Abstract
Study Design:
Meta-analysis.
Objectives:
To evaluate the long-term efficacy and safety of total disc replacement (TDR) compared with fusion in patients with functionally disabling chronic low back pain due to single-level lumbar degenerative disc disease (DDD) at 5 years.
Methods:
PubMed and Cochrane Central Register of Controlled Trials databases were searched for randomized controlled trials reporting outcomes at 5 years for TDR compared with fusion in patients with single-level lumbar DDD. Outcomes included Oswestry Disability Index (ODI) success, back pain scores, reoperations, and patient satisfaction. All analyses were conducted using a random-effects model; analyses were reported as relative risk (RR) ratios and mean differences (MDs). Sensitivity analyses were conducted for different outcome definitions, high loss to follow-up, and high heterogeneity.
Results:
The meta-analysis included 4 studies. TDR patients had a significantly greater likelihood of ODI success (RR 1.0912; 95% CI 1.0004, 1.1903) and patient satisfaction (RR 1.13; 95% CI 1.03, 1.24) and a significantly lower risk of reoperation (RR 0.52; 95% CI 0.35, 0.77) than fusion patients. There was no association with improvement in back pain scores whether patients received TDR or fusion (MD −2.79; 95% CI −8.09, 2.51). Most results were robust to sensitivity analyses. Results for ODI success and patient satisfaction were sensitive to different outcome definitions but remained in favor of TDR.
Conclusions:
TDR is an effective alternative to fusion for lumbar DDD. It offers several clinical advantages over the longer term that can benefit the patient and reduce health care burden, without additional safety consequences.
Keywords: total disc replacement, lumbar arthroplasty, lumbar arthrodesis, lumbar fusion, degenerative disc disease, long-term outcomes, meta-analysis
Introduction
Lumbar degenerative disc disease (DDD) is a common cause of debilitating low back pain, with a lifetime prevalence of approximately 62% to 84%.1-4 Strategies for the treatment of symptomatic lumbar DDD always begin with nonsurgical approaches consisting of a combination of rehabilitation and medication. For patients with functionally disabling mechanical discogenic back pain who fail to improve after several months of conservative therapy, surgical options such as spinal fusion or lumbar total disc replacement (TDR) may be considered.
The goal of spinal fusion has been to reduce pain by eliminating motion at the affected segment. Inherently, this changes the mechanics of the spine and can place increased stress on neighboring segments.5, 6 Complications of fusion include pseudoarthrosis, hardware-related issues (breakage, local pain), and possibly increased adjacent segment radiographic degeneration.5-8 While fusion rates and efficacy outcomes have improved in past decades, as many as one third of postfusion patients continue to experience symptoms, often leading to repeated operations in a predictable percentage of patients.9-11
Total disc replacement is an alternative treatment option that is indicated in a subset of fusion-eligible patients with discogenic low back pain due to single-level DDD who have failed conservative treatment. TDR devices approved by the US Food and Drug Administration allow segmental motion by attempting to mimic the function of an intervertebral disc. By allowing segmental motion at the affected level and restoring stability to the spine, TDR reduces back pain, improves functional performance, and, in contrast to fusion, reduces the incidence of radiographic degeneration of adjacent segments.12 The design of TDR devices has evolved over time, and more recent TDR devices that have been studied in randomized controlled trials, such as the activL Artificial Disc (Aesculap, Tuttlingen, Germany), further advance motion-preserving technology by supporting controlled translational and rotational movement.13 These advanced mechanisms translate into improved biomechanics that are designed to result in even less wear on facet joints and adjacent segments.13
The benefits of TDR over current care have been demonstrated at 2 years in several randomized trials14-18 and meta-analyses.19-22 Most of these randomized trials have since published findings after 5-year follow-up.23-26 These studies demonstrate that substantial improvements in efficacy outcomes such as disability and pain relief are maintained over several years. Pooling of long-term safety of TDR by Hiratzka and colleagues has shown a 2-fold reduction in the relative risk of adverse events (AEs) with TDR compared with fusion at 5 years.27 However, this meta-analysis did not assess efficacy outcomes and only included 2 randomized controlled trials. The objective of this article was to evaluate the long-term efficacy and safety of TDR compared with fusion in patients with functionally disabling chronic low back pain due to single-level lumbar DDD at 5 years.
Methods
Search Strategy
A comprehensive search strategy was devised to identify relevant literature from the PubMed/Medline and Cochrane Central Register of Controlled Trials (CENTRAL) databases. The search strategy also adapted well-established randomized control trial-enriching search methods.28 Search strategies and terms are provided in Supplemental Table S1 (all supplemental materials are available in the online version of the article). Searches were conducted between 2000 and 2015, with no restrictions on level of evidence or publication status. Only English-language articles were reviewed.
Two reviewers screened identified records for potentially relevant titles, abstracts, and full texts, with disagreements resolved by consensus or a third party.
Study Eligibility
Study eligibility was defined using a PICOS statement (ie, population, intervention, comparator, outcomes, and study design). Randomized controlled trials were eligible if they compared a TDR device with fusion for the treatment of discogenic low back pain due to lumbar spine DDD for 5 years. TDR devices of interest included Charité (DePuy Spine, Raynham, MA), ProDisc-L (Synthes Spine, West Chester, PA), Maverick (Medtronic, Memphis, TN), and FlexiCore (Stryker Spine, Allendale, NJ); fusion options included anterior lumbar interbody fusion (ALIF), posterior lumbar fusion (PLF), posterior lumbar interbody fusion (PLIF), and circumferential fusion. Studies excluded from the analysis compared a TDR device with another control TDR device (ie, activL, Kineflex-L), had >50% loss to follow-up, were single-arm or observational studies, or were subanalyses.
Data Extraction
Data from included studies was extracted onto a standardized extraction form by a single reviewer and then entered into Review Manager software v5.3 (Cochrane Collaboration, Copenhagen, Denmark). Extracted data was validated by a second reviewer.
