Abstract
Study Design:
Case series and literature review.
Objective:
There is a growing body of literature supporting that osteochondroma of the spine may not be as rare as previously documented. The purpose of this study was to perform an updated review and present our experience with 4 cases of solitary osteochondroma of the spine, including surgical treatment and subsequent outcomes.
Methods:
A review of 4 cases and an updated literature review.
Results:
All 4 cases were diagnosed as solitary osteochondroma of the spine based on clinical and histopathologic findings. Majority of the lesions arose from the posterior column with one case showing extension into the middle column with clinical neurologic sequelae. Treatment strategies for all cases included complete marginal excision of the lesions using a posterior approach. All 4 cases showed no radiographic evidence of recurrence. The literature review yielded 132 cases of solitary osteochondroma and 17 case associated with multiple hereditary exostosis. Out of the 132 cases, 36 presented with myelopathic symptoms.
Conclusion:
Osteochondroma of the spine may not be as rare as previously reported. The best approach to treatment in almost all symptomatic cases include wide surgical excision of the tumor. This should include complete resection of the cartilaginous cap of the tumor in an effort to prevent recurrence. When excision is performed properly, the outcomes are excellent with very low recurrence of the tumor.
Keywords: osteochondroma, literature review, case series, exostosis, surgical excision
Introduction
Osteochondroma (exostosis) is the most common benign bone tumor, accounting for 36% of benign bone tumors.1 Most often found in long bones, reports suggest osteochondroma of the spine to be relatively rare, accounting for only 4% to 7% of primary benign spinal tumors1-3 and less than 3% of all osteochondromas.3,4 Osteochondroma can arise as a solitary lesion or as part of an inherited condition known as multiple hereditary exostosis (MHE).1 Several studies have reported that solitary osteochondromas are more common in the spine when compared with osteochondroma associated with MHE.2,4,5 There is a growing body of evidence suggesting osteochondroma of the spine may not be as rare as previously reported.6-8 In this article, we describe our experience with the diagnosis, treatment, and natural history of osteochondroma of the spine of 4 cases, and the most up-to-date literature review of this topic since 2003.
Case Series
The authors have obtained the patients’ informed written consent for print and electronic publication of the case report.
Case 1
A 6-year-old female was brought to the emergency department for nonradiating neck pain that was localized to her left posterior neck. The patient had no neurological signs or symptoms. Radiographs demonstrated an osseous neck mass arising from the posterior cervical elements (Figure 1). Advanced imaging (magnetic resonance imaging and computed tomography scans) demonstrated an osseous lesion with a medullary cavity contiguous with the left C6 lamina. No signs of cord or root compression were seen. The patient has no known significant medical or family history of similar lesions.
Figure 1.

A 6-year-old female referred for a nonpainful mass in her neck noticed by her family. (A) Lateral radiograph showing ossified mass involving the C6 vertebrae and (B) sagittal magnetic resonance imaging showing the extent of the soft tissue involvement and the mass arising from the posterior cervical elements. (C) Hematoxylin and eosin slide of the cervical mass demonstrating a benign cartilage cap with subchondral bone, findings typical of an osteochondroma.
Case 2
A healthy 35-year-old male complained of 2 weeks of persistent mid-back pain after riding a go-cart. The patient denied any neurological symptoms during this time and was neurologically intact on physical examination. Imaging studies showed an osseous lesion about the low thoracic and thoracolumbar junction (Figure 2) without neurological involvement. The patient denies any medical or family history of similar lesions.
Figure 2.
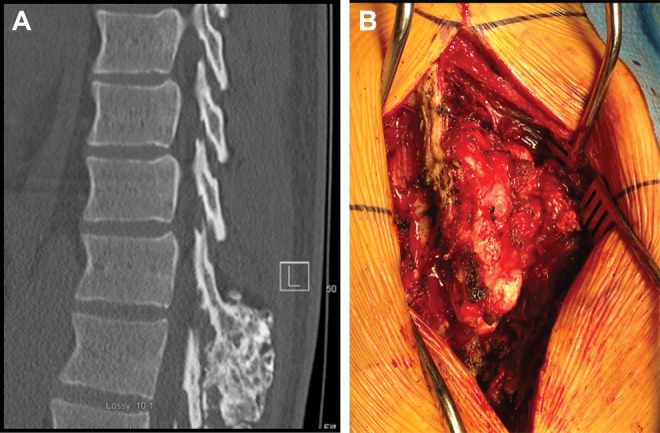
A 35-year-old male with mid-back pain following a minor injury. Plain radiographs demonstrated a mass arising from the posterior elements of T12. (A) Mid-Sagittal computed tomography scan of the thoracic spine demonstrating an osseous mass at the thoracolumbar junction. (B) Intraoperative clinical photograph of well-encapsulated thoracic mass.
Case 3
An 11-year-old male had progressive right posterior neck swelling for the past 10 months. A cervical computed tomography scan showed a mass at the C6 vertebrae with medullary continuity with the right lamina and spinous process. There was no evidence of cord or nerve root compression or vascular compromise. There was no significant medical or family history of similar lesions.
Case 4
A 36-year-old female had neck pain and progressive myelopathy (bowel and bladder dysfunction, gait abnormality, and progressive upper and lower extremity weakness). No history of antecedent trauma was reported. Plain radiographs showed an osseous lesion arising from the posterior column with significant canal compromise at the level of the C3 and C4 vertebrae (Figure 3). The patient had no medical or family history of similar lesions.
Figure 3.

(A) Lateral radiograph and (B) axial computed tomography scan showing osseous mass arising from the right C3 lamina and invading the spinal canal causing cord compression. Biopsy revealed an osteochondroma. (C) Postoperative lateral radiograph showing C3 vertebrectomy, anterior reconstruction with a titanium cage and plate, and a posterior instrumented fusion from C2 to C5 required to stabilize the spine following a wide resection of the osteochondroma.
Literature Review
Ovid MEDLINE and other nonindexed citations database search engines were used with the assistance of a medical librarian. The terms “osteochondroma” and “spine” and/or proxy descriptors were used to query PubMed. No limit in publication year, country, or language of publication was used. This yielded a list of all reported cases of osteochondroma of the spine since 1951. The list of articles was screened using the inclusion criterion—all reported cases from 2016 to 2004—and the following exclusion criteria: literature reviews, cases of primary tumor not arising from spine, non–case report accounts of cases, and nontumor processes (infection). Each case was reviewed for each parameter of clinical history and radiographic description whenever available in the case reports. Demographics, anatomic location of tumor, symptoms, treatment, and recurrence rates of tumor were almost always available and reported. A custom-built Excel database was used to organize and analyze the data. Descriptive statistics were used to summarize the results of the data.
Results
All 4 cases were diagnosed as solitary osteochondroma of the spine based on clinical findings and histopathologic features. All cases except for “Case 4” had no neurological symptoms—Case 4 was associated with cord compression and progressive myelopathy. Three of the 4 cases involved the cervical spine (includes case with cord compression) and 1 of 4 from the thoracolumbar region. All cases of osteochondroma in this series appeared to arise from the posterior column, with one case showing extension into the middle column and clinical neurologic sequelae. Treatment strategies for all cases included complete marginal excision of osteochondroma lesions using a posterior approach. Additionally, Case 4 (osteochondroma with cervical retrovertebral lesion and cord compression) required anterior corpectomy with placement of an interbody cage, followed by posterior decompression and instrumented fusion. All patients had complete symptomatic relief at their latest follow-up (up to 2 years) and showed no radiographic evidence of recurrence.
