Abstract
Study Design:
Retrospective cohort study.
Objectives:
Unplanned reoperation following lumbar spinal fusion is detrimental to patients, providers, and health systems. The aim of this study was to identify risk factors associated with unplanned reoperation following elective posterior lumbar spinal fusion and assess the reasons for reoperation.
Methods:
A retrospective analysis of 22 151 patients from the American College of Surgeons National Surgical Quality Improvement Program data set between 2012 and 2015 was completed. The primary outcome measure was unplanned reoperation within 30 days. Secondary outcome measures were specific diagnoses and procedures associated with unplanned reoperation, as well as time to reoperation from initial procedure. Multiple stepwise logistic regression was employed to determine preoperative variables predictive of unplanned 30-day reoperation.
Results:
Patients with disseminated cancer (OR = 3.44, P = .0049), weight loss >10% in 6 months prior to surgery (OR = 3.26, P = .0276), bleeding disorders (OR = 1.92, P = .0049), American Society of Anesthesiologists score of 3 (OR = 1.46, P < .0001), body mass index of 35.0 to 39.9 (OR = 1.50, P = .0037), body mass index of ≥40 (OR = 1.83, P < .0001), and multilevel fusion (OR = 1.24, P = .0069) exhibited increased odds of 30-day reoperation. The most common diagnosis associated with reoperation was postoperative infection (n = 121, 21.1% of reoperations).
Conclusions:
Predictors and causes of unplanned reoperation within 30 days following elective posterior lumbar spinal fusion are identifiable. In this study cohort, obesity, American Society of Anesthesiologists score, disseminated cancer, weight loss, bleeding disorders, and multilevel fusion were identified as significant risk factors for reoperation. Further research investigating risk factor modification on reoperation in elective posterior lumbar spinal fusion is warranted.
Keywords: posterior lumbar fusion, unplanned reoperation, ACS-NSQIP, return to OR
Introduction
Each year, more than 200 000 patients undergo lumbar spinal fusion in the United States.1 Unplanned return to the operating room is a highly undesirable outcome from both clinical and health resource utilization perspectives. Unplanned reoperation within 30 days has been associated with markedly increased mortality rates and charges; Birkmeyer et al reported a 6.9-fold increased risk of mortality and 4.6-fold higher charges associated with unplanned 30-day reoperation in general surgery.2 Accordingly, efforts to reduce the rate of reoperation in posterior lumbar fusion are warranted, and reduction of adverse events through risk factor modulation and proper patient selection for elective procedures is becoming increasingly important.
Previous studies have focused on either isolated contributors to reoperation3,4 or reoperation risk in broad, heterogeneous spine surgery cohorts.5–7 These investigations have demonstrated the importance of differentiating risk factors by anatomical location, procedure, and approach.5,7 Additionally, the cause of unplanned reoperation has been shown to be highly specific to the procedure studied.2 The aim of this study was to evaluate risk factors for unplanned 30-day reoperation after posterior lumbar spinal fusion. This study also examined the specific causes, types of procedures, and timing of reoperations.
Methods
Study Design and Setting
A retrospective analysis was performed using data from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data set for the years 2012 to 2015. The ACS NSQIP collects data on preoperative and intraoperative risk factors, as well as morbidity and mortality outcomes for 30 days following major surgical procedures.8 The data is collected by certified and trained staff and assessed for quality using an interrater reliability audit. Notably, the ACS NSQIP does not contain data for minor cases, patients <18 years old, patients with American Society of Anesthesiologists (ASA) score of 6 (brain-stem-dead organ donor),9 trauma cases, and solid organ transplant cases. This investigation was deemed exempt from institutional review board approval, given the publicly available and de-identified nature of the NSQIP data set.
Patients
Primary inclusion criterion for this study was the presence of a primary Common Procedural Terminology (CPT) code corresponding to posterior lumbar fusion (22612, 22630, or 22633). Patients were excluded if the procedure was nonelective, deemed emergent, conducted in an outpatient setting, involved any cervical or thoracic levels, or employed anterior, anterolateral, anterior interbody, or lateral excavitary techniques—all of which were evaluated based on presence of other listed CPT codes.
