Short abstract
Congenital anorectal malformation is the most common digestive tract malformation in newborns. It has been reported that FOXD3/FOXD4, a forkhead transcription factor, regulates the generation, migration, and differentiation of neural crest cells. However, whether FOXD3/FOXD4 takes part in anorectal malformation remains unclear. In the present study, we used ethylene thiourea to induce the animal models of anorectal malformation in rat embryos and to interrogate the role of FOXD3/FOXD4 in anorectal malformation pathogenesis. Hindgut samples of the animal models were collected at E15, E17, E19, and E21 days of age. The expression of FOXD3/FOXD4 was detected by immunohistochemistry, western blot, and quantitative real-time fluorescence PCR. By immunohistochemical staining, FOXD3/FOXD4 was observed in epithelial cells of the rectum and the anus both in normal and rat embryos with anorectal malformation. Expression level analysis by western blot indicated that FOXD3/FOXD4 expression increased in ethylene thiourea-induced anorectal malformation groups. mRNA expression as determined by quantitative real-time fluorescence PCR analysis was consistent with the western blot results. Tentative conclusions were drawn that FOXD3/FOXD4 is expressed in the hindgut in rat embryos and is upregulated in anorectal malformation. FOXD3/FOXD4 is required for the development of the hindgut, and its aberrant expression may be an important factor leading to the incidence of anorectal malformation.
Impact statement
Congenital anorectal malformation (ARM) is the most common digestive tract malformation in newborns. The pathophysiological ground remains unclear. In this study, we used animal models of ARM for the first time to interrogate the role of FOXD3/FOXD4 in ARM pathogenesis. The animal models of ARM were successfully induced by ethylene thiourea (ETU) in rat embryos providing a strong basis for pathogenesis study of this disease. Expression analysis of FOXD3/FOXD4 was carried out in these models, and the results shape a deeper understanding of FOXD3/FOXD4 being required for the normal development of the hindgut. The aberrant expression of FOXD3/FOXD4 may be an important factor leading to ARM incidence.
Keywords: Congenital anorectal malformation, ethylene thiourea, animal model, FOXD3/FOXD4, forkhead transcription factor, hindgut
Introduction
Congenital anorectal malformation (ARM) is the most common digestive tract malformation in newborns with an incidence of 1/5000 to 1/1500.1 ARM comprises a wide spectrum of diseases affecting the anus, rectum, and urogenital tracts, resulting from impeded hindgut development during the embryonic stage. However, the pathophysiological mechanisms of ARM remain unclear. Recent studies have demonstrated that the forkhead-box (FOX) FOXD3/FOXD4 signaling pathway is involved in the embryonic development of many systems in higher vertebrates. During gut development, it maintains the formation of intestinal epithelial growth as well as intestinal wall development along the anterior–posterior axis during regional differentiation and plays an important role in building the enteric nervous system.2
Human FOXD3/FOXD4 is an important member of the FOX gene family and is located on 1p31.3 and 9p24.3. It has been shown that, acting as an important transcription factor at the embryonic stage, FOXD3/FOXD4 is indispensable for embryonic development and maintenance of embryonic stem cells in mammals.3,4 The translation product of FOXD3 is a transcriptional regulatory factor that activates or inhibits the expression of target genes, which not only activates gene cascades during the embryonic development stage but also regulates the differentiation of select embryonic stem cell populations.5,6 Hanna et al.4 showed that FOXD3 is essential for the development of mammalian embryos. They also found that FOXD3 may play a role in maintaining the number of stem cells in the inner cell mass of the upper germ layer. FOXD3 is expressed in a specific pattern in the embryo, including the binding site between ectoderm and the embryonic inner layer. In short, FOXD3 plays an important role in the formation of ectoderm and mesoderm in the embryo. Recently, Mundel and Labosky7 further demonstrated that FOXD3 is essential for the development of mammalian embryos.
FOXD4 acts very early in the nascent neural ectoderm to promote the formation of the immature neural ectoderm, expand the neural plate, and delay the onset of neural differentiation.8,9 It both upregulates genes that maintain an immature, proliferative neural ectoderm and downregulates genes that promote the transition to neural progenitors and lead to neural differentiation.10 Determining how FOXD4 both upregulates and downregulates its various target genes is key to understanding the transcriptional network that regulates the critical developmental transition from an immature, proliferative neural ectoderm to a definitive neural plate that comprises neurally committed, differentiating cells. Duplications that gave rise to the FOXD4 group appear to be relatively recent, that is, during hominid evolution. Very little information is available on these proteins, but it has been shown that at least two FOXD4L genes are transcriptionally active; furthermore, evidence of purifying selection in the forkhead domains of these proteins suggests that they may play physiological roles.11
The FOXD3/FOXD4 genes have been shown to regulate the generation, migration, and differentiation of neural crest cells.12 However, whether they take part in ARM remains unclear. In the present study, we used ethylene thiourea (ETU) to induce animal models of ARM in Wistar rat embryos and then collected hindgut samples to detect FOXD3/FOXD4 expression during the development of the anus and rectum, providing evidence for FOXD3/FOXD4 in the pathogenesis of ARM.