The review literature yielded a total of 223 articles,2-92 of which 110 were from the 2016 to 2004 period. Twenty-six articles were excluded, leaving 84 articles in the final analysis. The 84 articles yielded 149 reported cases. One hundred and thirty-two (88.6%) were solitary osteochondromas and 17 (11.4%) were associated with MHE.
Table 1 lists all 132 cases of solitary osteochondroma of the spine from the literature. Table 1 highlights the interesting data from each case of solitary osteochondroma. The location, treatment, and outcome of the cases are shown, along with the demographic data. For solitary osteochondromas (Table 2), there was a female-to-male ratio of 1:1.6 and an average age of 35.2 years (range = 2-77). The most common spinal level involved was cervical, with 63 (52.2%) of the cases, followed by lumbar 35 (26.5%), thoracic 24 (18.2%), sacrum 9 (6.8%), and coccyx 1 (0.76%). The most frequent spinal anatomic column involved was the posterior column, with 85 cases (64.3%), followed by unknown 28 (21.2%), anterior column 19 (14.3%), and 0 in the middle column.
Table 1.
Reviewed Cases in Literature: Interesting Dataa.
| Author | Journal | Year | Age | Sex | Tumor Level | Location | Presentation | Radiculopathy | Myelopathy | Treatment | Clinical Outcomes | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ramzan et al | Pediatric Neurosurgery | 2016 | 8 | Male | C1 | Posterior C1 arch | Symptomatic | No | Yes | Complete excision | Complete resolution | N/A |
| Bauer et al | Skeletal Radiology | 2015 | 19 | Female | C1 | C1 posterior arch | Symptomatic | No | Yes | Complete excision | Complete resolution | No |
| Michael | Journal of Pediatric Orthopaedics | 2015 | 16 | Female | L5-S1 | Facet joints | Symptomatic | No | No | Complete excision | Complete resolution | No |
| Haque et al | European Spine Journal | 2015 | 21 | Male | S3-S4 | Sacrum L of midline | Asymptomatic | No | No | Complete Excision | Complete resolution | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 35 | Female | C7 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 48 | Male | S1 | N/A | N/A | N/A | N/A | En bloc resection | N/A | Yes |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 46 | Male | C7 | N/A | N/A | N/A | N/A | Intralesional excision | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 46 | Female | T9-T10 | N/A | N/A | N/A | N/A | Intralesional excision | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 61 | Male | L2 | N/A | N/A | N/A | N/A | Intralesional excision | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 65 | Male | C3-T2 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 48 | Male | S1 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 43 | Female | C6-C7 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 76 | Female | T11-T12 | N/A | N/A | N/A | N/A | Intralesional excision | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 21 | Male | S1 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 49 | Female | C5-C7 | N/A | N/A | N/A | N/A | Intralesional excision | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 17 | Male | S1 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 32 | Male | L4-L5 | N/A | N/A | N/A | N/A | Intralesional excision | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 60 | Male | T12 | N/A | N/A | N/A | N/A | Intralesional excision | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 68 | Female | L2 | N/A | N/A | N/A | N/A | Intralesional excision | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 13 | Female | T1 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 19 | Male | L5 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 38 | Male | L4-L5 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 20 | Male | L5 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 36 | Female | T11-T12 | N/A | N/A | N/A | N/A | En bloc resection | N/A | Yes |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 26 | Male | C4-C5 | N/A | N/A | N/A | N/A | Intralesional excision | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 33 | Female | L1 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 21 | Male | T7 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 18 | Male | T1-T2 | N/A | N/A | N/A | N/A | Unknown | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 13 | Male | L5-S3 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 17 | Female | C1-C3 | N/A | N/A | N/A | N/A | Intralesional excision | N/A | No |
| Sciubba et al | Journal of Neurosurgery: Spine | 2015 | 2 | Female | T8-T11 | N/A | N/A | N/A | N/A | En bloc resection | N/A | No |
| Kang et al | JAMA Otolaryngology—Head and Neck Surgery | 2015 | 60 | Female | C2 | N/A | Symptomatic | No | No | Complete excision | N/A | N/A |
| Eren et al | The Spine Journal | 2015 | 24 | Female | L4 | Spinous process | Symptomatic | No | No | N/A | N/A | N/A |
| Neal et al | Military Medicine | 2015 | 40 | Male | L5 | Right anteriosuperior endplate of L5 | Symptomatic | No | No | Complete excision | Complete resolution | No |
| Dormont et al | Clinical Neuroradiology | 2014 | 59 | Male | C4 | Posterior C4 arch | Symptomatic | No | No | Complete excision | Near complete resolution | No |
| Mahore et al | BMJ Case Reports | 2014 | 28 | Male | D2-D3 | Posterior arch | Symptomatic | No | Yes | Laminectomy | Complete resolution | No |
| Boucetta et al | Pan African Medical Journal | 2014 | 48 | Male | C6 | Posterior arch | Symptomatic | No | No | Complete excision | Complete resolution | N/A |
| Kantarsi et al | The Spine Journal | 2014 | 24 | Female | C3 | Lamina | Symptomatic | Yes | No | N/A | N/A | N/A |
| Fumiaki et al | Neurologia Medico Chirucgica | 2014 | 57 | Male | L4 | Inferior articular process | Symptomatic | Yes | No | Laminectomy | Complete resolution | No |
| Fumiaki et al | Neurologia Medico Chirucgica | 2014 | 63 | Female | S1 | Superior articular process of | Symptomatic | Yes | No | Hemilaminectomy | Complete resolution | No |
| Fumiaki et al | Neurologia Medico Chirucgica | 2014 | 48 | Female | L4 | Inferior articular process | Symptomatic | Yes | No | Hemilaminectomy | Near complete resolution | No |
| Fumiaki et al | Neurologia Medico Chirucgica | 2014 | 32 | Male | L4 | Inferior articular process | Symptomatic | Yes | No | Hemilaminectomy | Complete resolution | No |
| Fumiaki et al | Neurologia Medico Chirucgica | 2014 | 62 | Male | L4 | Inferior articular process | Symptomatic | Yes | No | Hemilaminectomy | Complete resolution | No |
| Barbagallo et al | European Review for Medical and Pharmacological Sciences | 2014 | 68 | Male | C4-C5 | Anterior arch | Symptomatic | No | No | Complete excision | Complete resolution | No |
| Parekh et al | BMJ Case Reports | 2014 | 20 | Male | C7-T1 | Posterior arch | Asymptomatic | No | No | Complete excision | N/A | N/A |
| Hopper et al | Journal of Belgian Society of Radiology | 2014 | 68 | Female | T9-L3 | Posterior arch | Symptomatic | No | No | Complete excision | Complete resolution | No |
| Mont et al | Orthopedics (Healio) | 2014 | 11 | Male | L2-L4 | Inferior articular process | Symptomatic | Yes | No | En bloc resection | Complete resolution | No |