Outcome Measures
Primary outcome, classified as a binary variable, was unplanned return to the operating room within 30 days of the original procedure. The specific reoperation procedure and associated diagnosis were assessed using CPT and International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes, respectively. Days from index procedure to unplanned reoperation were plotted, and the distribution was smoothed with a 3-day trailing average to facilitate trend visualization.
Risk Factors
All NSQIP preoperative patient characteristics (excluding laboratory values) available from 2012 to 2015 were assessed as covariates in the analysis, including patient age, sex, body mass index (BMI) that was calculated from available data on height and weight, and race. Comorbidities were also included as covariates, including diabetes, smoking, dyspnea, ventilator dependency, history of severe chronic obstructive pulmonary disease (COPD), ascites, congestive heart failure in 30 days before surgery, hypertension, acute renal failure, dialysis, disseminated cancer, open/infected wound, steroid use for chronic condition, >10% weight loss in prior 6 months, bleeding disorders, preoperative transfusion in 72 hours prior to surgery, systemic sepsis, and ASA classification. Notably, BMI was categorized as underweight (<18.5), normal weight (18.5-24.9), overweight (25.0-29.9), obesity class I (30.0-34.9), obesity class II (35.0-39.9), and obesity class III (≥40). Comorbidities were analyzed as unique risk factors—rather than as contributors to a comorbidity index (eg, Charlson Comorbidity Index)—as we sought to identify specific characteristics independently associated with unplanned reoperation. Procedural characteristics derived from patient-specific CPT codes were also included as covariates. Surgical approach was included as a covariate and was defined as posterior/posterolateral, posterior interbody, or combined posterior/posterolateral and interbody, based on the primary listed CPT code (22612, 22630, and 22633, respectively). The number of levels fused was assessed based on presence of CPT codes indicating additional fusion levels (22634, 22632, and 22614) and classified as single-level or multilevel (≥2 levels). NSQIP data set year was also included as a covariate.
Statistical Analysis
All statistical analysis was performed utilizing SAS 9.4 (SAS Institute, Cary, NC). Missing data was reported in descriptive statistics, and patients with missing data were excluded from further analyses. Two-tailed χ2 or Fisher’s exact test was performed for categorical variables, as appropriate. Bivariate risk factor analyses examined association of covariates with odds of unplanned 30-day reoperation. Multiple logistic regression was performed to assess independent risk factors associated with reoperation. Stepwise model building was employed, with a threshold α for variable entrance and retention of 0.2 and 0.1, respectively. Odds ratios (ORs) and 95% confidence intervals (CIs) were evaluated for risk factors deemed significantly predictive of unplanned reoperation. No collinearity, as assessed by variable tolerance in a generalized linear model, was observed between potential risk factors. Model goodness-of-fit was assessed using the Hosmer-Lemeshow goodness-of-fit test. Correlations between bleeding disorders, weight loss, and other comorbidities were analyzed. Statistical significance was maintained as P < .05.
Source of Funding
No funding was received in support of this study.
Results
In total, 22 151 patients undergoing elective posterior lumbar fusion were identified and included in the analysis. The majority of patients were overweight (BMI 25.0-29.9) or obese (BMI > 30; n = 18 259, 82.6%), white (n = 18 666, 84.3%), nondiabetic (n = 18 252, 82.4%), and undergoing single-level procedures (n = 13 056, 58.9%). Overall, 3.4% (n = 745) patients underwent an unplanned reoperation within 30 days of the original procedure (Tables 1 and 2).
Table 1.
