reporter gene assay and real-time PCR were used to confirm the target relationship between miR-145 and FoxO1. Furthermore, the modulatory effects of miR-145 and FoxO1 on hASCs osteoinductive differentiation were measured by real-time PCR , Western blot, ALP staining, ARS staining, and cell immunofluorescence assay. After osteogenic differentiation, miR-145 was gradually down-regulated, while FoxO1 was up-regulated in hASCs. MiR-145 could directly target FoxO1 3′UTR. FoxO1 was negatively regulated by miR-145. After osteoinductive differentiation, BSP, Ocn, and OPN expression was lowered with the overexpression of miR-145 or the knockdown of FoxO1. Furthermore, ALP and ARS staining assay results showed weakened ALP activity and extracellular matrix calcification. When overexpressing miR-145 and FoxO1 simultaneously, no obvious change in ALP activity and extracellular matrix calcification was seen. MiR-145 could suppress hASCs osteoinductive differentiation by suppressing FoxO1 directly.
Impact statement
Researching on ASCs was a promising strategy to study osteogenic differentiation. The regulatory role of miR-145 on hASCs osteogenic differentiation remained partially explored.
Our study revealed a novel mechanism of the osteogenic differentiation process and suggested that miR-145 and its target gene FoxO1 may be potential targets for the therapy of human osteogenic-related disorders.
Keywords: miR-145, osteogenic differentiation, human adipose-derived stem cells, FoxO1
Introduction
Mesenchymal stem cells (MSCs), a population of self-renewing multipotent cells, have been widely used experimentally for tissue engineering due to their strong ability to differentiate into bone, cartilage, and fat under appropriate induction conditions.1 Human mesenchymal stem cells (hMSCs) are derived from various tissues, including bone marrow and adipose tissues. HMSCs have multilineage differentiating potentials.2 Human adipose-derived stem cells (ASCs) also have multilineage differentiation potentials and they are prospective ancestor cells for bone regeneration.3,4 Interests in the therapeutic potential of stem cells isolated from adipose tissue, called ASCs has grown due to their abundant sources and easy access for some clinical uses which avoided further manipulation, suggesting a more favorable tissue source than bone marrow.5–8 Thus, researching on ASCs was a promising strategy to study osteogenic differentiation.
MicroRNAs (miRNAs) are a kind of endogenous small non-coding RNAs that function as post-transcriptional regulators by binding to 3′UTRs of target messenger RNAs.9 With the increasing application of miRNAs in the treatment and monitoring of different diseases, miRNAs have become an important tool in biological and medical research. miRNAs are involved in osteogenic differentiation of stem cells.4 For instance, miR-34 inhibited osteogenic proliferation and differentiation in mice by targeting SATB2.10 MiR-138 was reported down-regulated during osteogenic differentiation and negatively regulating the osteogenesis of hMSCs.11 Other studies showed that miR-141, miR-31, and miR-200a were negative regulators of the differentiation of bone marrow stromal cells.12,13 Of our particular interest, miR-145 has also been found to have influence on bone differentiation. For instance, Jia et al.14 reported that miR-145 suppressed osteogenic differentiation by targeting Sp7. In addition, Fukuda et al.15 determined that miR-145 regulated osteoblastic differentiation by targeting the transcription factor Cbfb. However, the regulatory role of miR-145 on hASCs osteogenic differentiation remained partially explored.
FoxO1 is the main regulator of redox balance among the three predominant members of the FoxO family (FoxO1, FoxO3a, and FoxO4). FoxO1 could stimulate proliferation and differentiation as well as inhibits apoptosis of osteogenic lineage cells.16 Previous studies have shown the target relationship between miRNA and FoxO1 as well as the effect of their interaction on osteogenic differentiation process. For instance, miR-182 could act as a FoxO1 inhibitor to antagonize osteogenic differentiation.16,17 In addition, miR-615–3p could hinder the osteogenic differentiation of human lumbar ligamentum flavum cells by suppressing GDF5 and FoxO1.18 However, the modulating regulation between miR-145 and FoxO1 as well as the role of FoxO1 in hASCs needs further investigation.
Herein, we aimed at elucidating the effect of miR-145 and FoxO1 on hASCs osteogenic differentiation and the regulatory mechanisms between them. In the present study, we detected the dynamic expression of miR-145 and FoxO1 before and after osteogenic differentiation. Our findings indicated that miR-145 was involved in the efficient regulation of hASCs osteogenic differentiation through targeting FoxO1. This evidence prompted us to conclude that miR-145 may serve as a novel therapeutic target for hASC osteogenesis-related disorders.