| Jameel et al | Journal of Clinical and Diagnostic Research | 2014 | 14 | Female | C5-C6 | Transverse process | Asymptomatic | No | No | Total excision | Complete resolution | No |
| David et al | Asian Spine Journal | 2014 | 52 | Male | C2-C6 | Transverse process | Symptomatic | Yes | No | Laminectomy | Complete resolution | No |
| Chow et al | Pediatric Neurology | 2013 | 9 | Male | C1-C2 | Inner surface of C2 arch | Symptomatic | Yes | No | C2 hemilaminectomy, resection of posterior C1 arch | Complete resolution | N/A |
| Scuotto et al | BMJ Case Reports | 2013 | 56 | Female | L2 | Lamina | Symptomatic | Yes | No | En bloc resection | Complete resolution | No |
| Garg et al | Kulak Burun Bogaz Ihtis Derg | 2013 | 22 | Male | C3-C4 | Vertebrae and pedicles | N/A | N/A | N/A | N/A | N/A | N/A |
| Jianru et al | Journal of Spinal Disorders Tech | 2013 | 43 | Male | L4 | Spinous process | Symptomatic | Yes | No | Complete excision | Complete resolution | N/A |
| Jianru et al | Journal of Spinal Disorders and Techniques | 2013 | 26 | Male | C1-C2 | Lateral mass | Symptomatic | No | Yes | Complete excision | Complete resolution | N/A |
| Jianru et al | Journal of Spinal Disorders and Techniques | 2013 | 11 | Male | T1 | Laminar mass | Symptomatic | Yes | No | Laminectomy, complete excision | Complete resolution | N/A |
| Jianru et al | Journal of Spinal Disorders and Techniques | 2013 | 60 | Female | C1 | Lateral mass | Symptomatic | No | Yes | Complete excision | Worsening of symptoms | N/A |
| Jianru et al | Journal of Spinal Disorders and Techniques | 2013 | 34 | Female | C1-C2 | Lateral mass | Symptomatic | No | Yes | Laminectomy, complete excision | Complete resolution | N/A |
| Jianru et al | Journal of Spinal Disorders and Techniques | 2013 | 17 | Female | C1 | Transverse process | Symptomatic | Yes | No | Complete excision | Complete resolution | N/A |
| Jianru et al | Journal of Spinal Disorders and Techniques | 2013 | 63 | Female | C5-C7 | Lamina | Symptomatic | Yes | No | Complete excision | Complete resolution | N/A |
| Jianru et al | Journal of Spinal Disorders and Techniques | 2013 | 17 | Female | T6 | Pedicle | Symptomatic | Yes | No | Laminectomy, complete excision | Complete resolution | N/A |
| Jianru et al | Journal of Spinal Disorders and Techniques | 2013 | 49 | Female | C2-C3 | Vertebral body | Symptomatic | No | Yes | Laminectomy, complete excision | Worsening of symptoms | N/A |
| Jianru et al | Journal of Spinal Disorders and Techniques | 2013 | 68 | Female | L2 | Lamina | Symptomatic | No | Yes | Laminectomy, complete excision | Complete resolution | N/A |
| Jianru et al | Journal of Spinal Disorders and Techniques | 2013 | 56 | Female | T5 | Vertebral body | Symptomatic | Yes | No | Laminectomy, complete excision | Partial functional resolution | N/A |
| Jianru et al | Journal of Spinal Disorders and Techniques | 2013 | 57 | Female | C5 | N/A | Symptomatic | Yes | No | Laminectomy, complete excision | Complete resolution | N/A |
| Madan et al | South Asian Journal of Cancer | 2013 | 9 | Male | T1 | Vertebral body, posterior arch | Symptomatic | No | Yes | Partial resection | Near complete resolution | No |
| Rai et al | Global Spine Journal | 2013 | 65 | Male | C2 | Vertebral body | Symptomatic | No | No | Complete excision | Complete resolution | N/A |
| Ghasemikhah et al | Iranian Journal of Radiology | 2013 | 19 | Male | T9 | Posterior arch | Symptomatic | No | Yes | Laminectomy | Complete resolution | N/A |
| Temiz et al | Acta Orthopaedica et Traumatologica Turcica | 2012 | 62 | Female | L2 | Inferior articular process | Symptomatic | No | Yes | Complete excision | Complete resolution | No |
| Chang et al | Skeletal Radiology | 2012 | 39 | Male | L4 | N/A | Symptomatic | No | No | N/A | N/A | N/A |
| Hussain et al | BMJ Case Reports | 2012 | 16 | Male | C1 | Posterior arch | Symptomatic | No | Yes | Laminectomy | Near complete resolution | No |
| Temiz et al | Turkish Neurosurgery | 2012 | 48 | Male | L3 | Inferior articular process | Symptomatic | Yes | No | Hemilaminectomy, complete excision | Near complete resolution | No |
| Kettner et al | Spine | 2012 | 21 | Female | C5 | Spinous process | Symptomatic | No | No | Laminectomy w/t en bloc resection | Complete resolution | N/A |
| Mamindla et al | Asian Journal of Neurosurgery | 2012 | 14 | Male | C3 | Lamina | Symptomatic | No | Yes | Laminectomy w/t en bloc resection | Complete resolution | N/A |
| Shin et al | Journal of Korean Neurosurgery | 2012 | 32 | Male | C4-C5 | Lamina and facet joint | Symptomatic | No | Yes | Hemilaminectomy | Near complete resolution | No |
| Kars et al | Asian Spine Journal | 2012 | 42 | Female | C1 | Lamina | Symptomatic | No | Yes | Laminectomy | Complete resolution | No |
| Nakamura et al | Skeletal Radiology | 2011 | 69 | Male | C7-T1 | N/A | Symptomatic | No | Yes | Laminectomy | Complete resolution | No |
| Rousseaux et al | Orthopaedics & Traumatology: Surgery & Research | 2011 | 23 | Male | C4 | Posterior arch | Symptomatic | No | Yes | Laminectomy | Complete resolution | No |
| Kettner et al | Journal of Manipulative and Physiological Therapeutics | 2011 | 24 | Male | C4 | Vertebral body | Symptomatic | No | No | Nonsurgical—Spinal manipulation | Complete resolution | No |
| Saglik et al | Archives of Orthopaedic and Trauma Surgery | 2011 | 26 | Male | L1 | Spinous process | Symptomatic | No | No | Complete excision | Complete resolution | No |
| Saglik et al | Archives of Orthopaedic and Trauma Surgery | 2011 | 9 | Male | C3/C4-T1 | Spinous process, posterior arch | Asymptomatic | No | No | Complete excision | Asymptomatic | No |
| Saglik et al | Archives of Orthopaedic and Trauma Surgery | 2011 | 36 | Female | T11-L1 | Lamina | Symptomatic | Yes | No | Laminectomy, complete excision | Near complete resolution | No |
| Saglik et al | Archives of Orthopaedic and Trauma Surgery | 2011 | 65 | Male | C4 | Vertebral body | Symptomatic | Yes | No | Anterior excision, followed by anterior cervical fusion | Complete resolution | No |
| Saglik et al | Archives of Orthopaedic and Trauma Surgery | 2011 | 19 | Male | C5-C6 | Spinous process | Symptomatic | No | No | Complete excision | Complete resolution | No |
| Saglik et al | Archives of Orthopaedic and Trauma Surgery | 2011 | 32 | Female | L3-L4 | Lamina | Asymptomatic | No | No | Nonsurgical | Asymptomatic | No |
| Schneider et al | Ethiopian Medical Journal | 2010 | 7 | Male | Coccyx | Coccyx | Symptomatic | No | No | En bloc excision | Complete resolution | N/A |
| Shimada et al | Neurologia Medico Chirucgica | 2010 | 58 | Male | C1-C2 | Spinous process | Symptomatic | Yes | Yes | En bloc excision | Complete resolution | No |
| Meshkini et al | Journal of Neurosurgery: Spine | 2010 | 29 | Male | L4 | Pedicle | Symptomatic | Yes | No | Laminectomy | Complete resolution | N/A |
| Meshkini et al | Journal of Neurosurgery: Spine | 2010 | 58 | Male | L5 | Vertebral body | Symptomatic | Yes | Yes | Laminectomy | Complete resolution | N/A |
| Meshkini et al | Journal of Neurosurgery: Spine | 2010 | 60 | Male | C5 | Lamina | Symptomatic | No | Yes | Hemilaminectomy | Complete resolution | N/A |
