Abstract
Introduction:
Splenic syndrome is a rare presentation of sickle cell disease. It is important to rule out this possibility when an ethnically vulnerable patient presents with an acute abdominal symptoms in a background of precipitating events.
Case Report:
A 26-year-old man who developed a severe abdominal pain at high altitude, found to have a tender splenomegaly. However, further inquiry revealed he is from an area where sickle cell disease is prevalent. Screening for sickle cell disease was positive. Radiological investigations confirmed a massive splenic infarction keeping with a diagnosis of splenic syndrome. Patient was managed conservatively.
Conclusion:
Sickle cell trait is considered a benign carrier state. However, rarely they can present with life-threatening conditions. Therefore, a high degree of clinical suspicion is required for early diagnosis of these specific entities to avoid increased morbidity and mortality of these patients.
Keywords: sickle cell trait, high altitude, splenic syndrome
Introduction
The prevalence of sickle cell trait (SCT) is low in Sri Lanka. Splenic syndrome at high altitude is uncommon albeit well-known to be associated with SCT. Unless this complication is kept in mind, these patients may be subjected to unnecessary interventions when they present with altitude-induced acute abdomen. Here, we report a case of splenic syndrome, presenting as an acute left abdominal pain with fever and respiratory symptoms.
Case Report
A 26-year-old man from Hambantota, had been well four days before when experienced a sudden severe left upper abdominal pain on his way back from a pilgrimage to Adam’s Peak. He had fever, sweating, and vomiting. He denied similar episodes in the past, hospital admissions or musculoskeletal symptoms. He was transferred from a peripheral hospital to a tertiary care center on the fourth day of the illness as symptoms were persistent. He was in distress due to pain and appeared ill. He had raised body temperature (103°F), icterus but no pallor. His pulse rate was 116/minutes, and blood pressure 150/80 Hgmm. Respiratory rate was 20/minutes and air entry reduced to the left lung base with a patch of bronchial breathing. There was tenderness on the left upper quadrant of the abdomen with rigidity and rebound tenderness. The spleen was felt 1 cm below the costal margin and tender. Central nervous system appeared normal. His hemoglobin was 12.9 g/dL, white blood cell count was 9600/µL, neutrophils 88%, platelets 150 000/µL, reticulocyte count 2.5% (0.5%-1.5%), and direct Coombs’ test was negative. Peripheral blood smear showed increased rouleaux formation, mild neutrophilia with toxic changes, and left shifted maturation, consistent with acute bacterial infection. There were no malaria parasites. There was an indirect hyperbilirubinemia (total 69 µmol/L [1-24 µmol/L], indirect 42 µmol/L [0-17 µmol/L]) with increased urine urobilinogen. Lactate dehydrogenase was 939 U/L (140-280 U/L) and C-reactive protein 188 mg/L (0-5 mg/L). Serum transaminases, creatinine, and electrolytes remained normal. Serology for dengue and salmonella infections were negative. Screening for melioidosis was not performed. Blood and urine cultures were negative for bacteremia. Chest radiograph showed atelectasis of lower segment of the left lung obliterating left hemi diaphragm with mild synpneumonic effusion. Ultrasound scan abdomen revealed mild splenomegaly with altered echo texture and a well-defined significant subcapsular infarction, measuring 12.1 cm × 3.4 cm with intact splenic capsule. These findings were further confirmed by a contrast enhanced computed tomography of abdomen (Figures 1 and 2).
Figure 1.