Materials and methods
Cell cultivation and transfection
hASCs HMSC-ad cell line (Sciencell, USA) was cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco), 100 units/ml penicillin, and 100 μg/ml streptomycin (Gibco) in 5% CO2 in a humidified incubator at 37°C. pcDNA3.1 plasmids were used to construct FoxO1 overexpression plasmids. The synthesis of miR-145 mimics, control mimics, pcDNA3.1-FoxO1 and pcDNA3.1-control plasmids was purchased from Invitrogen (Carlsbad, CA, USA). The synthesis of FoxO1 siRNA was conducted by Shanghai Sangon Company. The synthetics were transfected using Lipofectamine, 2000 (Invitrogen, Carlsbad, CA, USA).
Osteoblast differentiation induction
To induce osteoblast differentiation, HMSC-ad cells were plated in six-well plates (1 × 105 cells/well, 2 ml medium per well) and cultured normally. After five days cultivation (to 80%∼90% confluence), the media were replaced with osteoblasts-specific induction medium, the low glucose of Dulbecco's Modified Eagle's Medium with 10% FBS, 10 mM β-glycerophosphate, 0.1 uM dexamethasone, and 0.2 mM antiscorbic acid (Sigma–Aldrich, Louis, MO, USA) to induce osteoblast differentiation. Von Kossa staining was conducted after two weeks.
RT-qPCR
The total RNA was extracted from hASCs osteodifferentiated induction cells with TRIzol reagent (Life Technologies corporation, Gaithersburg, MD, USA) and reverse-transcribed into cDNA using PrimeScript II 1st Strand cDNA Synthesis Kit (TaKaRa, Tokyo, Japan). RT-qPCR was performed using SYBR Premix Ex Taq (TaKaRa, Tokyo, Japan). U6 was used as an endogenous normalization control for miR-145, while GAPDH was used as a FoxO1 mRNA control. The relative level of RNA was quantified using the 2−ΔΔCt method. The primers were synthesized by Shanghai Sangon Company and are listed in Table 1.
Table 1.
RT-qPCR primer sequence.
| Name | Primers |
|---|---|
| MiR-22 | F: 5′-GCAGTTCTTCAGTGGCAA-3′ |
| MiR-22 | R: 5′-GAATACCTCGGACCCTGC-3′ |
| U6 | F: 5′-CTCGCTTCGGCAGCACA-3′ |
| U6 | R: 5′-AACGCTTCACGAATTTGCGT-3′ |
| FoxO1 | F: 5′-TTAGCCAGTCCAACTCGG-3′ |
| FoxO1 | R: 5′-TCTTGACCATCCACTCGTA-3′ |
| GAPDH | F: 5′-TGGTCACCAGGGCTGCTT-3′ |
| GAPDH | R: 5′-AGCTTCCCGTTCTCAGCC-3′ |
Western blot
Quantification of total protein was performed using Bradford protein assay (Bio-Rad, Hercules, CA, USA). Proteins were resolved by SDS-PAGE and transferred to a PVDF membrane using the iBlot Dry Blotting Transfer System (Life Technologies corporation, Gaithersburg, MD, USA). After blocking in PBS with 5% milk and 0.1% Tween for 2 h, the membrane was incubated with primary antibodies for goat anti-FoxO1, anti-Ocn, anti-BSP, anti-OPN (1:1000 dilution) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or goat anti-GAPDH polyclonal antibody (1:1000) (GenScript, Nanjing, China) at 4°C overnight and washed and incubated with secondary antibodies horseradish peroxidase labeled anti-goat (Abcam, Cambridge, MA, USA) for 1–2 h. After incubation, PVDF membrane was washed with PBS before placing in a darkroom. Moderate amount of DAB substrate was applied and the membranes were put in the darkroom for exposure. Images were obtained using a chemiluminescent image analyzer (LAS-4000 mini; Fujifilm) and cumulative optical density value of each target band was semi-quantified by ImageJ software (NIH). GAPDH was used as a loading control to quantify the relative protein level.
Alkaline phosphatase staining and alizarin red S staining
Seven or fourteen days after osteogenic induction, DMEM media were discarded. Cells were washed three times with PBS and fixed with 4% paraformaldehyde. The cell layer was subsequently washed and dried in air. Reaction solution was prepared using an alkaline phosphatase (ALP) Assay Kit (Beyotime Biotechnology, Shanghai, China). After reaction at 37°C for 1 h, purplish red granules formed were detected under an optical microscope; 7 or 14 days post osteogenic differentiation, cells were fixed with ethanol for 10 min. Lastly, the cells were treated with 0.1% alizarin red S (ARS)-Tris-Hcl (pH 8.3) at 37°C for 1 h. To quantify calcium deposition, cells were washed with distilled water and dried in air. Absorbance was measured under an optical microscope.