Patient and Procedure Characteristics With Bivariate Analyses Examining Relationship to 30-Day Reoperation Rate.
| Variable | Patients | Percentage | Reoperation Rate (%) | P |
|---|---|---|---|---|
| N | 22 151 | — | 3.4 | — |
| Age (years) | .4068 | |||
| <65 | 12 595 | 56.9 | 3.2 | |
| 65-79 | 8423 | 38.0 | 3.6 | |
| 80+ | 1133 | 5.1 | 3.4 | |
| Sex | .0802 | |||
| Male | 10 001 | 45.2 | 3.1 | |
| Female | 12 150 | 54.9 | 3.6 | |
| BMI | <.0001 | |||
| Underweight | 153 | 0.7 | 1.3 | |
| Normal weight | 3695 | 16.7 | 2.6 | |
| Overweight | 7266 | 32.8 | 3.0 | |
| Obese, class I | 6065 | 27.4 | 3.0 | |
| Obese, class II | 3098 | 14.0 | 4.5 | |
| Obese, class III | 1830 | 8.3 | 5.7 | |
| Missing | 44 | 0.2 | — | |
| Race | .8442 | |||
| American Indian or Alaska Native | 146 | 0.7 | 3.4 | |
| Asian | 306 | 1.4 | 2.6 | |
| Black or African American | 1668 | 7.5 | 3.7 | |
| Native Hawaiian or Pacific Islander | 70 | 0.3 | 2.9 | |
| White | 18 666 | 84.3 | 3.3 | |
| Missing | 1295 | 5.8 | – | |
| ASA classification | <.0001 | |||
| 1, 2 | 11 202 | 50.6 | 2.63 | |
| 3 | 10 442 | 47.1 | 4.13 | |
| 4, 5 | 472 | 2.1 | 3.81 | |
| Missing | 35 | 0.2 | ||
| Levels fused | .0049 | |||
| Single-level procedure | 13 056 | 58.9 | 3.08 | |
| Multilevel procedure | 9095 | 41.1 | 3.77 | |
| Approach/technique | .3073 | |||
| Posterior/posterolateral technique | 11 251 | 50.8 | 3.55 | |
| Posterior interbody technique | 4929 | 22.3 | 3.16 | |
| Combined technique | 5971 | 27.0 | 3.18 | |
| Year | .6318 | |||
| 2012 | 3757 | 17.0 | 3.22 | |
| 2013 | 4947 | 22.3 | 3.21 | |
| 2014 | 6136 | 27.7 | 3.31 | |
| 2015 | 7311 | 33.0 | 3.58 |
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologists.
Table 2.
Patient Comorbidities With Bivariate Analyses Examining Relationship to 30-Day Reoperation Rate.
| Variable | Patients | Percentage | Reoperation Rate (%) | P |
|---|---|---|---|---|
| Diabetes | .0002 | |||
| None | 18 252 | 82.4 | 3.1 | |
| Yes, non-insulin therapy | 2656 | 12.0 | 4.2 | |
| Yes, insulin therapy | 1243 | 5.6 | 4.9 | |
| Smoking | .2431 | |||
| None | 17 563 | 79.3 | 3.3 | |
| Yes | 4588 | 20.7 | 3.6 | |
| Dyspnea | .7216 | |||
| None | 20 694 | 93.4 | 3.4 | |
| With moderate exertion | 1405 | 6.3 | 3.6 | |
| At rest | 52 | 0.2 | 1.9 | |
| Ventilator dependent | 1.0000 | |||
| None | 22 146 | 100.0 | 3.4 | |
| Yes | 5 | 0.0 | 0.0 | |
| COPD | .2014 | |||
| None | 21 063 | 95.1 | 3.3 | |
| Yes | 1088 | 4.9 | 4.0 | |
| Ascites | 1.0000 | |||
| None | 22 149 | 100.0 | 3.4 | |
| Yes | 2 | 0.0 | 0.0 | |
| Congestive heart failure | .0674 | |||
| None | 22 086 | 99.7 | 3.4 | |
| Yes | 65 | 0.3 | 7.7 | |
| Hypertension requiring medication | .0582 | |||
| None | 9369 | 42.3 | 3.1 | |
| Yes | 12 782 | 57.7 | 3.6 | |
| Acute renal failure | 1.0000 | |||
| None | 22 139 | 100.0 | 3.4 | |
| Yes | 12 | 0.1 | 0.0 | |
| Dialysis | 1.0000 | |||
| None | 22 122 | 99.9 | 3.4 | |
| Yes | 29 | 0.1 | 3.5 | |
| Disseminated cancer | .0141 | |||
| None | 22 092 | 99.7 | 3.4 | |
| Yes | 59 | 0.3 | 10.2 | |
| Open/infected wound | .4301 | |||
| None | 22 097 | 99.8 | 3.36 | |
| Yes | 54 | 0.2 | 5.56 | |
| Chronic steroid use | .0302 | |||
| None | 21 295 | 96.1 | 3.31 | |
| Yes | 856 | 3.9 | 4.67 | |
| Weight loss >10% | .0557 | |||
| None | 22 108 | 99.8 | 3.35 | |
| Yes | 43 | 0.2 | 9.3 | |
| Bleeding disorder | .0005 | |||
| None | 21 829 | 98.6 | 3.31 | |
| Yes | 322 | 1.5 | 6.83 | |
| Preoperative blood transfusion | 1.0000 | |||
| None | 22 134 | 99.9 | 3.37 | |
| Yes | 17 | 0.1 | 0 | |
| Systemic sepsis | .9666 | |||
| None | 22 105 | 99.8 | 3.37 | |
| SIRS | 42 | 0.2 | 2.38 | |
| Sepsis | 3 | 0.0 | 0 | |
| Septic shock | 1 | 0.0 | 0 |
Abbreviations: COPD, chronic obstructive pulmonary disease; SIRS, systemic inflammatory response syndrome.