Materials and methods
Animal model
Nulliparous healthy adult Wistar rats (220–250 g) were obtained from the animal experiment center of Shengjing Hospital of China Medical University. Specific pathogen free adults included 40 female and 10 male rats. Male and female rats were placed in cages at a ratio of 3:1 at 22:00 h, and a vaginal smear was obtained the next day at 8:00 h to interrogate the presence of sperm or vaginal suppository via microscopy, remember 0 days (E0). In the ETU-induced group, rats were gavage-fed a single dose of 125 mg/kg of 1% ETU (2-imidazolidinethionep; Aldrich Chemical Co., Germany) on E10; in the control group, rats received saline alone without the addition of ETU. Six hindgut samples (anatomical microscope observation of the location of the anus, there is no anal opening to determine the presence of ARMs) were selected at E15, E17, E19, and E21. Embryos were divided into three groups: saline-induced normal control (NS), ETU-induced normal control (NE), and ETU-induced ARM (AE) groups. Methods of aseptic operation were used to collect hindgut samples, which were immediately placed at −80°C. FOXD3/FOXD4 expression was detected by western blot, immunohistochemistry, and quantitative real-time PCR (qRT-PCR) at E15, E17, E19, and E21.
Immunohistochemistry
Rat embryos were fixed in 4% paraformaldehyde/0.1 M phosphate-buffered saline (PBS) for 12 to 24 h depending on size. Then, embryos from each age group were dehydrated, embedded in paraffin, and sectioned serially transversely, sagittally, and coronally at 4 µm thickness. Embryos were incubated 60°C for 2 h after room temperature preservation. Xylene I and Xylene II were used for dewaxing for 10 min each. Dehydration was performed using a gradient of ethanol (ethanol: 95%, 85%, and 50%) for 5 min incubations each. Samples were rinsed with PBS and then washed three times with PBS for 5 min. Samples were then placed in 3% hydrogen peroxide (H2O2) and protected from light for 10 min at room temperature. Samples were rinsed with PBS and then washed three times with PBS for 5 min. Samples were then incubated with 1–3 drops of Serum Blocking Reagent for 15 min, then incubated with 1–3 drops of Avidin Blocking Reagent for 15 min, and subsequently rinsed with PBS. Next, samples were incubated with 1–3 drops of Biotin Blocking Reagent for 15 min, rinsed with PBS, and then washed three times in PBS for 5 min. Samples were incubated with rabbit anti-FOXD3/FOXD4 (1:200 dilution; Beijing Biosynthesis Biotechnology Co., Ltd, Beijing, China) diluted in Incubation Buffer overnight (16 h) at 4°C. Samples were then rinsed with PBS and then washed three times in PBS for 5 min. Slides were then coated in a solution of goat anti-rabbit secondary antibody (1:2000 dilution; Beijing Biosynthesis Biotechnology Co., Ltd, Beijing, China) for 1 h. Samples were again rinsed with PBS, washed three times with PBS for 5 min, and then finally transferred to a solution of streptavidin–horseradish peroxidase (LSAB2 System; Agilent (Dako), Santa Clara, CA, USA) for 10 min. Then, sections were counterstained with hematoxylin for 1 min. The intensity of tissue staining was monitored under a microscope. Samples were rinsed with PBS, washed with PBS for 10 min, and finally rinsed with distilled water. Slides were examined under a microscope and the density of the positively stained area was calculated at 400× magnification.
To determine positive FOXD4/FOXD3 staining, 10 fields (400×) were randomly selected for analysis. In each field, 50 positively stained cells were analyzed. Staining intensity of positive cells was determined and the percentage of positively stained cells was calculated (yellow staining indicated positive cells and non-staining indicated negative cells). Positive cells were defined by the presence yellow staining and the following three conditions: (1) the structure of cellular organization is clear; (2) positive particles are well positioned; and (3) the staining is clear and its intensity is obviously higher than that of the background. Percentage of positive cell-staining was scored via the following method: 1 point is ≤10% positive cells, 2 points is 11% to 50%, 3 points is 51% to 75%, and 4 points is ≥75%. The intensity of positive staining was scored via the following method: 0 point is colorless, 1 point is light yellow, 2 points is brownish yellow, and 3 points is brown.