Outcomes of interest at 5 years for which data was extracted consisted of the following:
Oswestry Disability Index (ODI) success, defined as ≥15-point or ≥25% improvement in ODI score
Mean change in back pain score from baseline
Reoperations, defined as device-related failures resulting in the subsequent surgical interventions of reoperation, revision, removal, or supplemental fixation
Device-related serious AEs (SAEs)
Patient satisfaction or willingness to choose the same surgery again
Intention-to-treat (ITT) data was extracted; per-protocol estimates were included only when ITT data was not reported. For one study, the sponsor was contacted for additional outcomes data because the conference abstract did not contain sufficiently detailed data for the outcomes of interest.23 Some data was extracted by digitizing data (TechDig v2.0 digitizing software). A normal distribution was assumed for continuous outcomes; mean differences were calculated from percent change and follow-up data. Missing variance data was calculated from other effect estimates and dispersion measures where feasible and appropriate. Otherwise, missing variance measures were imputed per standard methods outlined by the Cochrane Collaboration29 and Hozo et al.30 All study populations consisted of prospectively randomized patients.
Although the reporting of SAEs typically includes serious medical/surgical events that occur during participation in the study, SAEs are not necessarily related to the intervention being tested (ie, childbirth, unrelated conditions requiring overnight hospitalization). To gain a more complete understanding of SAEs specifically related to the investigational device, it is important to assess and report device-related SAEs in clinical trials of TDR devices. However, studies differed in the reporting of SAEs; therefore, an analysis of this outcome could not be conducted.
Quality Assessment
Study quality was assessed independently by 2 reviewers using The Cochrane Collaboration’s tool for assessing risk of bias, which assesses the risks of selection bias (sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and other bias.31 Studies were scored for low, unclear, or high risk of bias. Discrepancies between the 2 reviewers were resolved by a third party.
Small-study effects, a trend for smaller studies to show larger treatment effects using funnel plots, was not assessed because the analysis consists of fewer than 10 studies and is therefore underpowered to make reasonable assertions.32
Statistical Analysis
Primary Analysis
The meta-analyses were performed using Review Manager software (Version 5.3, the Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark, 2014). The primary analyses were conducted using a random-effects model; dichotomous variables (ie, ODI success, reoperations, patient satisfaction) were analyzed for relative risk (RR) using the Mantel-Haenszel method, whereas continuous variables (ie, back pain score) were analyzed for mean difference (MD) using the inverse-variance method. Heterogeneity of included studies was assessed visually by examining forest plots and statistically using the χ2 test and I2 measure.
Sensitivity Analyses
Sensitivity analyses were conducted for the following: (1) outcome definitions, where studies that used different definitions or measures for outcomes of interest were excluded23,24,26; (2) follow-up rate, where studies with a >30% loss-to-follow-up rate were excluded25; and (3) low heterogeneity, where analyses were conducted using a fixed-effects model.
Results
Search Results
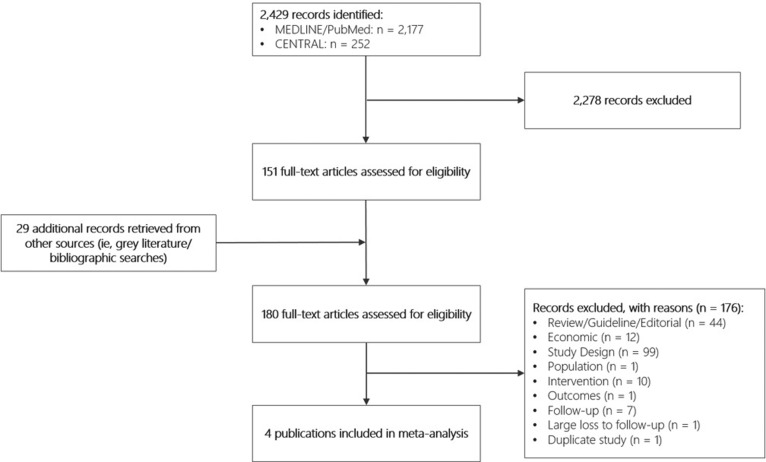
Figure 1 presents the results of the literature review. Searches identified 2429 potentially relevant records. After excluding 2278 records during the title and abstract review and including an additional 29 records identified from grey literature and bibliographic searches, 180 full-text articles were assessed for inclusion. A total of 4 publications were deemed eligible for the meta-analysis.23-26 Excluded studies were non–randomized controlled trials, non–clinical studies, conducted in a patient population that did not include single-level lumbar DDD with discogenic low back pain, did not examine one or more of the outcomes of interest, did not compare TDR with fusion, had follow-up period of less than 5 years, had a large loss to follow-up, or were subanalyses.
Figure 1.
PRISMA flowchart for the comprehensive literature search.
Study and Participant Characteristics
Table 1 details the characteristics of the included studies. Study sample sizes at baseline ranged from 152 to 577, with participants’ mean age in each study being 39 years. All studies compared TDR with fusion for the surgical treatment of lumbar DDD. Charité,25 ProDisc,24 and Maverick23 were each examined in single studies. One study included all 3 TDR devices.26 For the types of fusion, 2 studies used ALIF23,25; 1 study used circumferential fusion, which was ALIF with femoral ring allograft and posterolateral fusion with iliac crest bone autograft and pedicle screws24; and 1 study used PLF/PLIF.26 Single-level surgeries were performed in 100% of study participants in 3 studies23-25; in 1 study, 51% of participants were operated on at a single level.26 The majority of participants in 3 studies were treated at L5/S1 (65% to 79%).23-25 Mean ODI and back pain scores at baseline were similar across studies. In all studies, participants were followed for 5 years. Reported complications varied by study (Table 2), with 2 studies reporting only major or serious complications,23,25 1 study reporting all complications,26 and 1 study reporting both all and serious complications.24 Causes of reoperation were not consistently reported across studies. The proportion of participants who were lost to follow-up at 5 years ranged from 1% to 43%. All 4 studies reported ODI success, back pain score, reoperation, and patient satisfaction (Table 3).