Dual-luciferase reporter assay
The FoxO1 3′UTR fragment containing the predicted potential miR-145-binding sites was amplified and pGL3-WT plasmids were constructed by PCR method. pGL3-Mut was generated by site-directed mutagenesis, replacing the first six ribonucleotides of the miR-145 complementary sequence. HEK293T cells were grown in 24-well plates and cotransfected with miR-145 mimics and pGL3-WT (miR-WT group), miR-145 mimics and pGL3-Mut (MiR-Mut group), control mimics and pGL3-WT (NC-WT group), control mimics and pGL3-MuT (NC-Mut group). The media were replaced after 6 h; 24 h after cotransfection, the Dual-Luciferase Reporter Gene Test Kit (Beyotime Biotechnology, Shanghai, China) was used to detect the luciferase activities in different groups. The firefly and the renal luciferase reagent were added to detect the luciferase activity of each group.
Statistical analysis
Affymetrix Data Mining Tool version 3.1 (DMT 3.1) and scripts called within R environment (http://www.r-project.org/) were utilized to screen differentially expressed mRNAs and miRNAs. The filtration criteria were: Log2 (Fold Change) >2 and P < 0.001. MiRNA analysis was conducted using GSE72429 and GPL16770. MRNA analysis was conducted using GSE63754 and GPL17077 platform. Three osteogenic differentiation hASCs and three normal hASCs without osteogenic differentiation were selected to screen out differentially expressed miRNAs and mRNAs, respectively. The results represented the mean of three independent experiments and data were presented as mean ± standard deviation (x ± s). Comparisons between two groups were analyzed by Student’s t test. For the testing among multiple groups, a one-way analysis of variance (ANOVA) was conducted. Statistical analysis was performed using GraphPad Prism 6.0 software. P-values < 0.05 were considered to be statistically significant.
Results
FoxO1 was highly expressed after hASCs osteogenic differentiation
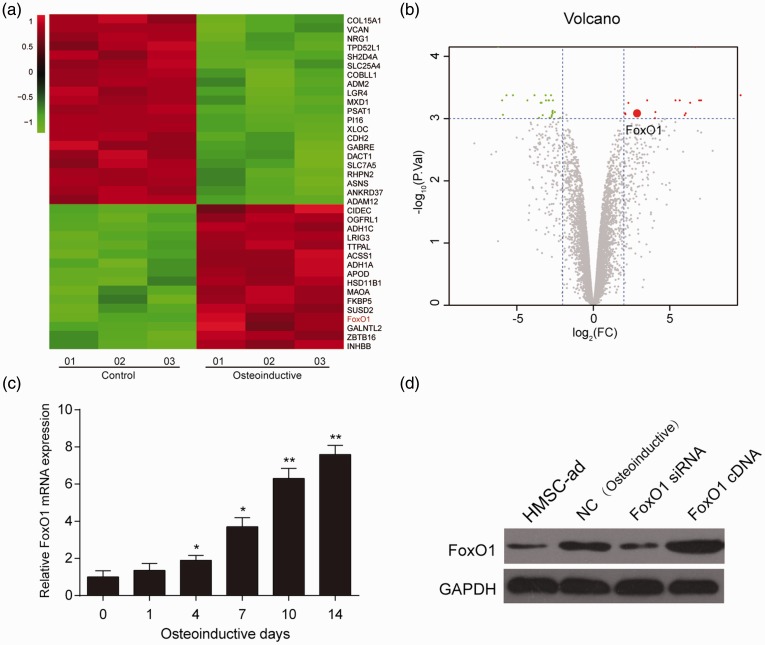
Three osteogenic HMSC-ad cell lines and three normal HMSC-ad without osteogenic differentiation cell lines were selected to screen out differentially expressed mRNAs. Among the 16 upregulated mRNAs with significant differences in osteoinductive HMSC-ad cell lines, there was FoxO1. The expression of FoxO1 in osteoinductive HMSC-ad cell lines was 2.29-fold higher in osteoinductive differentiation cell lines (Figure 1(a) and (b)). The expression of FoxO1 was gradually upregulated with the progression of osteogenic differentiation (Figure 1(c)). The protein expression of FoxO1 increased in hASCs after osteoinductive differentiation. Meanwhile, overexpression of FoxO1 (FoxO1 cDNA) and silence expression of FoxO1 (FoxO1 siRNA) was successfully constructed (Figure 1(d)).