The target image was collected into the computer by Qwin Leica image analysis system using an optical microscope (10× magnification). Images were taken of all slices using the Leica Qwin image analysis system. Six fields of view were randomly selected for each slice, and image analysis was performed used Image Pro Plus 6.0. The mean expression of FOXD4/FOXD3 was calculated using integral optical density.
Western blot
Protein was extracted with a Jiangsu Biyun Tianquan total protein extraction kit. Protein extracts (50 µg) were heated to 90°C for 10 min and size-fractionated on Bis-TRIS sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (Millipore, Billerica, MA, USA). After being blocked with 5% fat-free milk in TRIS-buffered saline (1 h, room temperature), extracts were subsequently incubated with rabbit anti-FOXD3 (bs-11518R-HRP; 53 kDa; 1:200 dilution; Beijing Biosynthesis Biotechnology Co., Ltd, Beijing, China), rabbit anti-FOXD4 (bs-7127R-HRP; 47 kDa; 1:200 dilution; Beijing Biosynthesis Biotechnology Co., Ltd, Beijing, China), or anti-β-actin (YT0099; 43 kDa; 1:2000 dilution; ImmunoWay Biotechnology Company, Plano, TX, USA) at 4°C overnight. Membranes were washed and incubated with secondary antibody, Goat anti-rabbit IgG/HRP (bs-0295G-HRP; 1:5000 dilution; Beijing Biosynthesis Biotechnology Co., Ltd, Beijing, China), for 2 h at room temperature. Following another wash, membranes were developed using a chemiluminescent substrate kit (Pierce, Pierce, Rockford, IL, USA). Densitometry analysis was performed using GEL-PRO 4.0 software (Media Cybernetics, LP, Waltham, MA, USA). The relative densities were calculated as the density of the tested protein band normalized to that of the loading control (β-actin) band.
Quantitative real-time fluorescence PCR Total RNA were extracted from rat embryos using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The purity of extracted total RNA was determined by the 260/280 nm ratio with expected values between 1.9 and 2.0, and then RNA was stored at −80°C for later use. Total RNA was reverse transcribed into complementary DNA (cDNA; PrimeScript™ RT Reagent Kit with gDNA Eraser, Takara Biotechnology, Shiga, Japan) per manufacturer’s instructions. qRT-PCR was performed using SYBR Premix Ex Taq (Takara Biotechnology, Shiga, Japan) on a 7900HT fast Real-time PCR system (Applied Biosystems, Foster City, CA, USA) under the following conditions: 50°C for 2 min, 95°C for 3 min, 40 cycles of 95°C for 10 s, and 59°C for 30 s. β-Actin was used as a reference gene. The relative levels of gene expression were determined as ΔCt = Ctgene − Ctreference, and the fold change in gene expression was calculated via the 2−ΔΔ Ct method.13 Experiments were performed in triplicate. The primers of qRT-PCR were as follows: FOXD3 forward, 5' CGAGCAAGCCCAAGAAC3', reverse, 5' TGCTGATGAACTCGCAGAT-3' (123 bp); FOXD4 forward, 5'TCATTAGTGACCGCTTCCC3', reverse, 5'TCCAGGCTCCAGTAGTTGC3' (145 bp); and β-actin forward, 5'GGAGATTACTGCCCT GGCTCCTA3', reverse, 5' GACTCATCGTACTCCTGCTTGCTG3' (139 bp).
Statistical analysis
The Statistical Program for Social Sciences version 13.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. Student’s t-test was used to compare FOXD3/FOXD4 levels between the normal group and the short tail group. All results are presented as mean ± standard deviation (S.D.), where P values of 0.05 or less are considered statistically significant.
Results
Description of the ARM animal model
Pregnant rats injected with saline produced 147 embryos with a survival rate of 100%, and no dysplasia, fetal stillbirth, or fetal intrauterine absorption embryos was observed. For comparison, only 119 embryos were produced by pregnant rats injected with ETU, within which ARMs were found in 73. The teratogenic rate was 61.34%. In addition to anal deformity, other malformations such as tail malformations (loss or short), spina bifida, gastroschisis, and omphalocele were observed.