Table 1.
Study and Baseline Participant Characteristics for Included Trials Reporting 5-Year Follow-up Data.
| Study; ClinicalTrials.gov Identifier | Treatment | Blinding | Analysis Set | N | 5-Year LTFU (%) | Mean Age (Years) | Prior Spinal Surgery | Mean BMI (kg/m2) | Single-Level Surgery (%) | L4/5 Level Treated (%) | L5/S1 Level Treated (%) | Mean Baseline ODI Scorea | Mean Baseline Back Pain Scoreb | Preoperative Work Status (% Working) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gornet 2010; NCT00635843 | Maverick | Open | ITT | 577 | 27% | 40 | 28% | — | 100% | 24% | 75% | 54% | 72 | 60% |
| ALIF | ||||||||||||||
| Guyer 2009; NCT00215332 | Charité | Singlec | ITT | 304 | 43% | 39.6 | 34% | 26 | 100% | 30% | 69% | 51% | 72 | 53% |
| ALIF | ||||||||||||||
| Skold 2013 | Charité, ProDisc, Maverick | singlea | ITT | 152 | 1% | 39.4 | 12% | — | 51% | — | — | 41% | 60 | 31% |
| PLIF/PLF | ||||||||||||||
| Zigler 2012; NCT00295009 | ProDisc-L | Singlec | ITT | 292 | 18% | 39 | 34% | 27 | 100% | 32% | 65% | 63% | 76 | — |
| CF |
Abbreviations: —, not reported; LTFU, loss to follow-up; BMI, body mass index; ODI, Oswestry Disability Index; ITT, intention to treat; ALIF, anterior lumbar interbody fusion; PLIF, posterior lumbar interbody fusion; PLF, posterior lumbar fusion; VAS, Visual Analogue Scale; NRS, Numerical Rating Scale.
aODI questionnaire v2.0 was used in Gornet 2010, Guyer 2009, and Skold 2013, and a modified ODI questionnaire was used in Zigler 2012.
bVAS pain scale (in mm) was used in Guyer 2009, Skold 2013, and Zigler 2012, and the NRS pain scale was used in Gornet 2010.
cParticipants were preoperatively blinded.
Table 2.
Description of Complications and Causes of Reoperation.
| Study | Complication Rate, n/N (%) | Description of Complications | Causes of Reoperationa | ||
|---|---|---|---|---|---|
| Fusion | TDR | Fusion | TDR | ||
| Gornet 2010 | 12/172 (7.0%) | 4/405 (1.0%) | Serious device or device/procedure-related adverse event | Not reported | Not reported |
| Guyer 2009 | 0% | 0% | Major complications, defined as major vessel injury resulting in >1500 cc blood loss, neurological damage, nerve root injury, death | Nonunion | Symptomatic spondylolisthesis |
| Pseudoarthrosis | Device subsidence with low back pain | ||||
| Facet joint arthrodesis | Facet degeneration | ||||
| Undefined persistent back pain | Early postoperative implant displacement with back pain | ||||
| Skold 2013 | 9/72 (12.5%) | 13/80 (16.3%) | Infection, hematoma, pseudoarthrosis, suspected facet joint pain, wound hernia, donor site pain, dural tear, nerve entrapment, meralgia paresthetica, subsidence | Not reported | Not reported |
| Zigler 2012 | Overall: 5.1 per patient | Overall: 5.4 per patient | Serious complications: blood loss >1500 cc, dural tears, retrograde ejaculation, posterior wound infections, deep venous thrombosis, death | Unresolved pain | Device migration |
| Serious: 0.58 per patient | Serious: 0.38 per patient | Technical error | |||
| Unresolved pain | |||||
Abbreviation: TDR, total disc replacement.
aCauses for reoperation were for device failures resulting in subsequent surgical interventions of reoperation, revision, removal, or supplemental fixation. For the TDR arm, Guyer 2009 reported causes of reoperation for supplemental fixation only.
Table 3.
Outcomes Data for Each Study at 5-Year Follow-up.
| Study | ODI Success,a n/N (%) | Back Pain Score,b Mean (SD) | Reoperation, n/N (%) | Patient Satisfaction, n/N (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fusion | TDR | Fusion | TDR | Fusion | TDR | Fusion | TDR | |||
| Baseline | 5 Years | Baseline | 5 Years | |||||||
| Gornet 2010 | 90/118 (76.3%) | 241/302 (79.3%) | 73.3 (19.4) | 22.7 (27.1) | 71.7 (18.9) | 18.9 (27.6) | 14/119 (11.8%) | 18/304 (5.9%) | 75/118 (63.6%) | 211/301 (70.1%) |
| Guyer 2009 | 28/43 (65%) | 61/90 (68%) | 71.8 | 29.9 (28.1) | 72.0 | 31.2 (23.2) | 7/43 (16%) | 7/90 (8%) | 31/43 (72%) | 70/90 (78%) |
| Skold 2013 | 46/71 (64.8%)c | 62/80 (77.5%)c | 58.5 (21.7) | 30.5 (26.9) | 62.3 (20.8) | 22.7 (29.2) | 20/71 (28.2%) | 9/80 (11.3%) | 50/72 (69%) | 63/80 (79%) |
| Zigler 2012 | 62.8% | 74.6% | 74.9 (14.7) | 40 (32.1) | 75.9 (16.4) | 37.1 (29.3) | 5/75 (6.7%) | 11/161 (6.8%) | 68.0%d | 82.5%d |
| Totale | 164/232 (70.7%) | 361/472 (76.4%) | — | — | — | — | 46/308 (14.9%) | 45/635 (7.1%) | 156/233 (66.9%) | 344/471 (73.0%) |
Abbreviations: —, not reported/not applicable; ODI, Oswestry Disability Index; TDR, total disc replacement; VAS, Visual Analog Scale.
aODI success defined as ≥15-point improvement in ODI score from baseline.
bBack pain assessed using VAS pain scale (in mm) in Guyer 2009, Skold 2013, and Zigler 2012. Numeric Rating Scale was used in Gornet 2010.
cODI success defined as ≥25% improvement in ODI score from baseline.
dPatient satisfaction defined as willingness to choose same surgery again.
eSum of available proportion data reported from studies.