Figure 1.
The expression of FoxO1 was up-regulated during hASCs osteogenesis. (a) Heat map revealed the FoxO1 was higher expressed after hASCs osteogenic differentiation. (b) Volcano plot showed the FoxO1 was highly expressed after hASCs osteogenic differentiation. (c) FoxO1 expression was up-regulated gradually along with the osteoinductive differentiation. (d) FoxO1 expression increased after osteoinductive differentiation in HMSC-ad cells. *P < 0.05, **P < 0.01, compared with day 0. (A color version of this figure is available in the online journal.)
FoxO1 promoted hASCs osteogenic differentiation
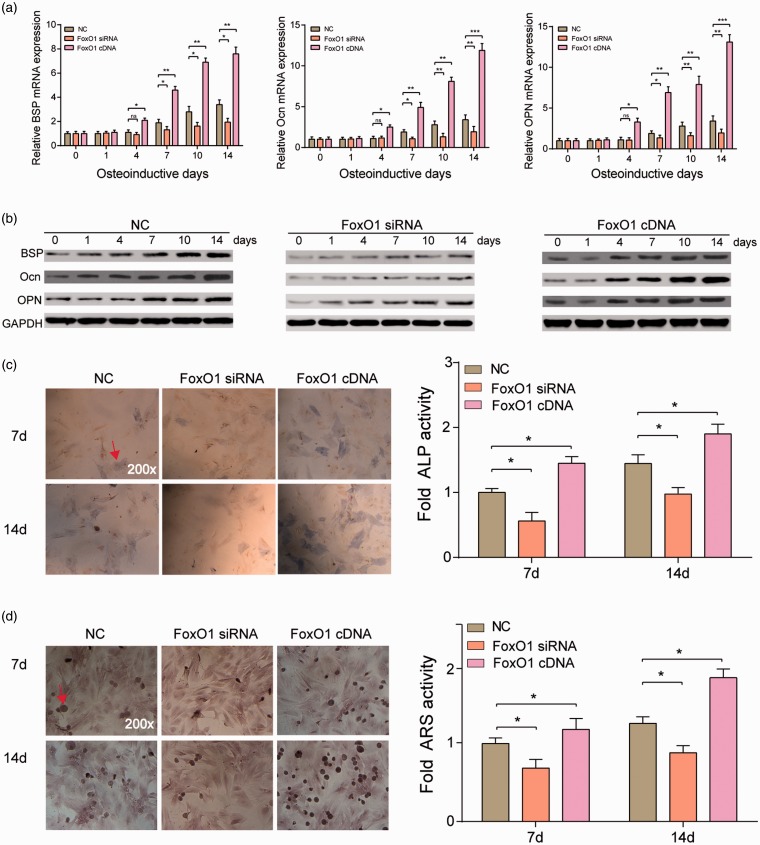
The result of RT-qPCR and Western blot was consistent. The expression of BSP, Ocn, and OPN in silence expression of FoxO1 group was downregulated since the fourth day and was more significant with the progression of osteogenic differentiation (P < 0.01). A remarkably opposite trend was observed in overexpression of FoxO1 group (P < 0.001) (Figure 2(a) and (b)).
Figure 2.
FoxO1 promoted hASCs osteogenesis. (a–b) RT-PCR and Western blot results showed that BSP, Ocn, and OPN mRNA expression in FoxO1 siRNA group were much lower than in NC group after osteoinductive differentiation, whereas those in FoxO1 cDNA group were much higher than in NC group after osteoinductive differentiation, especially four days after the differentiation induction. *P < 0.05, **P < 0.01, ***P < 0.001 compared with NC group. (c and d). ALP and ARS staining results showed that HMSC-ad osteogenesis differentiation after osteoinduction was much stronger in FoxO1 cDNA group yet much weaker in FoxO1 siRNA group at either time point (day 7 and day 14). *P < 0.05 compared with NC group. (A color version of this figure is available in the online journal.)
ALP staining and ARS staining showed that on days 7 and 14, ALP activity and calcification were lowered in FoxO1 siRNA group, but enhanced in FoxO1 cDNA group (P < 0.05) (Figures 2(c) and 3(d)). Collectively, these results revealed that FoxO1 could promote hASCs osteogenic differentiation.