The appearance and location of the anus were both normal in the saline- (NS) and ETU- (NE) induced control groups. However, in the ETU teratogenic ARM (AE) group, only a closed blind end was noted on the skin instead of an anal opening.
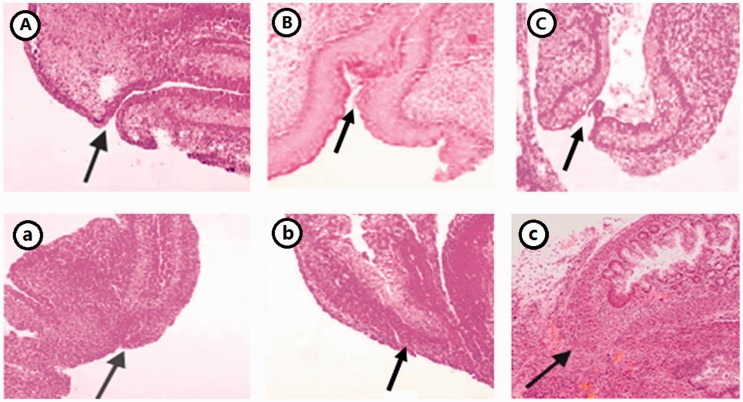
Histologic changes of the anorectal tissue were observed via hematoxylin staining. The location of the anus was normal in both the NS and NE groups, the contour of the anus was clear, and the layer of the rectum developed and improved with age. In contrast, in the AE group, the anal opening was closed, and atresia at the end of rectum displayed hyperplastic squamous epithelium and a thickened muscular layer (Figure 1).
Figure 1.
HE staining of hindgut of the normal and ARM rat embryos (×100). (A) NE15, (B) NE17, (C) NE21. (a) AE15, (b) AE17, (c) AE21. (A color version of this figure is available in the online journal.)
Immunohistochemical staining
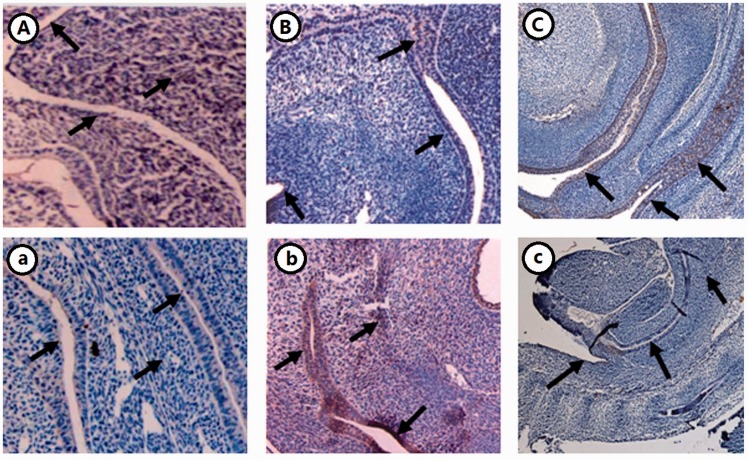
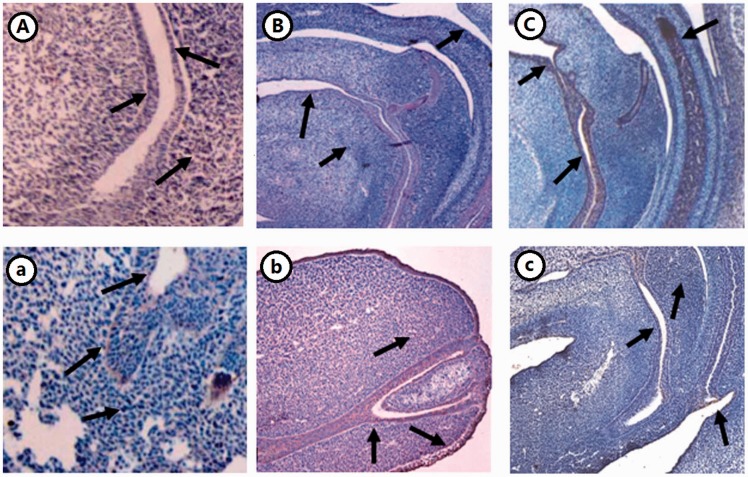
By immunohistochemical staining, FOXD3/FOXD4 was observed in epithelial cells of the rectum and the anus in NE17 and NE19 and was stronger at these sites in AE17 and AE19. The expression of FOXD3/FOXD4 was strongest in AE17 in the urorectal septum and periphery of the rectum (Figures 2 and 3).