Study Quality
The risk of bias was similar across studies (Figure 2). Methods for randomized sequence generation were reported in 3 studies,23-25 with all 3 studies using block randomization with a fixed block size of 6. Central allocation was reported as the method used in 3 studies,23-25 with sites notified of allocation using sealed envelopes in 2 studies23,25 and by telephone in 1 study.24 Sealed envelopes were also used as the method of allocation concealment in the study by Skold et al.26 In all studies, surgeons and/or staff were not blinded for preparatory purposes; participants were blinded to their preoperative randomization group in 3 studies.24-26 No studies reported on whether data collectors, outcomes adjudicators, and data analysts were blinded. In 3 studies, ITT analyses were also conducted24-26; 1 study reported very low loss of participants at follow-up (ie, 99% follow-up rate)26 (see Table 1).
Figure 2.

Risk of bias assessment for included trials.
Primary Analysis Results
ODI Success
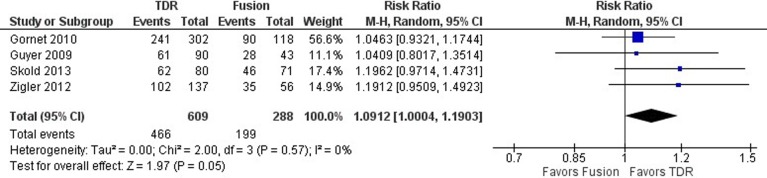
Oswestry disability index success was defined as a ≥15-point improvement in ODI score from preoperative baseline in 3 studies23-25 and as a ≥25% improvement in ODI score from preoperative baseline in 1 study.26 In Skold et al,26 a ≥25% improvement would approximate a minimum 10-point reduction in ODI score. As such, these 2 definitions for ODI success were deemed similar for the purposes of this study. When data from all 4 studies were pooled, ODI success was significantly better with TDR than with fusion, with a 9% relative increase in the likelihood of TDR patients achieving this endpoint (RR 1.0912; 95% CI 1.0004, 1.1903; P = .05; Figure 3). Heterogeneity across studies was low (I2 = 0%).
Figure 3.
Forest plot of pooled results comparing TDR with fusion for ODI success.
Back Pain Score
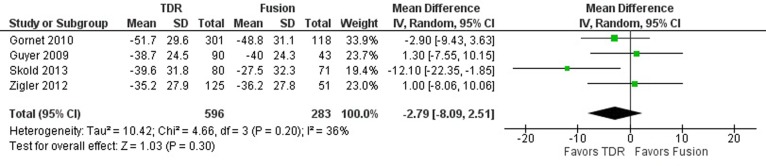
Back pain, reported in all 4 studies, was assessed using the Visual Analog Scale (VAS) in 3 studies24-26 and the Numeric Rating Scale (NRS) in 1 study.23 No differences were observed in the improvement in the mean back pain score from preoperative baseline to 5-year follow-up (MD −2.79; 95% CI −8.09, 2.51; P = .30; Figure 4). The 4 studies showed moderate heterogeneity (I2 = 36%).
Figure 4.
Forest plot of pooled results comparing TDR with fusion for back pain score.
Visual inspection of the forest plot revealed that results from 2 studies were inconclusive for back pain24,25 and that those from the other 2 studies favored TDR.23,26 Exploration of heterogeneity revealed differences in the definition of the outcome reported in the studies: in the former 2 studies, it is not clear whether pain scores reflect back pain, leg pain, or both,24,25 whereas in the latter 2 studies, back and leg pain scores were reported separately.23,26 From these latter studies, only back pain scores were included in the analysis. In addition, the VAS instruments used in each study were standardized differently, with VAS standardized to 100 mm in 2 studies25,26 and to 10 cm in 1 study.24
Reoperation
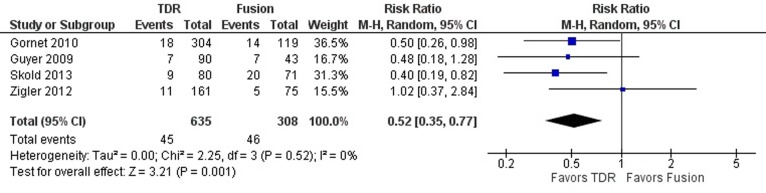
The definition for reoperations resulting from device-related failures was consistent in all 4 studies. Treatment of lumbar DDD with TDR resulted in a 48% relative reduction in the risk of reoperations than treatment with fusion (RR 0.52; 95% CI 0.35, 0.77; P = .001; Figure 5). Heterogeneity across studies was low (I2 = 0%).
Figure 5.
Forest plot of pooled results comparing TDR with fusion for reoperations.
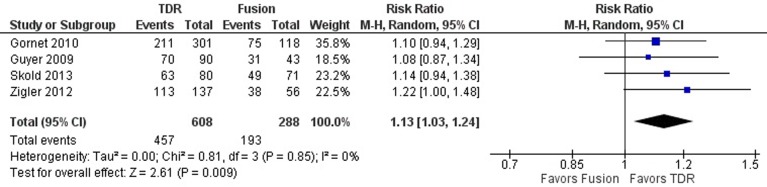
Patient Satisfaction
The proportion of patients satisfied with treatment was reported in 3 studies,23,25,26 whereas the proportion of patients willing to choose the same surgery again was reported in 1 study.24 In the pooled analysis of all 4 studies, there was a significantly greater likelihood of patients reporting satisfaction with an implanted TDR device than with fusion at 5 years (RR 1.13; 95% CI 1.03, 1.24; P = .009; Figure 6). Variation across included studies was low (I2 = 0%).
Figure 6.