Bivariate Analysis
Bivariate analysis was performed to determine the frequency of unplanned return to the operating room by preoperative characteristics. Patient BMI (P < .0001), ASA classification (P < .0001), levels fused (P = .0049), diabetes status (P = .0002), disseminated cancer (P = .0141), chronic steroid use (P = .0302), and bleeding disorders (P = .0005) exhibited significant differences in rates of unplanned reoperation (Tables 1 and 2).
Multivariate Analysis
Significant predictors of unplanned reoperation included disseminated cancer (OR = 3.44, 95% CI = 1.46-8.15; P = .0049), weight loss >10% of body weight (OR = 3.26, 95% CI = 1.14-9.34; P = .0276), bleeding disorder (OR = 1.92, 95% CI = 1.22-3.03; P = .0049), ASA score of 3 (OR = 1.46, 95% CI = 1.24-1.71; P < .0001), obesity class II (OR = 1.50, 95% CI = 1.14-1.98; P = .0037), obesity class III (OR = 1.83, 95% CI = 1.36-2.46; P < .0001), and multilevel fusion (OR = 1.24, 95% CI = 1.06-1.44; P = .0069; Table 3). Notably, diabetes status and chronic steroid use (both statistically significant in bivariate analyses) did not pass prespecified α thresholds for model entry/retention (Table 3).
Table 3.
Independent Risk Factors for 30-Day Unplanned Reoperation.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Disseminated cancer (ref = None) | |||
| Yes | 3.44 | 1.46-8.15 | .0049 |
| Weight loss >10% (ref = None) | |||
| Yes | 3.26 | 1.14-9.34 | .0276 |
| Bleeding disorder (ref = None) | |||
| Yes | 1.92 | 1.22-3.03 | .0049 |
| ASA classification (ref = 1, 2) | |||
| 3 | 1.46 | 1.24-1.71 | <.0001 |
| 4, 5 | 1.25 | 0.75-2.08 | .3871 |
| BMI (ref = Normal weight, BMI = 18.5-24.9) | |||
| Underweight, BMI < 18.5 | 0.43 | 0.10-1.81 | .2522 |
| Overweight, BMI 25.0-29.9 | 1.10 | 0.85-1.41 | .4793 |
| Obese, class I, BMI 30.0-34.9 | 1.05 | 0.81-1.36 | .7288 |
| Obese, class II, BMI 35.0-39.9 | 1.50 | 1.14-1.98 | .0037 |
| Obese, class III, BMI ≥40 | 1.83 | 1.36-2.46 | <.0001 |
| Levels fused (ref = Single-level) | |||
| Multilevel | 1.24 | 1.06-1.44 | .0069 |
Abbreviations: OR, odds ratio; CI, confidence interval; ASA, American Society of Anesthesiologists; BMI, body mass index.