Figure 2.
Expression of FOXD3 in the hindgut of ETU-induced rat embryos (×10). (A–C) NE15, 17, 21; (a–c) AE15, 17, 21. (A color version of this figure is available in the online journal.)
Figure 3.
Expression of FOXD4 in the hindgut of ETU-induced rat embryos (×10). (A–C) NE15, 17, 21; (a–c) AE15, 17, 21. (A color version of this figure is available in the online journal.)
Western blot
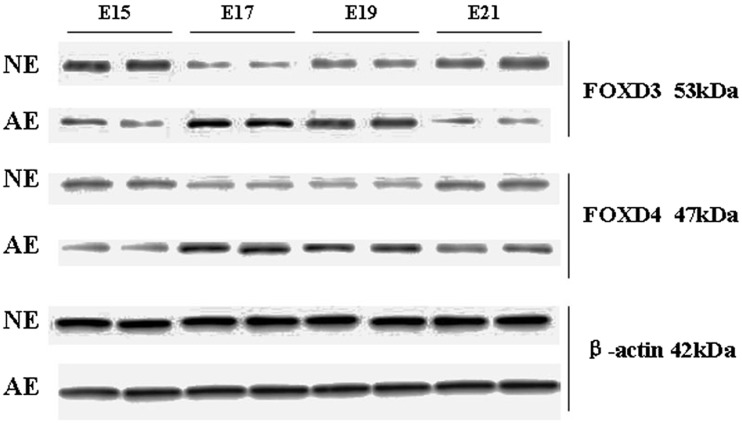
FOXD3 protein expression was higher in NE15 and NE21 in the NE group (70.23 ± 0.12 and 40.35 ± 0.09, respectively) but lower in NE17 and NE19 (7.94 ± 0.01 and 17.17 ± 0.03, respectively). In contrast, in the AE group, FOXD3 protein expression was lower in AE15 and AE21 (17.05 ± 0.03 and 9.14 ± 0.02, respectively) but higher in AE17 and AE19 (187.86 ± 0.36 and 66.48 ± 0.13, respectively). FOXD4 protein expression was higher in NE15 and NE21 (36.84 ± 0.07 and 31.91 ± 0.06, respectively) and lower in NE17 and NE19 (14.29 ± 0.03 and 31.91 ± 0.06, respectively). FOXD4 protein expression in AE15 and AE21 was lower in the AE group (12.31 ± 0.04 and 22.73 ± 0.06, respectively) and higher in AE17 and AE19 (103.67 ± 0.28 and 64.33 ± 0.17, respectively). These results show that the expression of FOXD3/FOXD4 protein significantly decreased at E17 and E19 in the NE group as compared to respective expression in the AE group; this difference was statistically significant (Figure 4).
Figure 4.
Western blot of FOXD3 and FOXD4 protein in the hindgut of two groups at different time points.
FOXD3/FOXD4 mRNA expression
Using NanoVue-Health care Bio-Sciences AB to determine the concentration and purity of RNA, the average A260/A280 value was between 1.8 and 2.0; thus, the concentration and purity of extracted RNA met the requirements for use for qRT-PCR.
FOXD3 and FOXD4 mRNA expression was 2.68- and 1.87-fold higher, respectively, in AE17 than in NE17. Similarly, at E19, FOXD3 and FOXD4 mRNA expression was 3.29- and 1.75-fold higher, respectively, in AE19 than in NE19; these differences were statistically significant (P = 0.034 and p = 0.045, respectively; Tables 1 and 2).
Table 1.
The relative expression value of FOXD3 gene in ARM.
| FOXD3 average Ct | β-Actin average Ct | △Ct | △△Ct | Expression multiple | |
|---|---|---|---|---|---|
| NE17 | 30.36 ± 1.23 | 14.97 ± 0.58 | 15.39 | 0 | 1 |
| AE17 | 32.37 ± 1.61 | 15.67± 0.93 | 16.70 | −1.31 | 2.68 |
| NE19 | 30.40 ± 1.74 | 14.46 ± 0.41 | 15.94 | 0 | 1 |
| AE19 | 34.83 ± 1.45 | 20.70 ±1.27 | 14.13 | 1.81 | 3.29 |
Table 2.