| Meshkini et al | Journal of Neurosurgery: Spine | 2010 | 34 | Male | C5-C6 | Lamina | Symptomatic | No | Yes | Laminectomy | Near complete recovery | N/A |
| Meshkini et al | Journal of Neurosurgery: Spine | 2010 | 55 | Male | T9 | Vertebral body | Symptomatic | No | Yes | Complete excision | Complete resolution | No |
| Meshkini et al | Journal of Neurosurgery: Spine | 2010 | 17 | Male | L3 | Inferior facet | Symptomatic | Yes | No | Hemilaminectomy | Complete resolution | N/A |
| Meshkini et al | Journal of Neurosurgery: Spine | 2010 | 34 | Female | C7 | Pedicle | Symptomatic | No | Yes | Hemilaminectomy | Near complete resolution | N/A |
| Meshkini et al | Journal of Neurosurgery: Spine | 2010 | 31 | Male | T8 | Superior facet | Symptomatic | No | Yes | Laminectomy | N/A | N/A |
| Kim et al | The Spine Journal | 2010 | 54 | Female | S1 | Sacral ala—anterior surface | Symptomatic | No | No | Complete excision | Complete resolution | No |
| Cha et al | Journal of Korean Neurosurgery | 2010 | 57 | Female | L3 | Lamina | Symptomatic | Yes | No | En bloc resection, laminectomy, facetectomy | Complete resolution | N/A |
| Horiuchi et al | Journal of Neurosurgery: Spine | 2009 | 77 | Female | C1 | Posterior arch | Symptomatic | No | Yes | Hemilaminectomy w/t en bloc resection | Complete resolution | No |
| Horiuchi et al | Journal of Neurosurgery: Spine | 2009 | 72 | Male | L4 | Inferior facet | Symptomatic | No | No | Marginal resection and facetectomy | Complete resolution | No |
| Horiuchi et al | Journal of Neurosurgery: Spine | 2009 | 69 | Male | L4-L5 | Inferior facet | Symptomatic | No | No | Intraarticular injection, biopsy, and ablation of articular facet joint | Complete resolution | No |
| Tian et al | Orthopedics | 2009 | 38 | Male | L5 | Lamina | Symptomatic | No | Yes | Laminectomy | Complete resolution | No |
| Jakheria et al | Journal of Pediatric Orthopaedics B | 2009 | 8 | Female | C2-C6 | Spinous process | Symptomatic | No | No | En bloc resection | Complete resolution | No |
| Chou et al | Case Reports/Journal of Clinical Neuroscience | 2009 | 16 | Female | C1-C2 | Vertebral Body | Symptomatic | No | No | Complete Excision | Complete resolution | No |
| Wenyuan et al | SAS Journal | 2009 | 28 | Male | T8 | Transverse process | Symptomatic | No | No | Radial excision | Complete resolution | N/A |
| Hassankhani et al | Cases Journal | 2009 | 16 | Female | L3 | Spinous process | Asymptomatic | No | No | En bloc resection | N/A | No |
| Srikantha et al | Journal of Neurosurgery: Spine | 2008 | 17 | Male | C3 | Spinolaminar Junction | Symptomatic | No | Yes | En bloc resection | Complete resolution | No |
| Srikantha et al | Journal of Neurosurgery: Spine | 2008 | 23 | Male | C4-C5 | Transverse processes, lamina, pedicles | Symptomatic | No | Yes | Partial resection, C4-C5 corpectomy, C3-C5 fusion | Complete resolution | No |
| Srikantha et al | Journal of Neurosurgery: Spine | 2008 | 40 | Female | C6 | Superior articular facet | Symptomatic | Yes | No | Medial facetectomy | Complete resolution | No |
| Byung-June et al | Joint Bone Spine | 2007 | 23 | Male | L5-S1 | Facet | Symptomatic | Yes | No | Partial laminectomy | Complete resolution | No |
| Song et al | European Journal of Pediatric Surgery | 2006 | 11 | Male | T4 | Superior articular process | Symptomatic | No | Yes | Laminectomy (T2-T3) | Complete resolution | No |
| Zhao et al | Spine | 2007 | 23 | Female | C7 | Transverse process | Symptomatic | No | No | En bloc resection | Complete resolution | No |
| Chatzidakis et al | Acta Neurochirurgica | 2007 | 22 | Male | C2 | Dens of C2 | Symptomatic | No | No | N/A | N/A | No |
| Ozturk et al | Acta Orthopaedica Belgica | 2007 | 46 | Male | C1 | Lamina | Symptomatic | Yes | No | Hemilaminectomy | Complete resolution | No |
| Maheshwari et al | Orthopaedic Surgery | 2006 | 20 | Male | C7 | Pedicle | Symptomatic | No | Yes | Laminectomy | Complete resolution | No |
| Moon et al | Pediatric Neurosurgery | 2005 | 16 | Male | C5-C7 | Spinous process | Symptomatic | Yes | Yes | Hemilaminectomy, complete excision of tumor | Complete resolution | No |
| Samartzis et al | Spine | 2006 | 11 | Male | S2 | Lamina | Symptomatic | Yes | No | Laminectomy S1-S4 | Complete resolution | No |
| McCall et al | Journal of Neurosurgery | 2006 | 13 | Female | C3 | Lamina | Asymptomatic | No | No | Complete excision | N/A | N/A |
| Yoshida et al | Acta Oto-Laryngologica | 2006 | 61 | Female | C1 | Anterior arch | Symptomatic | No | No | Complete excision | Complete resolution | No |
| Grivas et al | European Spine Journal | 2005 | 46 | Female | C7 | Pedicle | Symptomatic | Yes | No | Complete excision | Complete resolution | No |
| Brastianos et al | Neurosurgery | 2005 | 26 | Female | T12 | Vertebral body | Symptomatic | No | Yes | Complete excision, T12 corpectomy | Complete resolution | No |
| Agrawal et al | Pediatric Neurosurgery | 2005 | 14 | Male | L5-S1 | Illiac crest | Symptomatic | Yes | No | Laminectomy | Complete resolution | No |
| Faik et al | Joint Bone Spine | 2005 | 19 | Male | T4-T5 | Costovertebral angle, T4-T5 foramina | Symptomatic | Yes | No | Laminectomy, complete excision | Complete resolution | No |
| Miyamoto et al | Spinal Cord | 2005 | 23 | Male | C2 | Pedicle | Symptomatic | No | Yes | L hemilaminectomy, partial excision | Partial functional recovery | No |
| Kouwenhoven et al | European Spine Journal | 2004 | 42 | Male | C1-C2 | Neural arches | Symptomatic | Yes | No | Laminectomy, en bloc resection | Complete resolution | No |
| Gu rkanlar et al | Journal of Clinical Neuroscience | 2004 | 35 | Male | L4 | Lamina | Symptomatic | Yes | No | Complete Excision | Complete resolution | No |
| Schrot et al | Journal of Neurosurgery | 2004 | 15 | Male | C8 | Dermatome | Symptomatic | Yes | No | Hemilaminectomy, pediculectomy w/t complete excision of tumor | Complete resolution | No |
| Kulkarni et al | Neurologia Medico Chirucgica | 2004 | 15 | Male | T10-T11 | Facet | Symptomatic | No | Yes | Laminectomy | Complete resolution | No |
| Gille et al | Spine | 2004 | 18 | Female | C4 | Transverse process | Symptomatic | Yes | No | Cervicotomy | Complete resolution | No |
| Gille et al | Spine | 2004 | 15 | Male | C5 | Vertebral body | Symptomatic | No | Yes | Laminectomy and cervicotomy | Complete resolution | No |
| Gille et al | Spine | 2004 | 73 | Male | C2 | Posterior arch | Symptomatic | No | Yes | Laminectomy | Complete resolution | No |
| Gille et al | Spine | 2004 | 18 | Male | T11 | Pedicle | Asymptomatic | No | No | Laminectomy | Complete resolution | No |
| Gille et al | Spine | 2004 | 28 | Female | L4 | Posterior arch | Symptomatic | Yes | No | Laminectomy | Complete resolution | No |
| Gille et al | Spine | 2004 | 45 | Female | S1 | Vertebral body | Symptomatic | Yes | No | Lumbotomy | Complete resolution | No |
aAll 132 cases reviewed from literature are presented. Age, sex of the patient, location of lesion, type of surgery, symptoms, and recurrence are shown. If symptomatic w/o myelopathic or radiculopathic symptoms, symptomatic due to pain. N/A, data unavailable in the literature.