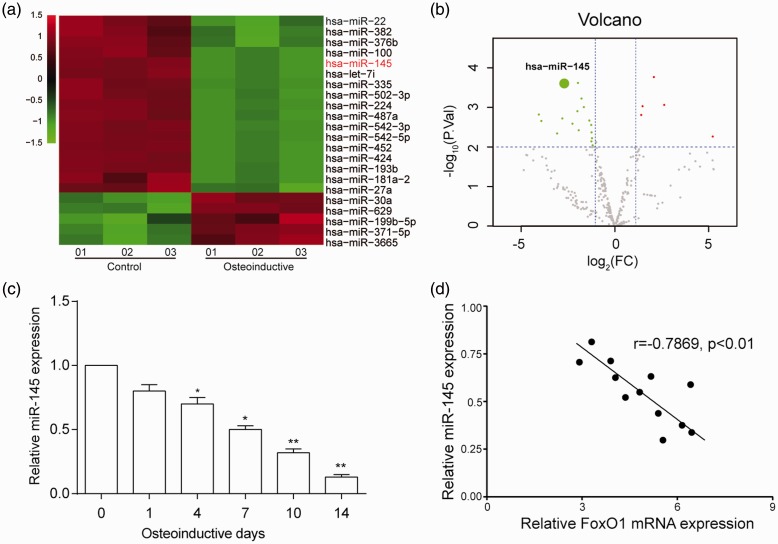
Figure 3.
MiR-145 expression was down-regulated during hASCs osteogenesis. (a) Heat map revealed the miR-145 was lower expressed after hASCs osteogenic differentiation. (b) Volcano plot showed the miR-145 was lower expressed after hASCs osteogenic differentiation. (c) MiR-145 expression was downregulated gradually along with osteoinductive differentiation. (d) There is a significantly negative correlation between miR-145 and FoxO1 expression. *P < 0.05, **P < 0.01 compared with day 0. (A color version of this figure is available in the online journal.)
MiR-145 was low expressed during hASCs osteogenic differentiation
Three osteogenic HMSC-ad cell lines and three normal HMSC-ad without osteogenic differentiation cell lines were selected to screen out differentially expressed miRNAs. Microarray analysis showed that miR-145 was low expressed in all three HMSC-ad osteogenic differentiation cell lines (Figure 3(a)). The expression of miR-145 in HMSC-ad was 2.09-fold lower after osteoinductive differentiation. MiR-145 expression was downregulated since the fourth day and was more significant along with the progression of osteogenic differentiation. Significant statistical difference was detected on day 14 (Figure 3(c)). MiR-145 and FoxO1 mRNA expression was detected in six cell lines on day 7 and 14 after osteo-differentiation induction, and the correlation analysis results showed that there was a significantly negative correlation between them (P < 0.01) (Figure 3(d)).
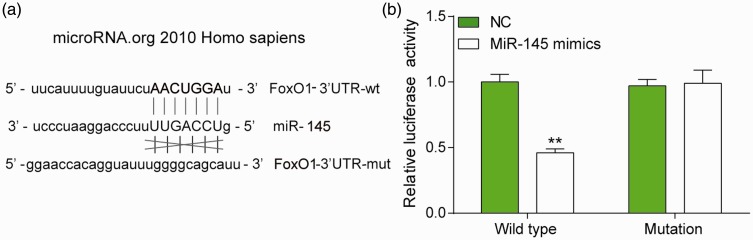
MiR-145 targeted the 3′UTR region of FoxO1
According to the database TargetScan, the binding site of miR-145 was located between bases 770 and 776 of the FoxO1 3′-UTR, suggesting that FoxO1 might be the target of miR-145 (Figure 4(a)). The luciferase reporter gene assay was conducted in 293T cells to verify their binding relationship. The result revealed that the luciferase activity of cells cotransfected with miR-145 mimics and pGL3-WT was significantly lower, whereas miR-145 mimics had no such effect on the fluorescence of the Mut recombinant plasmid (Figure 4(b)). Taken together, these results revealed that miR-145 directly targeted FoxO1 3′UTR.
Figure 4.
MiR-145 targeted FoxO1 3′UTR. (a) The binding sites of miR-145 and FoxO1 as well as the putative binding relationship were illustrated. (b) Dual luciferase reporter gene assay results demonstrated that the luciferase activity in miR-145 mimics + wild type group was significantly weaker than in NC + wild type group. **P < 0.01 compared with NC group. (A color version of this figure is available in the online journal.)