Forest plot of pooled results comparing TDR with fusion for patient satisfaction.
Sensitivity Analysis Results
Sensitivity analyses demonstrated that the results of the primary analysis were robust to the majority of variables tested (Supplemental Figures S1-S3). Results of sensitivity analyses for different outcome definitions were numerically in favor of TDR for ODI success and patient satisfaction, whereas those for back pain score were similar to those from the primary analysis (Supplemental Figure S1). Results of the primary analysis for all outcomes were robust to the use of a fixed-effects model (Supplemental Figure S2) and to high patient loss at follow-up (Supplemental Figure S3).
Discussion
Summary of Meta-Analytic Findings
There are now several published randomized studies reporting the long-term outcomes associated with TDR. As such, we conducted a meta-analysis of randomized studies to determine the impact of TDR compared with fusion on clinical and safety outcomes for the treatment of chronic lower back pain due to lumbar DDD at 5-year follow-up. Overall, the results demonstrated several statistically significant clinical benefits with TDR than fusion.
In our analysis, patients who had failed conservative care and then had surgery had a significantly greater likelihood of ODI success at 5 years. Furthermore, ODI success remained robust against sensitivity analyses but were sensitive to different outcome definitions. Previous meta-analyses comparing ODI outcomes between TDR and fusion also demonstrated superior clinical benefits with TDR.19-22,33,34
Long-term improvement in back pain scores were similar between TDR and fusion in our analysis, with significant improvements from baseline maintained from mid- to long-term follow-up in both treatment groups.23,25,35
Patients in our meta-analysis that underwent TDR were significantly more likely to be satisfied with their procedure at 5 years. Results for patient satisfaction were sensitive to different outcome definitions but were robust to other factors tested. Similar findings from previous meta-analyses support the benefits of patient satisfaction with TDR.19-22,33,34
In the original 2-year studies, the results for reoperations showed no difference between TDR and fusion.19-22,33,34 In contrast, a recent systematic review and meta-analysis comparing safety outcomes of overall AEs and reoperations between TDR and fusion at 2- and 5-year follow-up demonstrated that TDR had a significantly lower risk of AEs and reoperations than fusion at 2 years.27 The lower risk for these outcomes continued to trend in favor of TDR at 5 years. Our study, which incorporated more recent data from additional studies,23,25 demonstrated a significantly lower risk of reoperation with TDR than with fusion at 5 years. These long-term findings are corroborated by another qualitative systematic review that reported a lower range for reoperation rates with TDR (3.7% to 11.4%) than with fusion (5.4% to 26.1%),11 as well as by several long-term observational prospective studies.9,10,13,36,37
Other safety considerations such as device-related SAEs are also important for surgical interventions for lumbar DDD. Recent trials comparing different TDR devices at 2- and 5-year follow-up have reported device-related SAEs of 5.8% to 18.9% and 8.8% to 9.5%, respectively.38-40 While we were unable to pool results across included studies because of differences in reporting, all trials reported better safety outcomes with TDR than with fusion. Particularly, in 2 included studies, adjacent segment degeneration was lower at 5 years with TDR (1.1% to 9%) than with fusion (4.7% to 14%).24,25 Post hoc analysis of prospectively collected radiographic data from the ProDisc study showed significantly fewer adjacent-level degenerative changes with TDR (9.2%) than with fusion (28.6%; P = .004).12 Similarly, a systematic review of prospective single-arm studies with follow-up of 3 to 22 years demonstrated a significantly lower prevalence of adjacent segment degeneration with TDR than with fusion (9% vs 34%; P < .0001).41 Over the long term (ie, ≥5-year follow-up), several studies have demonstrated significantly lower SAEs, low rates of overall complications, and very low prevalence of device migration and/or subsidence with TDR.24,25,27,36,37,42-44
When compared with conservative treatment such as rehabilitation, exercise, activity restriction, and pharmacological pain management, TDR has demonstrated comparable or superior outcomes.14,45-47 Failure of conservative treatment to improve outcomes of chronic low back pain has been attributed to low adherence to clinical practice guideline recommendations,48 but this reflects real-world experience in both the variability of quality and overall availability of conservative care. Importantly, even in those patients who have received conservative treatment, chronic low back pain and associated functional limitations often persist or worsen, substantiating the need for alternative treatment options that are accessible for these patients.49
Strengths and Limitations of Study
This meta-analysis has several strengths. First, this is the only meta-analysis to examine the long-term efficacy of TDR as a class compared with fusion for lumbar DDD. Second, the analysis included only those studies with high-quality study designs (ie, randomized trials). Third, a comprehensive review of the literature was conducted that not only identified those randomized studies included in a previous meta-analysis but also those that were not included or have been published since. Fourth, previously unpublished long-term data comparing TDR with fusion from the Maverick IDE (investigational device exemption) trial were included in this analysis, substantially improving the power of the analysis. Results for outcomes from the Maverick IDE trial at 5-year follow-up continue to demonstrate the benefits of TDR over fusion in participants with single-level lumbar DDD (see Table 3). And fifth, detailed assessment of study quality and heterogeneity reported little variation between studies, which was supported by the majority of sensitivity analyses.