The relative expression value of FOXD4 gene in ARM.
| FOXD4 average Ct | β-Actin Average Ct | △Ct | △△Ct | Expression multiple | |
|---|---|---|---|---|---|
| NE17 | 32.06 ± 1.44 | 15.35 ± 0.69 | 16.71 | 0 | 1 |
| AE17 | 35.83 ± 2.35 | 22.81 ± 1.04 | 13.02 | 3.69 | 1.87 |
| NE19 | 32.06 ± 0.89 | 15.35 ± 1.06 | 16.71 | 0 | 1 |
| AE19 | 39.04 ± 3.17 | 23.83 ± 1.75 | 15.21 | 1.6 | 1.75 |
Discussion
ARM is the most common congenital malformation in newborns. The pathophysiological mechanisms remain unclear. It is well known that ARM is associated with abnormalities in embryogenesis and development. FOX is a family of recently discovered transcription factors that plays a key role in this process. FOX protein family members are expressed widely from Escherichia coli to humans and play important roles in cell proliferation, differentiation, growth and development, the cell cycle, and apoptosis.14,15 However, whether they participate in ARM pathogenesis remains unclear.
In the present study, the expression of FOXD3/FOXD4 was detected in the hindguts of rat embryos in an ETU-induced embryonic ARM animal model by qRT-PCR and western blot methods, which demonstrated that mRNA and protein expression in the two groups were very similar. The expression of FOXD3/FOXD4 mRNA and protein in the ETU-induced teratogenic ARM group was significantly higher than that in the normal group at E17 and E19. Our data show that the change in FOXD3/FOXD4 signal after applying the ETU at the fetal age significantly disrupted rectal terminal development during the most critical period, E15–E21. We assert that this change in the FOXD3/FOXD4 signal in the rectum end is not observed during normal development and led to the occurrence of deformity.
Anorectal development involves multifactorial and highly complex embryological events, which require histocytes to accept the correct quantity and quality of signal during a specific timeframe to properly differentiate into target cells and localize to the proper position. This process involves many cytologic and biochemical regulatory mechanisms. Adverse events—for example, gene mutation and external factors such as drugs or blood supply obstacles—can interfere with these links and impede the normal development, leading to deformity. FOX proteins are transcription regulatory factors that play key roles in maintaining cell life activity.16 Intra- or extracellular stimulation—including hormones, pro-splitting agents, viral coding products, stress response, and pathophysiological conditions—leads to activation of signal pathways that are controlled by transcriptional regulatory factors.11 Thus, inhibiting the expression of upstream transcription factors can achieve positive or negative regulation of downstream gene expression. At present, studies on the function of FOXD3 are mainly focused on the role of neural crest cells.12,17 FOXD3 is known to maintain pluripotent stem cell niches and promote cell proliferation.4,17,18 It was initially reported that FOXD3 is expressed in mouse embryonic stem cells and its malignant equivalents, which are called “Genesis” in the beginning, and that FOXD3 expression can also be detected in mouse epiblasts and during late embryonic development of neural crest cells.12,17,19
As an important transcription regulator, FOXD3/FOXD4 has a variety of biological functions, such as the maintenance of vertebrate embryonic development and embryonic stem cell pluripotentency; regulation of neural crest cell line formation, migration, and differentiation; and an extremely important role as transcription factors and in disease impact of disease.20,21 At present, research on the role of FOXD3/FOXD4 in embryonic development is mainly focused on vertebrates. For example, we report that FOXD3 function in the Xenopus laevis gastrula is essential for dorsal mesodermal development and for Nodal expression in the Spemann organizer.22 A study using FOXD3 gene knockout mice model showed that FOXD3 loss leads to incomplete in vitro ESC establishment and FOXD3 inactive teratomas, which suggests that FOXD3 is a necessary factor for survival and pluripotency of embryonic stem cells in mouse embryos and is essential for the establishment of an ESC system in vitro.6 Many years ago, researchers found that FOXD3 is specifically expressed in embryonic stem cells, and its expression decreases as the cells differentiate. This suggests that FOXD3 is likely to be a transcriptional inhibitor that can maintain pluripotent embryonic stem cells by inhibiting the expression of genes that promote differentiation in embryonic stem cells.23 Thus, FOXD3 plays a very important role in maintaining embryonic stem cells, and its loss can lead to the loss of pluripotent embryonic stem cells.
Additional studies have found that FOXD3 is expressed in neural crest cells before and after migration. As a gene downstream of PAX3, mutations in the PAX3 gene led to defects in neural crest development at sites where FOXD3 was not expressed, which means that FOXD3 plays an important role in regulating neural crest differentiation. Additionally, abnormal expression of FOXD3 in the chicken neural tube can affect neural crest development and inhibit the differentiation of intermediate neurons.24 Our data demonstrate that normal development of the anus and rectum rely on the regulation of FOXD3/FOXD4 and that FOXD3/FOXD4 signal disruption interferes with the differentiation of epithelial cells in the rectum and anus during intestinal development, which is closely related ARM occurrence.