MiR-145 prevented hASCs osteogenic differentiation via suppressing FoxO1 expression
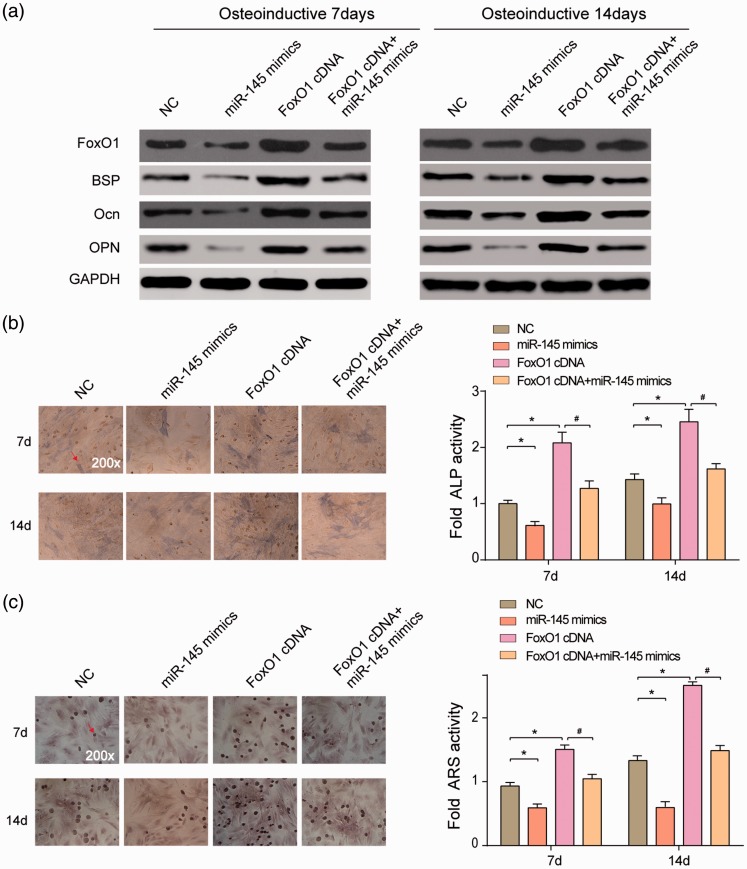
The protein expression of the cells after osteoinductive differentiation was detected on day 7 and 14. The protein expression of BSP, Ocn and OPN showed identical tendencies on day 7 and 14. In FoxO1 cDNA cells, BSP, Ocn, and OPN levels were high, whereas in miR-145 mimics cells, their levels were lower than in NC cells (Figure 5(a)). APL activity and ARS activity (calcification) were optimal in FoxO1 cDNA group, while the worst in miR-145 mimics group (P < 0.05) (Figure 5(b) and (c)). The above results demonstrated that miR-145 inhibited expression of osteogenic differentiation protein markers BSP, Ocn, and OPN via suppressing FoxO1, preventing hASCs osteogenic differentiation.
Figure 5.
MiR-145 inhibited FoxO1 to suppress hASCs osteogenesis. (a) FoxO1, BSP, Ocn, and OPN expression in miR-145 mimics group was much lower, whereas those in FoxO1 cDNA group was much higher than in NC group. There was no significant difference between the NC group and the (b–c) ALP and ARS staining results demonstrated that miR-145 mimics significantly reduced HMSC-ad osteogenesis differentiation after osteoinduction and FoxO1 cDNA significantly increased HMSC-ad osteogenesis differentiation. The simultaneous transfection of FoxO1 cDNA and miR-145 mimics showed no significant difference from the NC group. *P < 0.05, compared with NC group; #P < 0.05, compared with miR-145 mimics group. (A color version of this figure is available in the online journal.)
Discussion
We have uncovered that miR-145 was down-regulated during hASCs osteogenic differentiation and it directly regulated FoxO1 expression. Importantly, the dynamic expression of miR-145 and FoxO1 led to efficient modulation of hASCs osteogenic differentiation. Our findings would contribute to the comprehension of the regulatory network on hASCs osteogenic differentiation, and provided the evidence that miR-145 might be developed as a potential therapy target for osteogenic-related diseases.
MiRNAs had been detected dysregulated expression and verified to act as an important role during osteogenic differentiation of various types of human cells. For example, miR-615–3p was downregulated in many human cell lineages such as human BMSCs and osteoblasts during osteogenic differentiation.18 MiR-145 was also downregulated during osteogenic differentiation in C2C12 (mouse myoblast) and MC3T3-E1 (mouse preosteoblast) cells,14 which was in accordance with our result. Our findings showed that miR-145 was low expressed after hASCs osteogenic differentiation, indicating that miR-145 contributed to the prevention of hASCs osteogenic differentiation. Similarly, recent studies also have identified that miR-145 could regulate osteoblast differentiation by targeting transcription factors, core-binding factor subunit beta (Cbfb) and osterix (Osx).19 These findings underpinned the potential of systemic miR-145 therapy as a promising strategy by regulating the process of osteogenic differentiation.