Table 2.
Demographic Data of Solitary Osteochondroma of the Spine, 132 Cases, Without a Known Hereditary Genetic Disorder.
| Sex, males | 61.3% |
| Age, years (mean, range) | 35.2 (2-77) |
| Spinal level of tumor | |
| Cervical | 65 (52%) |
| Thoracic | 24 (18%) |
| Lumbar | 35 (27%) |
| Sacrum | 9 (7%) |
| Coccyx | 1 (1%) |
| Involved spinal column | |
| Posterior | 85 (64%) |
| Anterior | 19 (14%) |
| Middle | 0 (0%) |
| Unknown | 28 (21%) |
There were 36 (27.2%) cases that involved solitary osteochondroma with myelopathic symptoms (Table 3). This group had a female-to-male ratio of 1:2.6 and average age of 35.1 years (range = 8-77). The most common spinal level involved was cervical in 24 (66.6%) cases, followed by thoracic 8 (22.2%) and lumbar 4 (11.1%). The most frequent spinal anatomic column involved was posterior column, with 29 cases (80.5%), followed by anterior column 6 (16.6%), unknown 1 (2.7%), and middle column 0 (0%). The osteochondroma began in the posterior arch in 20 (55.5%) of the cases, followed by the lamina in 7 (19.4%), vertebral body in 5 (16.6%), spinous process in 2 (5.5%), and unknown location in 1 (2.7%) of the cases.
Table 3.
Demographic Data on Solitary Osteochondroma With Spinal Cord Compressiona.
| Sex, males | 72.2% |
| Age, years (mean, range) | 35.1 (8-77) |
| Spinal level of tumor | |
| Cervical | 24 (66.6%) |
| Thoracic | 12 (22.2%) |
| Lumbar | 5 (11.1%) |
| Sacrum | 0 (0%) |
| Involved spinal column | |
| Posterior | 29 (81.5%) |
| Anterior | 6 (16.6%) |
| Middle | 0 (0%) |
| Unknown | 1 (2.7%) |
| Origin of tumor | |
| Pedicle | 3 (8.3%) |
| Laminae | 7 (19.4%) |
| Spinous process | 2 (5.5%) |
| Posterior arch other than pedicle, laminae, spinous process | 17 (47.2%) |
| Vertebral body | 6 (16.6%) |
| Unknown location | 1 (2.7%) |
| Treatment | |
| Anterior approach | 3 (8.3%) |
| Posterior approach | 29 (80.5%) |
| Combined anterior-posterior approach | 2 (5.5%) |
| Unknown approach | 2 (5.5%) |
| Patients requiring excision | 36 (100%) |
aA case series of 27 patients with unknown locations of osteochondroma and unknown symptoms, which was part of the data, had to be excluded from the results due to lack of data.
All 36 patients underwent surgery, of whom 29 (80.5%) underwent a posterior approach, 3 (8.3%) underwent an anterior approach, 2 (5.5%) underwent a combined anterior-posterior approach, and 2 (5.5%) approaches were unknown. The clinical outcomes showed improvement of symptoms in 34 (94.4%) of the patients, with 28 people showing a complete recovery and 6 with a partial recovery. Two cases showed worsening symptoms after surgery. There were 2 recurrences among all cases recorded, and none among solitary lesions with myelopathic symptoms.
Discussion
The first solitary osteochondroma was reported in 1843 by Reid.64 Many reports in the literature show that solitary osteochondroma is more common than lesions associated with MHE. The prevalence of osteochondroma in the spine is likely higher than previously thought. There seems to be a rise in the amount of case reports of osteochondroma published in the recent years (2004 to 2016). When Albrecht et al2 reviewed the relevant English literature from 1843 to 1992, it yielded 96 cases of solitary spinal osteochondroma. When Gille et al8 updated the review, they identified 54 additional cases of solitary spinal osteochondroma from 1992 to 2003. Our study yielded 132 new cases reported from 2004 to 2016, representing a 2.4-fold increase since 2003.
This increase in the number of cases in a smaller period of time is likely due to a higher rate of case reports being published on the topic rather than an actual increase in the incidence of these tumors. Nevertheless, the higher number of reported cases in the past decade likely underestimates the true prevalence of osteochondroma, because a significant portion of these tumors/lesions remain asymptomatic and, thus, may not be seen by a health care provider and/or require surgical treatment.
The review and analysis of the reported cases corroborate some of the trends seen in the literature, such as cervical spine being the most common site for a solitary osteochondroma of the spine, complete surgical excision being the most common method of surgical treatment, and the good outcomes and low recurrence rates after excision.
Additionally, the review of literature indicated that 27.2% of the cases with solitary osteochondromas of the spine had myelopathic features. This is in concordance with previous reports of 30% by Albrecht et al.2 It is proposed that the myelopathic symptoms seen in osteochondroma are due to progressive compression of the spinal structures, but may include a potentiated effect as the tumor grows over several years; likewise, the onset of age-related degenerative changes seen with spinal stenosis may also contribute.8
Osteochondroma is a form of exostosis that can be seen in any age group. It is generally reported that the age range for symptomatic presentation for solitary osteochondroma is between 10 and 30 years for peripheral lesions, but it appears that spine patients develop symptoms at an average age of 32, distinctly different from the peripheral lesions seen in children. By definition, osteochondroma has a characteristic cartilage cap on histology and a medullary continuity with the host bone, and can be sessile or pedunculated. MHE involves many exostoses in a single patient, unlike in the case of solitary osteochondroma, which is more common. An incidence of 1.3% to 4.1% has been reported as the percentage of solitary osteochondromas that affect the spine; however, 9% of MHE lesions are found in the spine.2 In the current review, 11.4% of all the cases of osteochondromas of the spine reviewed were associated with MHE.