The data in this study also demonstrate that FOXD3/FOXD4 protein is mainly expressed in the epithelial cells of the rectum and anus. The localization of ETU in the normal group was gradually clear, and cell staining of FOXD3/FOX4 gradually increased with increasing age from positive at NE15 (brown) to negative at NE17 and NE19 (light yellow); with the continuous development of the intestinal tract, expression increased gradually, and NE21 showed positive expression (brown). In the ETU teratogenic ARM group, staining increased to positive at AE17 and AE19 (brown) from negative at AE15 (light yellow); with the development of the intestinal tract, expression decreased, with AE21 finally showing negative expression (light yellow).
Abnormal expression can cause abnormal FOXD3/FOXD4 signal transduction and subsequently affect the development of the epithelial cells of the rectum and anus. The present study demonstrates that FOXD3/FOXD4 participate in the development of ARM, affect the development of the rectal terminal, play a regulatory role in intestinal nerve muscle development, and may be related to the FOXD3/FOXD4 signaling pathway. However, we still do not understand its detailed mechanisms and regulation. How are FOXD3 and FOXD4 acting to promote the development of the enteric nervous system? How is the signal path completed? How do they promote the differentiation of epithelial cells in the rectum and anus? How to avoid single differentiation? Whether a variety of nutritional factors and the support of related gene technology are needed? What is the detailed regulatory mechanism in spatiotemporal terms? How do other members of the FOXD3/FOXD4 signaling pathway participate in the development of the intestinal wall? All these questions remain unclear and further study and discussion are warranted.
Current study of the FOXD3 and FOXD4 genes mainly focuses on maintaining embryonic stem cell development as well as neural crest cell pluripotentency, formation, migration, and differentiation; interaction with other transcription factors; and disease pathogenesis. Although some progress has been made in understanding upstream and downstream regulation of FOXD3/FOXD4, it is far from complete to fully reveal all their biological functions. As an important transcription factor in embryonic development, specific developmental studies of FOXD3/FOXD4 and other aspects remain sparse. According to relevant research results, we hypothesize that FOXD3/FOXD4 plays an important role in pathologic embryonic development and proliferation, apoptosis, and differentiation. Moreover, understanding its regulatory pathways may contribute to more complete elucidation of the biological functions of FOXD3/FOXD4, which is likely to be an important research direction in the future. In conclusion, we have demonstrated a relationship between FOXD3/FOXD4 gene expression and human digestive tract development and congenital malformation, which has not previously been reported in the field of molecular biology of human embryonic development.
Together, our results shape a deeper understanding of FOXD3/FOXD4 being required for the normal development of the hindgut. FOXD3/FOXD4 expression is increased in the embryonic rectum in the ETU teratogenic ARM group, which demonstrates that ETU interferes with the expression of FOXD3/FOXD4 signal. In the course of anorectal development, the expression of FOXD3/FOXD4 in the short tail area of the ETU teratogenic ARM group was increased, which leads to abnormal rectal development. We thus conclude that FOXD3/FOXD4 signal expression is an important factor involved in ARM occurrence.
Authors’ contributions
L-JW conducted the expression of FOXD3/FOXD4 in the hindgut of rat embryos with ethylene thiourea study and drafted the manuscript. HG and Y-ZB performed western blot and RT-PCR analyses. W-LW participated in the design of the study and contributed to sample collection. S-CZ conceived of the study, participated in its design and coordination, and helped draft the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The study was supported by National Natural Science Foundation of China (Nos 30700917 and 81570465).