A challenge of long-term lumbar TDR studies is loss of participants to follow-up. Included studies varied in the proportion of participants lost to follow-up at 5 years, ranging from 1% to 43%. Despite the risk high participant loss poses to a study’s validity,50 results of sensitivity analyses excluding studies with substantial loss to follow-up (ie, >30%) were similar to those of the primary analysis. Because few randomized controlled trials comparing TDR with fusion have reported long-term data, we opted for an inclusive approach to each outcome definition. Sensitivity analyses conducted to account for differences in the definitions and measures used for ODI success, back pain, and patient satisfaction consistently favored TDR over fusion despite the loss of statistical significance for some outcomes. Similarly, the analysis could not control for heterogeneity, a typical issue when addressing surgical outcomes. Nuances such as surgical technique and postoperative compliance could not be addressed by our study design. However, given the magnitude of centers involved in the 4 randomized controlled trials incorporated in this analysis, it seems reasonable that many variations in technique are accounted for. The analysis incorporated the most recent literature available, but data from newer TDR devices such as the activL Artificial Disc was not included, since findings at 5 years have yet to be reported.38 Two-year follow-up results from the activL IDE study aligned with the findings of the current analysis. Analyses including the long-term data for activL are expected to demonstrate similar or better findings favoring TDR than those shown in the current 5-year meta-analysis.51
Implications on Clinical Practice
The long-term findings of this study have important implications on the health care and economic burden of patients with symptomatic lumbar DDD. In the short term, TDR significantly reduces operating time and length of hospital stay,52,53 in addition to having a significantly lower rate of reoperation than fusion as reported in this analysis. It is expected that use of these long-term data in economic analyses will demonstrate a benefit with TDR when compared with current care.
Overall, our study demonstrates that TDR achieves clinical results at 5 years that are at least as good as or better than fusion in patients who suffer from single-level lumbar DDD and have failed conservative care. There is clearly a role for surgery in this patient population. Furthermore, TDR may reduce the need for future adjacent-level surgery after primary surgery by reducing the occurrence of radiographic adjacent-level disease at 5 years,12 a generally accepted precursor of the future need for additional adjacent-level surgery. With more than 119 000 TDR implants worldwide over the past 30 years, major universal issues of wear, breakage, breakdown, and other complications would certainly be common knowledge at this point, yet they are only rarely reported.54
Conclusions
Total disc replacement is an effective treatment compared with spinal fusion in lumbar DDD. It offers several clinical advantages that can benefit the patient, without the addition of safety consequences. The evidenced longer-term durability of lumbar disc replacement coupled with patients’ relief of symptoms and motion preservation makes TDR a desirable treatment alternative to spinal fusion.
Supplemental Material
Supplemental Material, activL_5-year_Meta-analysis_Manuscript-Supplemental-Mar_9_2017 for Comparison of Lumbar Total Disc Replacement With Surgical Spinal Fusion for the Treatment of Single-Level Degenerative Disc Disease: A Meta-Analysis of 5-Year Outcomes From Randomized Controlled Trials by Jack Zigler, Matthew F. Gornet, Nicole Ferko, Chris Cameron, Francine W. Schranck, and Leena Patel in Global Spine Journal
Acknowledgments
The authors would like to thank David F. Wootten, PhD, of Medtronic (Memphis, TN) for his support in obtaining data from Medtronic’s Maverick IDE trial, and David Banko of B Braun (Bethlehem, PA) and Katie Kleinschuster of Aesculap (Center Valley, PA) for constructive discussions on study design.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LP, NF, and CC are employees of Cornerstone Research Group Inc, a consultancy that received fees from Aesculap during the conduct of the study. JZ reports personal fees from Aesculap and DePuy Synthes outside the submitted work. MFG reports other funding from Medtronic, K2M, Bonovo, International Spine & Orthopedic Institute, LLC, Nocimed, OuroBoros, and Viscogliosi Bros Venture Partners, LLC outside the submitted work. FWS reports personal fees from Aesculap during the conduct of the study; personal fees from Medtronic, Zimmer/Biomet, and Nocimed and other from Nocimed outside the submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Aesculap Implant Systems, LLC.
Supplemental Material: The supplemental material is available in the online version of the article.
References
- 1. Walker BF, Muller R, Grant WD. Low back pain in Australian adults: prevalence and associated disability. J Manipulative Physiol Ther. 2004;27:238–244. doi:10.1016/j.jmpt.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 2. Fujii T, Matsudaira K. Prevalence of low back pain and factors associated with chronic disabling back pain in Japan. Eur Spine J. 2013;22:432–438. doi:10.1007/s00586-012-2439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Capkin E, Karkucak M, Cakirbay H, et al. The prevalence and risk factors of low back pain in the eastern Black Sea region of Turkey. J Back Musculoskelet Rehabil. 2015;28:783–787. doi:10.3233/bmr-150584. [DOI] [PubMed] [Google Scholar]
- 4. Cassidy JD, Carroll LJ, Côté P. The Saskatchewan health and back pain survey. The prevalence of low back pain and related disability in Saskatchewan adults. Spine (Phila Pa 1976). 1998;23:1860–1866. [DOI] [PubMed] [Google Scholar]
- 5. Gibson JN, Waddell G. Surgery for degenerative lumbar spondylosis. Cochrane Database Syst Rev. 2005;(4):CD001352 doi:10.1002/14651858.CD001352.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan A, Hai Y, Yang J, Zhou L, Chen X, Guo H. Adjacent segment degeneration after lumbar spinal fusion compared with motion-preservation procedures: a meta-analysis. Eur Spine J. 2016;25:1522–1532. doi:10.1007/s00586-016-4415-6. [DOI] [PubMed] [Google Scholar]