Malignant transformation is low in solitary osteochondroma (<3%), but can be as high as 10% when associated with inherited genetic mutations as seen with MHE. MHE has an autosomal dominant inheritance pattern and involves mutations in the EXT 1, 2, and 3 genes on chromosome 8, 11, and 19, respectively. Malignant degeneration leads to a low-grade peripheral chondrosarcoma, which is managed with complete surgical resection. Malignant transformation of solitary osteochondroma is most frequently reported in the pelvis and rarely occurs in the spine.
A treatment algorithm for these lesions should begin with a thorough history and physical examination, to evaluate for genetic inheritance of similar lesions and to rule out neurovascular compromise that will necessitate surgery. Moreover, the majority of these lesions remain benign and are painless. In benign cases, observation with radiographic surveillance (computed tomography and magnetic resonance imaging and other advanced imaging may be used as indicated to better characterize the lesion and its local effects). Osteochondromas do have a tendency to increase in size and, depending on its location, may be associated with neurologic sequelae. In cases where unrelenting pain and/or evidence of neurovascular compromise (radiculopathy, myelopathy, or vascular compression) exists, surgical management may be warranted. Surgical treatment may include in situ marginal or wide excision, via a posterior, anterior, or combined approach, with or without instrumentation. In some cases, that is, Case 4, a need for cord or nerve root decompression along with instrumented stabilization with or without fusion may be required. Tumor excision may sometimes require both an anterior approach and a posterior approach. Of paramount importance during surgical excision is complete resection of the characteristic cartilage cap seen with these tumors. Incomplete resection of the cartilage cap may increase the risk of recurrence, and the pediatric population is more susceptible to tumor recurrence given their higher growth potential/age at presentation.
The recurrence rate in the review of the literature was 1.3% for all cases, and 0% for solitary spinal osteochondromas with myelopathic symptoms. Nevertheless, the current review of literature demonstrates a lower recurrence rate than previously reported (4%).8 However, there may be a number of unreported recurrences, given that not all cases in the literature explicitly reported this parameter. There is also the impact of a better understanding of the biology of the tumor, advanced imaging, and surgical techniques allowing for more expedient treatment in the recent years.
Conclusion
Osteochondroma is a relatively common bone tumor, accounting for 36% of all benign bone tumors,1 but occurs infrequently in the spine accounting for less than 3% of all osteochondromas.3,4 The solitary lesions in the spine may cause neurologic symptoms including radiculopathy and myelopathy, 29.5% and 27%, respectively, as reported in this review. The best approach to treatment in almost all symptomatic cases is marginal excision of the tumor. Meticulous surgical excision, with complete resection of the cartilaginous cap of the tumor, is important in preventing recurrence. When tumor excision is performed adequately, the outcomes are excellent with very low recurrence rates.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RY and IO have no relationships to disclose. JRD is an employee of Norton Healthcare; board member, Scoliosis Research Society; receives consulting fees from Medtronic and DePuy; receives payments for lectures from Medtronic, DePuy, and Norton Healthcare; holds patents and receives royalties from Medtronic; is on the editorial review board of JBJS Highlights, Spine, Spine Deformity, JAAOS, and Global Spine. Nuvasive provided funds directly to database company. No funds are paid directly to individual or individual’s institution 06/2012 to 04/2015. LYC is an employee of Norton Healthcare; member, Editorial Advisory Board, Spine and Spine Journal; institutional review board member, University of Louisville Institutional Review Board; research committee member, Scoliosis Research Society; receives research funds from the Orthopedic Research and Educational Fund, 2013 to present; receives Scoliosis Research Society Research Funding, 2013 to present; received funds for travel for Study Planning Meetings from the Center for Spine Surgery and Research of the University of Southern Denmark; received funds for travel for annual required Continuing Education for Institutional Review Board Members, University of Louisville Institutional Review Board; Nuvasive provided funds directly to database company. No funds are paid directly to individual or individual’s institution 06/2012 to 4/2015.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. Springfield, IL: Charles C Thomas; 1986. [Google Scholar]
- 2. Albrecht S, Crutchfield JS, SeGall GK. On spinal osteochondromas. J Neurosurg. 1992;77:247–252. [DOI] [PubMed] [Google Scholar]
- 3. O’Brien MF, Bridwell KH, Lenke LG, Schoenecker PL. Intracanalicular osteochondroma producing spinal cord compression in hereditary multiple exostoses. J Spinal Disord. 1994;7:236–241. [DOI] [PubMed] [Google Scholar]
- 4. Roblot P, Alcalay M, Cazenave-Roblot F, Levy P, Bontoux D. Osteochondroma of the thoracic spine. Report of a case and review of the literature. Spine (Phila Pa 1976). 1990;15:240–243. [DOI] [PubMed] [Google Scholar]
- 5. Khosla A, Martin DS, Awwad EE. The solitary intraspinal vertebral osteochondroma. An unusual cause of compressive myelopathy: features and literature review. Spine (Phila Pa 1976). 1999;24:77–81. [DOI] [PubMed] [Google Scholar]
- 6. Bess RS, Robbin MR, Bohlman HH, Thompson GH. Spinal exostoses: analysis of twelve cases and review of the literature. Spine (Phila Pa 1976). 2005;30:774–780. [DOI] [PubMed] [Google Scholar]
- 7. Gelb DE, Bridwell KH. Benign tumors of the spine In: Bridwell KH, DeWald RL, eds. The Textbook of Spinal Surgery. 2nd ed Philadelphia, PA: Lippincott-Raven; 1997:1959–1981. [Google Scholar]
- 8. Gille O, Pointillart V, Vital JM. Course of spinal solitary osteochondromas. Spine (Phila Pa 1976). 2005;30:E13–E19. [PubMed] [Google Scholar]
- 9. Agrawal A, Dwivedi SP, Joshi R, Gangane N. Osteochondroma of the sacrum with a correlative radiographic and histological evaluation. Pediatr Neurosurg. 2005;41:46–48. [DOI] [PubMed] [Google Scholar]
- 10. Akhaddar A, Boucetta M. Solitary osteochondroma of the cervical spine presenting as recurrent torticollis. Pan Afr Med J. 2014;17:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Altaf F, Movlik H, Brew S, Rezajooi K, Casey A. Osteochondroma of C1 causing vertebral artery occlusion. Br J Neurosurg. 2013;27:130–131. [DOI] [PubMed] [Google Scholar]