Bleeding Disorder and Weight Loss Correlations
Bleeding disorders were positively correlated with diabetes (r = 0.047, P < .0001), dyspnea (r = 0.030, P < .0001), COPD (r = 0.021, P = .0016), congestive heart failure (r = 0.049, P < .0001), hypertension requiring medication (r = 0.057, P < .0001), open/infected wound (r = 0.017, P = .0117), chronic steroid use (r = 0.034, P < .0001), and were negatively correlated with smoking (r = −0.017, P = .0096). Weight loss >10% was positively associated with smoking (r = 0.018, P = .0075), congestive heart failure (r = 0.017, P = .0137), disseminated cancer (r = 0.018, P = .0087), and preoperative blood transfusion (r = 0.036, P < .0001; Table 4).
Table 4.
Correlation of Bleeding Disorder and Weight Loss >10% With Other Comorbidities.a
| Comorbidity | Bleeding Disorder | Weight Loss >10% | ||
|---|---|---|---|---|
| r | P | r | P | |
| Diabetes | 0.047 | <.0001 | 0.006 | .3927 |
| Smoking | −0.017 | .0096 | 0.018 | .0075 |
| Dyspnea | 0.030 | <.0001 | −0.008 | .2594 |
| Ventilator dependent | −0.002 | .7859 | −0.001 | .9214 |
| Chronic obstructive pulmonary disease | 0.021 | .0016 | 0.004 | .5306 |
| Ascites | −0.001 | .8636 | 0.000 | .9503 |
| Congestive heart failure | 0.049 | <.0001 | 0.017 | .0137 |
| Hypertension requiring medication | 0.057 | <.0001 | 0.000 | .9539 |
| Acute renal failure | −0.003 | .6739 | −0.001 | .8786 |
| Dialysis | −0.004 | .5128 | −0.002 | .8122 |
| Disseminated cancer | 0.008 | .2134 | 0.018 | .0087 |
| Open/infected wound | 0.017 | .0117 | −0.002 | .7456 |
| Chronic steroid use | 0.034 | <.0001 | 0.002 | .7888 |
| Bleeding disorder | — | — | 0.003 | .6325 |
| Weight loss >10% | 0.003 | .6325 | — | — |
| Preoperative blood transfusion | −0.003 | .6164 | 0.036 | <.0001 |
| Systemic sepsis | 0.009 | .1848 | −0.002 | .7767 |
aBoldface values indicate P < .05.
Characteristics of Unplanned Reoperation
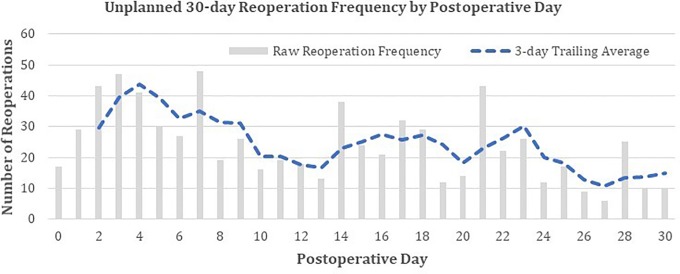
The most common procedures included hematoma drainage (n = 66, 9.9% of procedures), drainage of lumbar spine abscess (n = 61, 9.1%), exploration of spinal fusion (n = 59, 8.8%), and drainage of complex, postoperative wound infection (n = 55, 8.2%). The most common diagnoses included postoperative infection (n = 121, 21.1% of procedures), hematoma (n = 57, 9.9%), mechanical complication of internal orthopedic device (n = 51, 8.9%), seroma (n = 42, 7.3%), and accidental dura puncture/laceration (n = 21, 3.7%; Tables 5 and 6). Unplanned reoperation exhibited local maxima on 4, 16, and 23 days postoperatively, as assessed visually (Figure 1).
Table 5.