- 7. Ren C, Song Y, Liu L, Xue Y. Adjacent segment degeneration and disease after lumbar fusion compared with motion-preserving procedures: a meta-analysis. Eur J Orthop Surg Traumatol. 2014;24(suppl 1):S245–S253. doi:10.1007/s00590-014-1445-9. [DOI] [PubMed] [Google Scholar]
- 8. Zhang C, Berven SH, Fortin M, Weber MH. Adjacent segment degeneration versus disease after lumbar spine fusion for degenerative pathology: a systematic review with meta-analysis of the literature. Clin Spine Surg. 2016;29:21–29. doi:10.1097/BSD.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 9. Martin BI, Mirza SK, Comstock BA, Gray DT, Kreuter W, Deyo RA. Are lumbar spine reoperation rates falling with greater use of fusion surgery and new surgical technology? Spine (Phila Pa 1976). 2007;32:2119–2126. doi:10.1097/BRS.0b013e318145a56a. [DOI] [PubMed] [Google Scholar]
- 10. Greiner-Perth R, Boehm H, Allam Y, Elsaghir H, Franke J. Reoperation rate after instrumented posterior lumbar interbody fusion: a report on 1680 cases. Spine (Phila Pa 1976). 2004;29:2516–2520. [DOI] [PubMed] [Google Scholar]
- 11. van den Eerenbeemt KD, Ostelo RW, van Royen BJ, Peul WC, van Tulder MW. Total disc replacement surgery for symptomatic degenerative lumbar disc disease: a systematic review of the literature. Eur Spine J. 2010;19:1262–1280. doi:10.1007/s00586-010-1445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zigler JE, Glenn J, Delamarter RB. Five-year adjacent-level degenerative changes in patients with single-level disease treated using lumbar total disc replacement with ProDisc-L versus circumferential fusion. J Neurosurg Spine. 2012;17:504–511. doi:10.3171/2012.9.spine11717. [DOI] [PubMed] [Google Scholar]
- 13. Yue JJ, Garcia R, Jr, Miller LE. The activL® Artificial Disc: a next-generation motion-preserving implant for chronic lumbar discogenic pain. Med Devices (Auckl). 2016;9:75–84. doi:10.2147/MDER.S102949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hellum C, Johnsen LG, Storheim K, et al. Surgery with disc prosthesis versus rehabilitation in patients with low back pain and degenerative disc: two year follow-up of randomised study. BMJ. 2011;342:d2786 doi:10.1136/bmj.d2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976). 2007;32:1155–1162. doi:10.1097/BRS.0b013e318054e377. [DOI] [PubMed] [Google Scholar]
- 16. Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine (Phila Pa 1976). 2005;30:1565–1575. [DOI] [PubMed] [Google Scholar]
- 17. Berg S, Tullberg T, Branth B, Olerud C, Tropp H. Total disc replacement compared to lumbar fusion: a randomised controlled trial with 2-year follow-up. Eur Spine J. 2009;18:1512–1519. doi:10.1007/s00586-009-1047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gornet MF, Burkus JK, Dryer RF, et al. Lumbar disc arthroplasty with Maverick disc versus stand-alone interbody fusion: a prospective, randomized, controlled, multicenter investigational device exemption trial. Spine (Phila Pa 1976). 2011;36:E1600–E1611. doi:10.1097/BRS.0b013e318217668f. [DOI] [PubMed] [Google Scholar]
- 19. Nie H, Chen G, Wang X, Zeng J. Comparison of total disc replacement with lumbar fusion: a meta-analysis of randomized controlled trials. J Coll Physicians Surg Pak. 2015;25:60–67. doi:01.2015/JCPSP.6067. [PubMed] [Google Scholar]
- 20. Noshchenko A, Hoffecker L, Lindley EM, Burger EL, Cain CM, Patel VV. Long-term treatment effects of lumbar arthrodeses in degenerative disc disease: a systematic review with meta-analysis. J Spinal Disord Tech. 2014;28:E493–E521. doi:10.1097/BSD.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 21. Jacobs WC, van der Gaag NA, Kruyt MC, et al. Total disc replacement for chronic discogenic low back pain: a Cochrane review. Spine (Phila Pa 1976). 2013;38:24–36. doi:10.1097/BRS.0b013e3182741b21. [DOI] [PubMed] [Google Scholar]
- 22. Rao MJ, Cao SS. Artificial total disc replacement versus fusion for lumbar degenerative disc disease: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2014;134:149–158. doi:10.1007/s00402-013-1905-4. [DOI] [PubMed] [Google Scholar]
- 23. Gornet MF, Dryer RF, Peloza JH, Schranck FW. Lumbar disc arthroplasty vs. anterior lumbar interbody fusion: five-year outcomes for patients in the Maverick® disc IDE study. Spine J. 2010;10:S64 doi:10.1016/j.spinee.2010.07.174. [DOI] [PubMed] [Google Scholar]
- 24. Zigler JE. Five-year results of the ProDisc-L multicenter, prospective, randomized, controlled trial comparing ProDisc-L with circumferential spinal fusion for single-level disabling degenerative disk disease. Semin Spine Surg. 2012;24:25–31. doi:10.1053/j.semss.2011.11.006. [Google Scholar]
- 25. Guyer RD, McAfee PC, Banco RJ, et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: five-year follow-up. Spine J. 2009;9:374–386. doi:10.1016/j.spinee.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 26. Skold C, Tropp H, Berg S. Five-year follow-up of total disc replacement compared to fusion: a randomized controlled trial. Eur Spine J. 2013;22:2288–2295. doi:10.1007/s00586-013-2926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hiratzka J, Rastegar F, Contag AG, Norvell DC, Anderson PA, Hart RA. Adverse event recording and reporting in clinical trials comparing lumbar disk replacement with lumbar fusion: a systematic review. Global Spine J. 2015;5:486–495. doi:10.1055/s-0035-1567835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lefebvre C, Manheimer E, Glanville J. Searching for studies In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews for Interventions Version 5.1.0. Hoboken, NJ: John Wiley; 2011:95–150. [Google Scholar]
- 29. Anonymous. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley; 2011. [Google Scholar]