MiRNAs have been discovered to regulate FoxO1 expression directly and negatively during osteogenic differentiation according to several recent studies. For instance, Yin et al.18 found miR-615–3p suppressed osteogenic differentiation by directly inhibiting GDF and FoxO1 in human lumbar ligamentum flavum cells. Kim et al.16 identified FoxO1 as a target of miR-182 in C3H10T1/2 MSCs and MC3T3E1 preosteoblasts, negatively regulating osteoblast differentiation. The target relationship of miR-145 and FoxO1 in other medical processes was explored. A recent study showed that miR-145 was frequently downregulated in human bladder cancer and suppressed tumor formation by directly inhibiting FoxO1 functioning.20 However, the regulation of miR-145 targeting FoxO1 in hASCs remained to be elucidated. In the current study, we found FoxO1 a potential target of miR-145 and confirmed that miR-145 targeted the 3′UTR region of FoxO1 directly. Hence, based on previous studies, we believe that FoxO1 plays a key role during osteo-differentiation. FoxO1 can be targeted by various miRNAs during osteogenic differentiation, yet its suppression by miR-145 in human ASCs has not been elucidated until the present study. We thus speculated that miR-145 might reduce bone differentiation by suppressing FoxO1 directly.
FoxO1 has revealed high expression during osteogenic differentiation. For instance, FoxO1 mRNA levels remarkably increased after 14 days during osteoblast differentiation.21 Teixeira et al.22 approved that FoxO1 activity and expression increased during osteogenic differentiation. Coincidentally, we found that FoxO1 was highly expressed after hASCs osteogenic differentiation, suggesting that FoxO1 may induce hASCs osteogenic differentiation. Similarly, FoxO1 has recently been reported to inhibit osteoblast differentiation, indirectly suggesting that FoxO1 has a positive effect on promoting differentiation.21 Yin et al.18 previously proved that knockdown of FoxO1 could inhibit the osteogenic differentiation of human lumbar ligamentum flavum cells. However, the role of FoxO1 in differentiation of mesenchymal cells was also unraveled in recent studies. It has been shown that activation of FoxO1 prevented mesenchymal cells from differentiating into fat or muscle cells.22 It was possible that this apparent discrepancy was due to the approach used or that FoxO1 had different effects on osteogenic differentiation depending on the degree.
It was known that one gene can be targeted by multiple miRNAs, and in turn, one miRNA can also regulate multiple target genes. A limitation of this study was the fact that no other genes were taken into consideration, compromising the credit of the target relationship between miR-145 and FoxO1. The lack of animal experiments in our study should also be noted.
Collectively, our findings unveiled a novel mechanism of the osteogenic differentiation process and suggested that miR-145 and its target gene FoxO1 may be potential targets for the therapy of human osteogenic-related disorders.
Authors' contributions: All authors participated in the design, interpretation of studies and analysis of data and review of manuscript; Lugang Zhou, Yujie Sun and Tian He conducted the experiments; Hao Su, Jianqiang Ni and Peng Shi supplied critical reagents; Wei Hao and Hongzhi Liu drafted the manuscript; Xin Wang critically revised the manuscript and received the grant.
Approval of final manuscript: all authors.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This study was supported by Shandong Provincial Natural Science Foundation, China (ZR2016HM54).