Diagnoses Associated With 30-Day Unplanned Reoperation.
| ICD-9-CM Code | Diagnosis Description | N | % of Reoperations |
|---|---|---|---|
| 998.59 | Other postoperative infection | 121 | 21.1% |
| 998.12 | Hematoma complicating a procedures | 57 | 9.9% |
| 996.49 | Other mechanical complication of other internal orthopedic device, implant, or graft | 51 | 8.9% |
| 998.13 | Seroma as procedural complication | 42 | 7.3% |
| 349.31 | Accidental puncture or laceration of dura during a procedure | 21 | 3.7% |
| 998.32 | Disruption of external operation wound | 19 | 3.3% |
| 996.78 | Other complications due to other internal orthopedic device, implant, and graft | 14 | 2.4% |
| 724.4 | Thoracic or lumbosacral neuritis or radiculitis, unspecified | 13 | 2.3% |
| 997.09 | Other nervous system complications | 12 | 2.1% |
| 722.1 | Displacement of thoracic or lumbar intervertebral disc without myelopathy | 11 | 1.9% |
| — | Missing | 172 | — |
Abbreviation: ICD-9-CM, International Classification of Disease, Ninth Revision, Clinical Modification.
Table 6.
Procedure Types Associated With 30-Day Unplanned Reoperation.
| CPT Code | Procedure Description | N | % of Reoperations |
|---|---|---|---|
| 10140 | Incision and drainage of hematoma | 66 | 9.9% |
| 22015 | Incision and drainage of deep abscess of lumbar spine | 61 | 9.1% |
| 22830 | Exploration of spinal fusion | 59 | 8.8% |
| 10180 | Incision and drainage, complex, postoperative wound infection | 55 | 8.2% |
| 22849 | Reinsertion of spinal fixation device | 43 | 6.4% |
| 63707 | Repair of dural/cerebrospinal leak, without laminectomy | 42 | 6.3% |
| 11042 | Debridement of subcutaneous tissue | 35 | 5.2% |
| 22612 | Arthrodesis, single level, lumbar, posterior or posterolateral technique | 30 | 4.5% |
| 63042 | Laminectomy, with decompression of nerve root(s) | 28 | 4.2% |
| 11043 | Debridement of muscle | 25 | 3.7% |
| — | Missing | 75 | — |
Abbreviation: CPT, Common Procedural Terminology.
Figure 1.
Distribution of days to unplanned reoperation from day of procedure.
Discussion
This analysis identified disseminated cancer, weight loss >10% in the prior 6 months, bleeding disorders, ASA score of 3, BMI ≥35, and multilevel fusion as risk factors for unplanned return to the operating room within 30 days. The most common diagnosis associated with reoperation was postoperative infection. This data provides important information for providers regarding the risk factors and characteristics of unplanned reoperation following posterior lumbar spinal fusion.
In a study of lumbar fusion for degenerative diseases, Martin et al identified higher rates of 90-day reoperation among female patients, patients with higher numbers of comorbidities, procedures with anterior approach, and procedures utilizing bone morphogenetic protein.10 Our analysis of posterior lumbar fusion patients did not find a significant difference in 30-day unplanned reoperation rates by patient sex, though it is possible (but unlikely) that the increased risk among female patients in Martin et al’s study manifests primarily in the 30- to 90-day postoperative period. Furthermore, our investigation identified ASA score of 3 (vs 1 and 2) to be a risk factor for reoperation; ASA score of 4 and 5 did not exhibit a significant effect. It is possible that patients with particularly high preoperative disease burden receive more intensive pre- and postoperative care, decreasing likelihood of reoperation. However, it is also possible that low sample size in this group (n = 472, 2.1%) may have limited our results. Future studies of lumbar spinal fusion patients with significant preoperative comorbidity burden may further clarify this finding.