- 30. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13 doi:10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928 doi:10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002 doi:10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 33. Wei J, Song Y, Sun L, et al. Comparison of artificial total disc replacement versus fusion for lumbar degenerative disc disease: a meta-analysis of randomized controlled trials. Int Orthop. 2013;37:1315–1325. doi:10.1007/s00264-013-1883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yajun W, Yue Z, Xiuxin H, Cui C. A meta-analysis of artificial total disc replacement versus fusion for lumbar degenerative disc disease. Eur Spine J. 2010;19:1250–1261. doi:10.1007/s00586-010-1394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zigler JE, Delamarter RB. Five-year results of the prospective, randomized, multicenter, Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential arthrodesis for the treatment of single-level degenerative disc disease. J Neurosurg Spine. 2012;17:493–501. doi:10.3171/2012.9.spine11498. [DOI] [PubMed] [Google Scholar]
- 36. Aghayev E, Etter C, Bärlocher C, et al. Five-year results of lumbar disc prostheses in the SWISSspine registry. Eur Spine J. 2014;23:2114–2126. doi:10.1007/s00586-014-3418-4. [DOI] [PubMed] [Google Scholar]
- 37. Siepe CJ, Heider F, Wiechert K, Hitzl W, Ishak B, Mayer MH. Mid- to long-term results of total lumbar disc replacement: a prospective analysis with 5- to 10-year follow-up. Spine J. 2014;14:1417–1431. doi:10.1016/j.spinee.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 38. Garcia R, Jr, Yue JJ, Blumenthal S, et al. Lumbar total disc replacement for discogenic low back pain: two-year outcomes of the activL multicenter randomized controlled IDE clinical trial. Spine (Phila Pa 1976). 2015;40:1873–1881. doi:10.1097/BRS.0000000000001245. [DOI] [PubMed] [Google Scholar]
- 39. Guyer RD, Pettine K, Roh JS, et al. Comparison of 2 lumbar total disc replacements: results of a prospective, randomized, controlled, multicenter Food and Drug Administration trial with 24-month follow-up. Spine (Phila Pa 1976). 2014;39:925–931. doi:10.1097/brs.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 40. Guyer RD, Pettine K, Roh JS, et al. Five-year follow-up of a prospective, randomized trial comparing two lumbar total disc replacements. Spine (Phila Pa 1976). 2016;41:3–8. doi:10.1097/BRS.0000000000001168. [DOI] [PubMed] [Google Scholar]
- 41. Harrop JS, Youssef JA, Maltenfort M, et al. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976). 2008;33:1701–1707. doi:10.1097/BRS.0b013e31817bb956. [DOI] [PubMed] [Google Scholar]
- 42. Lemaire JP, Carrier H, Sariali el-H, Skalli W, Lavaste F. Clinical and radiological outcomes with the Charité artificial disc: a 10-year minimum follow-up. J Spinal Disord Tech. 2005;18:353–359. [DOI] [PubMed] [Google Scholar]
- 43. Siepe CJ, Mayer HM, Wiechert K, Korge A. Clinical results of total lumbar disc replacement with ProDisc II: three-year results for different indications. Spine (Phila Pa 1976). 2006;31:1923–1932. doi:10.1097/01.brs.0000228780.06569.e8. [DOI] [PubMed] [Google Scholar]
- 44. Katsimihas M, Bailey CS, Issa K, et al. Prospective clinical and radiographic results of CHARITÉ III artificial total disc arthroplasty at 2- to 7-year follow-up: a Canadian experience. Can J Surg. 2010;53:408–4145. [PMC free article] [PubMed] [Google Scholar]
- 45. Hellum C, Berg L, Gjertsen Ø, et al. Adjacent level degeneration and facet arthropathy after disc prosthesis surgery or rehabilitation in patients with chronic low back pain and degenerative disc: second report of a randomized study. Spine (Phila Pa 1976). 2012;37:2063–2073. doi:10.1097/BRS.0b013e318263cc46. [DOI] [PubMed] [Google Scholar]
- 46. Jacobs W, Van der Gaag NA, Tuschel A, et al. Total disc replacement for chronic back pain in the presence of disc degeneration. Cochrane Database Syst Rev. 2012;(9):CD008326 doi:10.1002/14651858.CD008326.pub2. [DOI] [PubMed] [Google Scholar]
- 47. Johnsen LG, Brinckmann P, Hellum C, Rossvoll I, Leivseth G. Segmental mobility, disc height and patient-reported outcomes after surgery for degenerative disc disease: a prospective randomised trial comparing disc replacement and multidisciplinary rehabilitation. Bone Joint J. 2013;95-B:81–89. doi:10.1302/0301-620x.95b1.29829. [DOI] [PubMed] [Google Scholar]
- 48. Chou R, Atlas SJ, Stanos SP, Rosenguist RW. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine (Phila Pa 1976). 2009;34:1078–1093. doi:10.1097/BRS.0b013e3181a103b1. [DOI] [PubMed] [Google Scholar]
- 49. Mirza SK, Deyo RA, Heagerty PJ, Turner JA, Martin BI, Comstock BA. One-year outcomes of surgical versus nonsurgical treatments for discogenic back pain: a community-based prospective cohort study. Spine J. 2013;13:1421–1433. doi:10.1016/j.spinee.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dettori JR. Loss to follow-up. Evid Based Spine Care J. 2011;2:7–10. doi:10.1055/s-0030-1267080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yue J. Long-term prospective randomized outcomes following lumbar total disc replacement: a single site experience with 6 years follow-up. Paper presented at: Conference I-tA ed; March 2016; Las Vegas, NV. [Google Scholar]
- 52. Fritzell P, Berg S, Borgstrom F, et al. Cost effectiveness of disc prosthesis versus lumbar fusion in patients with chronic low back pain: randomized controlled trial with 2-year follow-up. Eur Spine J. 2011;20:1001–1011. doi:10.1007/s00586-010-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kurtz SM, Lau E, Ianuzzi A, et al. National revision burden for lumbar total disc replacement in the United States: epidemiologic and economic perspectives. Spine (Phila Pa 1976). 2010;35:690–696. doi:10.1097/BRS.0b013e3181d0fabb. [DOI] [PubMed] [Google Scholar]
- 54. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP). https://www.ahrq.gov/research/data/hcup/index.html. Accessed October 3, 2017. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, activL_5-year_Meta-analysis_Manuscript-Supplemental-Mar_9_2017 for Comparison of Lumbar Total Disc Replacement With Surgical Spinal Fusion for the Treatment of Single-Level Degenerative Disc Disease: A Meta-Analysis of 5-Year Outcomes From Randomized Controlled Trials by Jack Zigler, Matthew F. Gornet, Nicole Ferko, Chris Cameron, Francine W. Schranck, and Leena Patel in Global Spine Journal