References
- 1.Xie Q, Wang Z, Zhou H, Yu Z, Huang Y, Sun H, Bi X, Wang Y, Shi W, Gu P, Fan X. The role of miR-135-modified adipose-derived mesenchymal stem cells in bone regeneration. Biomaterials 2016; 75:279–94 [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284:143–7 [DOI] [PubMed] [Google Scholar]
- 3.Lin CY, Chang YH, Li KC, Lu CH, Sung LY, Yeh CL, Lin KJ, Huang SF, Yen TC, Hu YC. The use of ASCs engineered to express BMP2 or TGF-beta3 within scaffold constructs to promote calvarial bone repair. Biomaterials 2013; 34:9401–12 [DOI] [PubMed] [Google Scholar]
- 4.Deng Y, Zhou H, Zou D, Xie Q, Bi X, Gu P, Fan X. The role of miR-31-modified adipose tissue-derived stem cells in repairing rat critical-sized calvarial defects. Biomaterials 2013; 34:6717–28 [DOI] [PubMed] [Google Scholar]
- 5.Toupadakis CA, Wong A, Genetos DC, Cheung WK, Borjesson DL, Ferraro GL, Galuppo LD, Leach JK, Owens SD, Yellowley CE. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am J Vet Res 2010; 71:1237–45 [DOI] [PubMed] [Google Scholar]
- 6.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006; 24:1294–301 [DOI] [PubMed] [Google Scholar]
- 7.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 2005; 33:1402–16 [DOI] [PubMed] [Google Scholar]
- 8.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 2003; 5:362–9 [DOI] [PubMed] [Google Scholar]
- 9.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 2009; 10:126–39 [DOI] [PubMed] [Google Scholar]
- 10.Wei J, Shi Y, Zheng L, Zhou B, Inose H, Wang J, Guo XE, Grosschedl R, Karsenty G. miR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J Cell Biol 2012; 197:509–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskildsen T, Taipaleenmaki H, Stenvang J, Abdallah BM, Ditzel N, Nossent AY, Bak M, Kauppinen S, Kassem M. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc Natl Acad Sci U S A 2011; 108:6139–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sangani R, Periyasamy-Thandavan S, Kolhe R, Bhattacharyya MH, Chutkan N, Hunter M, Isales C, Hamrick M, Hill WD, Fulzele S. MicroRNAs-141 and 200a regulate the SVCT2 transporter in bone marrow stromal cells. Mol Cell Endocrinol 2015; 410:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Q, Wang Z, Bi X, Zhou H, Wang Y, Gu P, Fan X. Effects of miR-31 on the osteogenesis of human mesenchymal stem cells. Biochem Biophys Res Commun 2014; 446:98–104 [DOI] [PubMed] [Google Scholar]
- 14.Jia J, Tian Q, Ling S, Liu Y, Yang S, Shao Z. miR-145 suppresses osteogenic differentiation by targeting Sp7. FEBS Lett 2013; 587:3027–31 [DOI] [PubMed] [Google Scholar]
- 15.Fukuda T, Ochi H, Sunamura S, Haiden A, Bando W, Inose H, Okawa A, Asou Y, Takeda S. MicroRNA-145 regulates osteoblastic differentiation by targeting the transcription factor Cbfb. FEBS Lett 2015; 589:3302–8 [DOI] [PubMed] [Google Scholar]
- 16.Kim KM, Park SJ, Jung SH, Kim EJ, Jogeswar G, Ajita J, Rhee Y, Kim CH, Lim SK. miR-182 is a negative regulator of osteoblast proliferation, differentiation, and skeletogenesis through targeting FoxO1. J Bone Miner Res 2012; 27:1669–79 [DOI] [PubMed] [Google Scholar]
- 17.Huang S, Wang S, Bian C, Yang Z, Zhou H, Zeng Y, Li H, Han Q, Zhao RC. Upregulation of miR-22 promotes osteogenic differentiation and inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells by repressing HDAC6 protein expression. Stem Cells Dev 2012; 21:2531–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin J, Zhuang G, Zhu Y, Hu X, Zhao H, Zhang R, Guo H, Fan X, Cao Y. MiR-615-3p inhibits the osteogenic differentiation of human lumbar ligamentum flavum cells via suppression of osteogenic regulators GDF5 and FOXO1. Cell Biol Int 2017; 41:779–86 [DOI] [PubMed] [Google Scholar]
- 19.Jia J, Zhou H, Zeng X, Feng S. Estrogen stimulates osteoprotegerin expression via the suppression of miR-145 expression in MG-63 cells. Mol Med Rep 2017; 15:1539–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang G, Huang C, Li J, Huang H, Jin H, Zhu J, Wu XR, Huang C. Role of STAT3 and FOXO1 in the divergent therapeutic responses of non-metastatic and metastatic bladder cancer cells to miR-145. Mol Cancer Ther 2017; 16:924–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siqueira MF, Flowers S, Bhattacharya R, Faibish D, Behl Y, Kotton DN, Gerstenfeld L, Moran E, Graves DT. FOXO1 modulates osteoblast differentiation. Bone 2011; 48:1043–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teixeira CC, Liu Y, Thant LM, Pang J, Palmer G, Alikhani M. Foxo1, a novel regulator of osteoblast differentiation and skeletogenesis. J Biol Chem 2010; 285:31055–65 [DOI] [PMC free article] [PubMed] [Google Scholar]