References
- 1.Wang C, Li L, Cheng W. Anorectal malformation: the etiological factors. Pediatr Surg Int 2015; 31:795–804 [DOI] [PubMed] [Google Scholar]
- 2.Teng L, Mundell NA, Frist AY, Wang Q, Labosky PA. Requirement for FOXD3 in the maintenance of neural crest progenitors. Development 2008; 135:1615–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson BC, Carpenter C, Nebert DW, Vasiliou V. Update of human and mouse forkhead box (FOX) gene families. Hum Genomics 2010; 4:345–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanna LA, Foreman RK, Tarasenko IA, Kessler DS, Labosky PA. Requirement for FOXD3 in maintaining pluripotent cells of the early mouse embryo. Genes Dev 2002; 16:2650–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkhateeb A, Fain PR, Spritz RA. Candidate functional promoter variant in the FOXD3 melanoblast developmental regulator gene in autosomal dominant vitiligo. J Invest Dermatol 2005; 125:388–91 [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Labosky PA. Regulation of embryonic stem cell self-renewal and pluripotency by FOXD3. Stem Cells 2008; 26:2475–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mundell NA, Labosky PA. Neural crest stem cell multipotency requires FOXD3 to maintain neural potential and repress mesenchymal fates. Development 2011; 138:641–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fetka I, Doederlein G, Bouwmeester T. Neuroectodermal specification and regionalization of the Spemann organizer in Xenopus. Mech Dev 2000; 93:49–58 [DOI] [PubMed] [Google Scholar]
- 9.Solter M, Koster M, Hollemann T, Brey A, Pieler T, Knochel W. Characterization of a subfamily of related winged helix genes, XFD-12/12'/12” (XFLIP), during Xenopus embryogenesis. Mech Dev 1999; 89:161–5 [DOI] [PubMed] [Google Scholar]
- 10.Neilson KM, Klein SL, Mhaske P, Mood K, Daar IO, Moody SA. Specific domains of FOXD4/5 activate and repress neural transcription factor genes to control the progression of immature neural ectoderm to differentiating neural plate. Dev Biol 2012; 365:363–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan Y, Newman T, Linardopoulou E, Trask BJ. Gene content and function of the ancestral chromosome fusion site in human chromosome 2q13-2q14.1 and paralogous regions. Genome Res 2002; 12:1663–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dottori M, Gross MK, Labosky P, Goulding M. The winged-helix transcription factor FOXD3 suppresses interneuron differentiation and promotes neural crest cell fate. Development 2001; 128:4127–38 [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–8 [DOI] [PubMed] [Google Scholar]
- 14.Fang L, Wang H, Zhou L, Yu D. Akt-FOXO3a signaling axis dysregulation in human oral squamous cell carcinoma and potent efficacy of FOXO3a-targeted gene therapy. Oral Oncol 2011; 47:16–21 [DOI] [PubMed] [Google Scholar]
- 15.Habashy HO, Rakha EA, Aleskandarany M, Ahmed MA, Green AR, Ellis IO, Powe DG. FOXO3a nuclear localisation is associated with good prognosis in luminal-like breast cancer. Breast Cancer Res Treat 2011; 129:11–21 [DOI] [PubMed] [Google Scholar]
- 16.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet 2007; 8:93–103 [DOI] [PubMed] [Google Scholar]
- 17.Hromas R, Ye H, Spinella M, Dmitrovsky E, Xu D, Costa RH. Genesis, a Winged Helix transcriptional repressor, has embryonic expression limited to the neural crest, and stimulates proliferation in vitro in a neural development model. Cell Tissue Res 1999; 297:371–82 [DOI] [PubMed] [Google Scholar]
- 18.Xu D, Yoder M, Sutton J, Hromas R. Forced expression of Genesis, a winged helix transcriptional repressor isolated from embryonic stem cells, blocks granulocytic differentiation of 32D myeloid cells. Leukemia 1998; 12:207–12 [DOI] [PubMed] [Google Scholar]
- 19.Labosky PA, Kaestner KH. The winged helix transcription factor Hfh2 is expressed in neural crest and spinal cord during mouse development. Mech Dev 1998; 76:185–90 [DOI] [PubMed] [Google Scholar]
- 20.Wijchers PJ, Burbach JP, Smidt MP. In control of biology: of mice, men and Foxes. Biochem J 2006; 397:233–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer 2007; 7:847–59 [DOI] [PubMed] [Google Scholar]
- 22.Steiner AB, Engleka MJ, Lu Q, Piwarzyk EC, Yaklichkin S, Lefebvre JL, Walters JW, Pineda-Salgado L, Labosky PA, Kessler DS. FOXD3 regulation of Nodal in the Spemann organizer is essential for Xenopus dorsal mesoderm development. Development 2006; 133:4827–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katoh M, Katoh M. Human FOX gene family (Review). Int J Oncol 2004; 25:1495–500 [PubMed] [Google Scholar]
- 24.Sutton J, Costa R, Klug M, Field L, Xu D, Largaespada DA, Fletcher CF, Jenkins NA, Copeland NG, Klemsz M, Hromas R. Genesis, a winged helix transcriptional repressor with expression restricted to embryonic stem cells. J Biol Chem 1996; 271:23126–33 [DOI] [PubMed] [Google Scholar]