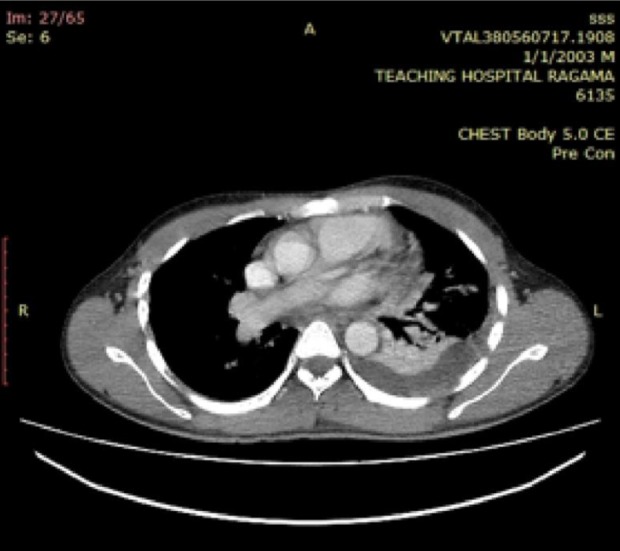
Contrast enhanced computed tomography scan (CECT) Lower chest. Atelectasis of lower segment of the left lung with mild synpneumonic effusion.
Figure 2.
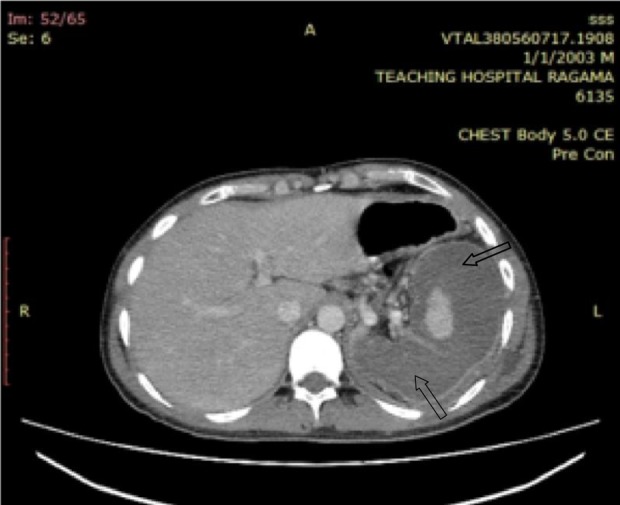
CECT abdomen. Spleen is enlarged with intact capsule and shape is maintained. Massive subcapsular splenic infarction sparing only a small central area of viable tissue. Intrasplenic blood vessels are visible.
Sickling test was positive and Hemoglobin-High performance liquid chromatography (Hb-HPLC) showed 38.6% of Haemoglobin S (HbS) with 50.6% of Haemoglobin A (HbA) confirming SCT. Abdominal pain was thought to arise from splenic syndrome secondary to acute splenic infarction at high altitude driven by hypoxia and strenuous exercise, which was subsequently complicated by left synpneumonic effusion.
Patient was managed with oxygen, hydration, chest physiotherapy, analgesics, and intravenous third generation cephalosporins. Pain in the left hypochondrium subsided gradually. However, body temperature remained elevated (99°F-103°F) due to left lower lobe pneumonia, which was further complicated with an abscess formation needing ultrasound-guided aspiration and more potent intravenous meropenem therapy. He was discharged on oral penicillin prophylaxis and immunization against encapsulated organisms of Streptococcus pneumonia, Haemophilus influenza b, and Neisseria meningitidis. His platelets rose gradually warranting antiplatelets.
Discussion
Sickle cell disease exists as genotypes of homozygous (Sickle cell anaemia-HbSS), heterozygous (Sickle cell trait-HbAS), or compound heterozygous (Sickle cell/haemoglobin C disease-HbSC, Sickle cell/beta thalassaemia-HbSβ, etc) A patient is said to have the heterozygous state of the sickle or SCT when he or she inherits normal hemoglobin-A gene from one parent and an abnormal hemoglobin-S gene from the other parent (1). The prevalence of SCT is high, especially in equatorial Africa and in African Americans. A rate of up to 50% has been reported in some enclaves of Africa and up to 10% in African Americans (2 –4).
Sickle cell trait is the third commonest hemoglobin abnormality in Sri Lanka especially in relation to ancient port cities like Hambanthota. Although SCT was initially considered as a benign condition, data are accumulating of serious morbidities including renal papillary necrosis, renal medullary carcinoma, thromboembolic disorders, splenic syndrome at high altitude, exercise-related rhabdomyolysis, and sudden death (1,5,6), warranting a high degree of clinical vigilance. No literature was found regarding the prevalence of splenic syndrome in Sri Lanka, though only few cases were reported (7).