- 12. Baruah RK, Das H, Haque R. Solitary sacral osteochondroma without neurological symptoms: a case report and review of the literature. Eur Spine J. 2015;24(suppl 4):S628–S632. [DOI] [PubMed] [Google Scholar]
- 13. Bonic EE, Kettner NW. A rare presentation of cervical osteochondroma arising in a spinous process. Spine (Phila Pa 1976). 2012;37:E69–E72. [DOI] [PubMed] [Google Scholar]
- 14. Brastianos P, Pradilla G, McCarthy E, Gokaslan ZL. Solitary thoracic osteochondroma: case report and review of the literature. Neurosurgery. 2005;56:E1379. [DOI] [PubMed] [Google Scholar]
- 15. Burki V, So A, Aubry-Rozier B. Cervical myelopathy in hereditary multiple exostoses. Joint Bone Spine. 2011;78:412–414. [DOI] [PubMed] [Google Scholar]
- 16. Byung-June J, Seung-Eun C, Sang-Ho L, Hyeop JS, Suk PS. Solitary lumbar osteochondroma causing sciatic pain. Joint Bone Spine. 2007;74:400–401. [DOI] [PubMed] [Google Scholar]
- 17. Calvo CE, Cruz M, Ramos E. An unusual complication in a 9-year-old patient with hereditary multiple osteochondromatosis. PM R. 2013;5:348–350. doi:10.1016/j.pmrj.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 18. Certo F, Sciacca G, Caltabiano R, et al. Anterior, extracanalar, cervical spine osteochondroma associated with DISH: description of a very rare tumor causing bilateral vocal cord paralysis, laryngeal compression and dysphagia. Case report and review of the literature. Eur Rev Med Pharmacol Sci. 2014;18(1 suppl):34–40. [PubMed] [Google Scholar]
- 19. Chatzidakis E, Lypiridis S, Kazdaglis G, Chatzikonstadinou K, Papatheodorou G. A rare case of solitary osteochondroma of the dens of the C2 vertebra. Acta Neurochir. 2007;149:637–638. [DOI] [PubMed] [Google Scholar]
- 20. Chin KR, Kim JM. A rare anterior sacral osteochondroma presenting as sciatica in an adult: a case report and review of the literature. Spine J. 2010;10:e1–e4. [DOI] [PubMed] [Google Scholar]
- 21. Choi BK, Han IH, Cho WH, Cha SH. Lumbar osteochondroma arising from spondylolytic L3 lamina. J Korean Neurosurg Soc. 2010;47:313–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chooi YS, Siow YS, Chong CS. Cervical myelopathy caused by an exostosis of the posterior arch of C1. J Bone Joint Surg. 2005;87:257–259. [DOI] [PubMed] [Google Scholar]
- 23. Eap C, Litre CF, Noudel R, Duntze J, Theret E, Rousseaux P. Spinal cord compression due to C4 vertebral arch osteochondroma. Orthop Traumatol Surg Res. 2011;97:94–97. [DOI] [PubMed] [Google Scholar]
- 24. Er U, Simsek S, Yigitkanli K, Adabag A, Kars HZ. Myelopathy and quadriparesis due to spinal cord compression of C1 laminar osteochondroma. Asian Spine J. 2012;6:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ezra N, Tetteh B, Diament M, Jonas AJ, Dickson P. Hereditary multiple exostoses with spine involvement in a 4-year-old boy. Am J Med Genet A. 2010;152A:1264–1267. [DOI] [PubMed] [Google Scholar]
- 26. Fadili S, Clarencon F, Bonneville F, Savatovsky J, Deltour S, Dormont D. Occlusion of vertebral artery due to transverse canal osteochondroma. Clin Neuroradiol. 2014;24:395–397. [DOI] [PubMed] [Google Scholar]
- 27. Faik A, Mahfoud Filali S, Lazrak N, El Hassani S, Hajjaj-Hassouni N. Spinal cord compression due to vertebral osteochondroma: report of two cases. Joint Bone Spine. 2005;72:177–179. [DOI] [PubMed] [Google Scholar]
- 28. Giudicissi-Filho M, de Holanda CV, Borba LA, Rassi-Neto A, Ribeiro CA, de Oliveira JG. Cervical spinal cord compression due to an osteochondroma in hereditary multiple exostosis: case report and review of the literature. Surg Neurol. 2006;66(suppl 3):S7–S11. [DOI] [PubMed] [Google Scholar]
- 29. Grivas TB, Polyzois VD, Xarchas K, Liapi G, Korres D. Seventh cervical vertebral body solitary osteochondroma. Report of a case and review of the literature. Eur Spine J. 2005;14:795–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gulati A, Mittal A, Singal R, Gupta S, Garg V. A unique case of cervical osteochondroma causing dysphagia. Kulak Burun Bogaz Ihtisas Dergisi/J Ear Nose Throat: KBB. 2013;23:246–248. [DOI] [PubMed] [Google Scholar]
- 31. Gunay C, Atalar H, Yildiz Y, Saglik Y. Spinal osteochondroma: a report on six patients and a review of the literature. Arch Orthop Trauma Surg. 2010;130:1459–1465. [DOI] [PubMed] [Google Scholar]
- 32. Gurkanlar D, Aciduman A, Gunaydin A, Kocak H, Celik N. Solitary intraspinal lumbar vertebral osteochondroma: a case report. J Clin Neurosci. 2004;11:911–913. [DOI] [PubMed] [Google Scholar]
- 33. Han IH, Kuh SU. Cervical osteochondroma presenting as Brown-Séquard syndrome in a child with hereditary multiple exostosis. J Korean Neurosurg Soc. 2009;45:309–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hancock GE, Mariathas C, Fernandes JA, Breakwell LM, Cole AA, Michael AL. Osteochondroma arising from a lumbar facet joint in a 16-year-old. J Pediatr Orthop B. 2015;24:251–254. doi:10.1097/BPB.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 35. Hassankhani EG. Solitary lower lumbar osteochondroma (spinous process of L3 involvement): a case report. Cases J. 2009;2:9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huda N, Julfiqar M, Pant A, Jameel T. Giant cervical spine osteochondroma in an adolescent female. J Clin Diagn Res. 2014;8:LD01–LD02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kahveci R, Ergungor MF, Gunaydin A, Sanli AM, Temiz A. Solitary lumbar osteochondroma presenting with foot-drop: a case report. Turkish Neurosurg. 2012;22:386–388. [DOI] [PubMed] [Google Scholar]
- 38. Kahveci R, Ergungor MF, Gunaydin A, Temiz A. Lumbar solitary osteochondroma presenting with cauda equina syndrome: a case report. Acta Orthop Traumatol Turc. 2012;46:468–472. [DOI] [PubMed] [Google Scholar]
- 39. Kim JH, Kang JW. Oropharyngeal mass causing obstructive sleep apnea. Osteochondroma. JAMA Otolaryngol Head Neck Surg. 2015;141:393–394. [DOI] [PubMed] [Google Scholar]
- 40. Kouwenhoven JW, Wuisman PI, Ploegmakers JF. Headache due to an osteochondroma of the axis. Eur Spine J. 2004;13:746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kulkarni AG, Goel A, Muzumdar D. Solitary osteochondroma arising from the thoracic facet joint—case report. Neurol Med Chir (Tokyo). 2004;44:255–257. [DOI] [PubMed] [Google Scholar]
- 42. Kuraishi K, Hanakita J, Takahashi T, Watanabe M, Honda F. Symptomatic osteochondroma of lumbosacral spine: report of 5 cases. Neurol Med Chir (Tokyo). 2014;54:408–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee JY, Im SB, Park KW, Shin DS. Subclinical cervical osteochondroma presenting as Brown-Séquard syndrome after trivial neck trauma. J Korean Neurosurg Soc. 2012;51:233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lotfinia I, Vahedi P, Tubbs RS, Ghavame M, Meshkini A. Neurological manifestations, imaging characteristics, and surgical outcome of intraspinal osteochondroma. J Neurosurg Spine. 2010;12:474–489. [DOI] [PubMed] [Google Scholar]