Bekelis et al examined NSQIP data from 2005 to 2010 to determine risk factors associated with 30-day return to the operating room in a diverse cohort of patients undergoing discectomy, corpectomy, and lumbar fusion of cervical, thoracic, and lumbar levels, finding corpectomy, non-anterior approach, weight loss, bleeding disorders, dialysis, peripheral vascular disease, COPD, and tobacco use to be significant risk factors.6 Notably, the NSQIP definition for “return to the operating room” in Bekelis et al’s study included both planned and unplanned reoperations, while our investigation only considers unplanned events. Our investigation aligns reasonably well with this study supporting increased risk borne by patients with weight loss and bleeding disorders. Furthermore, our correlation analysis suggests that iatrogenic bleeding disorders secondary to anticoagulation therapy may play a prominent role in reoperation risk, although detailed analysis of complications is not possible due to limitations in the granularity of NSQIP data. We also observed that patients with preoperative weight loss are frequently those with metastatic cancer. As both cancer and weight loss appeared as independent risk factors in multivariate analysis, it is possible that these 2 comorbidities act synergistically to increase risk of reoperation following lumbar spinal fusion and indicate physiologic frailty of the patient. In both bivariate- and covariate-adjusted analyses, however, we did not observe significant differences in 30-day unplanned reoperation rates by dialysis, COPD, or smoking status. Additionally, peripheral vascular disease is not included in the 2015 NSQIP public use file, and was therefore not included in our analysis. As our cohort was restricted to posterior lumbar arthrodesis, it is possible that these additional factors are not relevant to the posterior lumbar fusion population specifically. It is also possible that careful surgeon patient selection in elective lumbar spinal surgery may have mitigated these risks in more recent years, as compared to the 2005 to 2010 period covered by Bekelis et al’s study. Finally, it is possible that patients with these characteristics may be predisposed to planned, but not unplanned, reoperations.
Infection was the most common cause of 30-day reoperation in our analysis, involved in >20% of such events. Previous studies have identified diabetes status, smoking status, ASA classification ≥3, weight loss, dependent functional status, disseminated cancer, obesity, and greater number of levels fused as predictive of increased risk for surgical site infection following spine surgery.11–14 As expected, many of these factors overlap with those we identified as predictive of reoperation. It is notable, however, that dependent functional status, diabetes, and smoking status did not emerge as independent risk factors in our cohort.
Comparison of this study to previous investigations highlights the need for longitudinal risk factor modeling in orthopedic spine surgery as the prevalence of (and attention given to) various comorbidities changes over time. Retrospective analysis of large health care data sets will identify risk factors for complications in the context of the dominant standard of care and over the time period of interest. Comorbidities that are widely well-managed may not emerge as predictive of complications in retrospective analyses. The corpus of literature on risk factors thus provides a perspective on areas for potential improvement that is delimited both clinically and temporally. Frequent reexamination of retrospective risk factor analyses is warranted.
The necessity of modulating preoperative risk factors may increase as providers continue to share greater economic responsibility for patient care. This is particularly true within bundled payment windows, such as the 90-day period in Medicare’s Total Joint Replacement program.15 Iorio et al published a 2016 analysis of an urban, tertiary academic medical center’s experience with the program and demonstrated that decreased length of stay potentially led to downstream cost-savings. It is possible that, with success of this program, Medicare may implement Bundled Payment for Care Improvement programs across additional orthopedic and neurosurgical procedures. Aggressive modulation of preoperative risk factors—where possible—may be instrumental to financial sustainability under the model.
This study was constrained to the information provided in the ACS NSQIP data set. As a result, follow-up beyond 30 days was not obtained, constraining our ability to make inferences regarding long-term outcomes (eg, revision fusion rate, late infection, etc). Missing data occurred at high frequency for reoperation procedure type (10.1%, n = 75) and diagnosis associated with reoperation (23.1%, n = 172). Additionally, the variables contained in the NSQIP are general in nature and do not provide specific information on patient preoperative medications and disease progression, as well as other important clinical characteristics. As with many retrospective data set analyses, our pool of potential risk factors was limited, and it is possible that additional risk factors exist that were not present in the NSQIP data set. However, these limitations are present in most extant data set research, and we believe that the NSQIP data quality and large sample size sufficiently compensate for these factors. Finally, despite this study’s large sample size, select risk factors were present in very few patients. Out of the identified risk factors for 30-day reoperation, disseminated cancer (n = 59, 0.3%), and weight loss >10% in previous 6 months (n = 43, 0.2%) had particularly low incidence. While the likelihood of type 1 error is not affected by sample size (as threshold α was set at P < .05 a priori), it remains possible that these estimates may be biased by low sample size. Future analyses with larger data sets may address this remaining question.