Splenic syndrome occurs at hypoxic states such as high altitudes and general anesthesia. It is a medically manageable condition, but it is often initially misdiagnosed resulting in emergency laparotomy for acute abdomen followed by splenectomy and retrospective histopathological diagnosis (6). Typical presentation of splenic syndrome in healthy person is development of abdominal pain localizing to splenic region at a high altitude or following severe exertion (6,7). With hypoxia, circulation reaches a point of arrest within the anoxic and acidotic milieu in the red pulp of the spleen facilitating HbS polymerization, red cell sickling, vaso-occlusion, and ischemic infarction resulting in splenic syndrome. These initial occlusions trigger a vicious cycle which facilitates further propagation of the infarctions (autosplenectomy) (1). Prompt evacuation to sea level may hasten recovery and spare further splenic trauma (1,7). Unfortunately it had taken several hours for this patient to climbed down causing a massive splenic infarction.
Many of these patients develop left-sided pleural effusions with lower segmental atelectasis resulting in shallow, painful breathing (7).
Interestingly, splenic syndrome does not occur in sickle cell anemia (HbSS) due to autosplenectomy as a result of repeated vaso-occlusive crises at an early ages of their lives (1) Even within the SCT spectrum, the degree of susceptibility to splenic syndrome depends on the proportion of HbS. Higher the HbS percentage more the risk of developing splenic syndrome (1,7).
Conclusion
Although SCT is an uncommon genetic abnormality in Sri Lanka, the rare possibility of splenic syndrome should be considered when ethnically vulnerable patient presents with an acute abdomen at high altitude. This would prevent unnecessary surgical interventions in an otherwise medically manageable condition.
Author Biographies
CHKA Fernando is a senior registrar in Clinical Haematology, Department of Haematology, Colombo North Teaching Hospital, Ragama, Sri Lanka.
S Mendis is a consultant physician, Department of Adult Medicine, Colombo North Teaching Hospital, Ragama, Sri Lanka.
AP Upasena is a consultant radiologist, Department of Radiology, Colombo North Teaching Hospital, Ragama, Sri Lanka.
YJ Costa is a consultant haematologist, Department of Haematology, Colombo North Teaching Hospital, Ragama, Sri Lanka.
HS Williams is a senior lecturer and consultant haematologist, Department of Haematology, Faculty of Ragama, University of Kelaniya, Sri Lanka.
D Moratuwagama is a senior lecturer and consultant haematologist, Department of Haematology, Faculty of Ragama, University of Kelaniya, Sri Lanka.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Tantawy AAG. The scope of clinical morbidity in sickle cell trait. EJMHG. 2014;15:319–26. [Google Scholar]
- 2. Kark JA, Posey DM, Schumacher HR, Ruehle CJ. Sickle-cell trait as a risk factor for sudden death in physical training. New Engl J Med. 1987;317:781–7. [DOI] [PubMed] [Google Scholar]
- 3. Sears DA. The morbidity of sickle cell trait: a review of the literature. Am J Med. 1978;64:1021–36. [DOI] [PubMed] [Google Scholar]
- 4. Sheikha A. Splenic syndrome in patients at high altitude with unrecognized sickle cell trait: splenectomy is often unnecessary. Can J Surg. 2005;48:377–81. [PMC free article] [PubMed] [Google Scholar]
- 5. John N. A review of clinical profile in sickle cell traits. Oman Med J. 2010;25:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y. Complications associated with sickle cell trait: a brief narrative review. Am J Med. 2009;122:507–12. [DOI] [PubMed] [Google Scholar]
- 7. Abeysekera WY, de Silva WD, Pinnaduwa SS, Banagala AS. Acute massive splenic infarction with splenic vein thrombosis following altitude exposure of a Sri Lankan male with undetected sickle cell trait. High Alt Med Biol. 2012,13:288–90. [DOI] [PubMed] [Google Scholar]