Conclusions
This analysis examined risk factors for unplanned 30-day reoperation in patients undergoing elective, posterior lumbar spinal fusion. Disseminated cancer, weight loss >10% in the prior 6 months, bleeding disorders, ASA score of 3, BMI ≥35, and multilevel fusion were identified as risk factors for reoperation. Furthermore, the increased risk of reoperation in patients with bleeding disorders may be related to anticoagulation therapy. As return to the operating room is clinically adverse and costly to health systems, health care teams may elect to prioritize management of these comorbidities prior to elective surgery, where possible.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976). 2012;37:67–76. [DOI] [PubMed] [Google Scholar]
- 2. Birkmeyer JD, Hamby LS, Birkmeyer CM, Decker MV, Karon NM, Dow RW. Is unplanned return to the operating room a useful quality indicator in general surgery? Arch Surg. 2001;136:405–411. [DOI] [PubMed] [Google Scholar]
- 3. Basques BA, Anandasivam NS, Webb ML, et al. Risk factors for blood transfusion with primary posterior lumbar fusion. Spine (Phila Pa 1976). 2015;40:1792–1797. [DOI] [PubMed] [Google Scholar]
- 4. Kim BD, Hsu WK, De Oliveira GS, Jr, Saha S, Kim JY. Operative duration as an independent risk factor for postoperative complications in single-level lumbar fusion: an analysis of 4588 surgical cases. Spine (Phila Pa 1976). 2014;39:510–520. [DOI] [PubMed] [Google Scholar]
- 5. Buerba RA, Fu MC, Gruskay JA, Long WD, 3rd, Grauer JN. Obese class III patients at significantly greater risk of multiple complications after lumbar surgery: an analysis of 10,387 patients in the ACS NSQIP database. Spine J. 2014;14:2008–2018. [DOI] [PubMed] [Google Scholar]
- 6. Bekelis K, Desai A, Bakhoum SF, Missios S. A predictive model of complications after spine surgery: the National Surgical Quality Improvement Program (NSQIP) 2005-2010. Spine J. 2014;14:1247–1255. [DOI] [PubMed] [Google Scholar]
- 7. McCutcheon BA, Ciacci JD, Marcus LP, et al. Thirty-day perioperative outcomes in spinal fusion by specialty within the NSQIP database. Spine (Phila Pa 1976). 2015;40:1122–1131. [DOI] [PubMed] [Google Scholar]
- 8. American College of Surgeons. User guide for the 2015. ACS NSQIP Participant Use Data File (PUF) (Revised Version). https://www.facs.org/quality-programs/acs-nsqip/program-specifics/participant-use. Accessed September 23, 2017.
- 9. Fitz-Henry J. The ASA classification and peri-operative risk. Ann R Coll Surg Engl. 2011;93:185–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin BI, Mirza SK, Franklin GM, Lurie JD, MacKenzie TA, Deyo RA. Hospital and surgeon variation in complications and repeat surgery following incident lumbar fusion for common degenerative diagnoses. Health Serv Res. 2013;48:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Veeravagu A, Patil CG, Lad SP, Boakye M. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine (Phila Pa 1976). 2009;34:1869–1872. [DOI] [PubMed] [Google Scholar]
- 12. Marquez-Lara A, Nandyala SV, Sankaranarayanan S, Noureldin M, Singh K. Body mass index as a predictor of complications and mortality after lumbar spine surgery. Spine (Phila Pa 1976). 2014;39:798–804. [DOI] [PubMed] [Google Scholar]
- 13. Haleem A, Chiang HY, Vodela R, et al. Risk factors for surgical site infections following adult spine operations. Infect Control Hosp Epidemiol. 2016;37:1458–1467. [DOI] [PubMed] [Google Scholar]
- 14. De la Garza-Ramos R, Bydon M, Abt NB, et al. The impact of obesity on short-and long-term outcomes after lumbar fusion. Spine (Phila Pa 1976). 2015;40:56–61. [DOI] [PubMed] [Google Scholar]
- 15. Iorio R, Clair AJ, Inneh IA, Slover JD, Bosco JA, Zuckerman JD. Early results of Medicare’s bundled payment initiative for a 90-day total joint arthroplasty episode of care. J Arthroplasty. 2016;31:343–350. [DOI] [PubMed] [Google Scholar]