- 45. Maheshwari AV, Jain AK, Dhammi IK. Osteochondroma of C7 vertebra presenting as compressive myelopathy in a patient with nonhereditary (nonfamilial/sporadic) multiple exostoses. Arch Orthop Trauma Surg. 2006;126:654–659. [DOI] [PubMed] [Google Scholar]
- 46. Mardi K, Madan S. Pediatric solitary osteochondroma of T1 vertebra causing spinal cord compression: a case report. South Asian J Cancer. 2013;2:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McCall TD, Liu JK, Kestle JR. Sporadic osteochondroma of the cervical spine. Case illustration. J Neurosurg. 2006;104(4 suppl):S293. [DOI] [PubMed] [Google Scholar]
- 48. Mehrian P, Karimi MA, Kahkuee S, Bakhshayeshkaram M, Ghasemikhah R. Solitary osteochondroma of the thoracic spine with compressive myelopathy: a rare presentation. Iran J Radiol. 2013;10:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Cervical myelopathy caused by atlas osteochondroma and pseudoarthrosis between the osteochondroma and lamina of the axis: case report. Neurol Med Chir. 2010;50:346–349. [DOI] [PubMed] [Google Scholar]
- 50. Miyamoto K, Sakaguchi Y, Hosoe H, et al. Tetraparesis due to exostotic osteochondroma at upper cervical cord in a patient with multiple exostoses-mental retardation syndrome (Langer-Giedion syndrome). Spinal Cord. 2005;43:190–194. [DOI] [PubMed] [Google Scholar]
- 51. Moon KS, Lee JK, Kim YS, et al. Osteochondroma of the cervical spine extending multiple segments with cord compression. Pediatr Neurosurg. 2006;42:304–307. [DOI] [PubMed] [Google Scholar]
- 52. Mudumba V, Mamindla RK. Cervical osteochondroma presenting with acute quadriplegia. Asian J Neurosurg. 2012;7:101–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Natale M, Rotondo M, D’Avanzo R, Scuotto A. Solitary lumbar osteochondroma presenting with spinal cord compression. BMJ Case Rep. 2013. doi:10.1136/bcr-2013-010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ogul H, Tuncer K, Can CE, Kantarci M. An unusual cause of spinal compression in a young woman: cervical osteochondroma. Spine J. 2014;14:1356. [DOI] [PubMed] [Google Scholar]
- 55. Okamoto T, Neo M, Fujibayashi S, Takemoto M, Nakamura T. Paraarticular osteochondroma of a cervico-thoracic facet joint presenting as myelopathy. Skeletal Radiol. 2011;40:1629–1632. [DOI] [PubMed] [Google Scholar]
- 56. Ozturk C, Tezer M, Hamzaoglu A. Solitary osteochondroma of the cervical spine causing spinal cord compression. Acta Orthop Belg. 2007;73:133–136. [PubMed] [Google Scholar]
- 57. Pandya NK, Auerbach JD, Baldwin K, Lackman RD, Chin KR. Spinal cord compression in a patient with multiple hereditary exostoses caused by breast adenocarcinoma metastatic to osteochondromas of the spine: case report. Spine (Phila Pa 1976). 2006;31:E920–E924. [DOI] [PubMed] [Google Scholar]
- 58. Potocki K, Prutki M, Kralik M, Palezac L, Skavic P, Padovan RS. Spinal cord compression caused by multiple spinal osteochondromas. Wien Klin Wochenschr. 2008;120:538. [DOI] [PubMed] [Google Scholar]
- 59. Pourtaheri S, Emami A, Stewart T, et al. Hip flexion contracture caused by an intraspinal osteochondroma of the lumbar spine. Orthopedics. 2014;37:e398–e402. [DOI] [PubMed] [Google Scholar]
- 60. Rahman A, Bhandari PB, Hoque SU, Ansari A, Hossain AT. Solitary osteochondroma of the atlas causing spinal cord compression: a case report and literature review. BMJ Case Rep. 2012. doi:10.1136/bcr.12.2011.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ramdasi RV, Mahore A. Solitary thoracic osteochondroma presenting as Brown-Séquard syndrome. BMJ Case Rep. 2014. doi:10.1136/bcr-2014-206656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rao H, Jakheria S. Giant cervical exostosis: a case report with review of literature. J Pediatr Orthop B. 2009;18:103–105. [DOI] [PubMed] [Google Scholar]
- 63. Reckelhoff KE, Green MN, Kettner NW. Cervical spine osteochondroma: rare presentation of a common lesion. J Manipulative Physiol Ther. 2010;33:711–715. [DOI] [PubMed] [Google Scholar]
- 64. Reid J. Disease of the spinal cord from exostosis of the second cervical vertebra. Lond Edinb Mon J Med Sci. 1843;3:194–198. [Google Scholar]
- 65. Ruivo C, Hopper MA. Spinal chondrosarcoma arising from a solitary lumbar osteochondroma. JBR-BTR. 2014;97:21–24. [DOI] [PubMed] [Google Scholar]
- 66. Rustagi T, Katz DA, Lavelle WF. C2 compressive osteochondroma with transient neurologic symptoms in a pediatric patient. Spine J. 2014;14:2516–2517. [DOI] [PubMed] [Google Scholar]
- 67. Rymarczuk GN, Dirks MS, Whittaker DR, Neal CJ. Symptomatic lumbar osteochondroma treated via a multidisciplinary military surgical team: case report and review of the literature. Military Med. 2015;180:e129–e133. [DOI] [PubMed] [Google Scholar]
- 68. Sade R, Yuce I, Karaca L, Ogul H, Kantarci M, Eren S. Lumbar osteochondroma presented with low back pain. Spine J. 2015;15:e35. [DOI] [PubMed] [Google Scholar]
- 69. Sadek AR, Vajramani G, Barker S, Walker M, Kennedy C, Nader-Sepahi A. Multiple spinal osteochondromata and osteosarcoma in a patient with Gorlin’s syndrome. Clin Neurol Neurosurg. 2014;118:5–8. [DOI] [PubMed] [Google Scholar]
- 70. Samartzis D, Marco RA. Osteochondroma of the sacrum: a case report and review of the literature. Spine (Phila Pa 1976). 2006;31:E425–E429. [DOI] [PubMed] [Google Scholar]
- 71. Schrot RJ, Kim KD, Fedor M. Trevor disease of the spine. Case report. J Neurosurg Spine. 2004;1:342–346. [DOI] [PubMed] [Google Scholar]
- 72. Sciubba DM, Macki M, Bydon M, et al. Long-term outcomes in primary spinal osteochondroma: a multicenter study of 27 patients. J Neurosurg Spine. 2015;22:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sekharappa V, Amritanand R, Krishnan V, David KS. Symptomatic solitary osteochondroma of the subaxial cervical spine in a 52-year-old patient. Asian Spine J. 2014;8:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sharma C, Acharya M, Kumawat BL, Parekh J. Giant spinal exostosis. BMJ Case Rep. 2014. doi:10.1136/bcr-2014-203819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Song KJ, Lee KB. Solitary osteochondroma of the thoracic spine causing myelopathy. Eur J Pediatr Surg. 2007;17:210–213. [DOI] [PubMed] [Google Scholar]
- 76. Srikantha U, Bhagavatula ID, Satyanarayana S, Somanna S, Chandramouli BA. Spinal osteochondroma: spectrum of a rare disease. J Neurosurg Spine. 2008;8:561–566. [DOI] [PubMed] [Google Scholar]
- 77. Strovski E, Ali R, Graeb DA, Munk PL, Chang SD. Malignant degeneration of a lumbar osteochondroma into a chondrosarcoma which mimicked a large retropertioneal mass. Skeletal Radiol. 2012;41:1319–1322. [DOI] [PubMed] [Google Scholar]
- 78. Sultan M, Khursheed N, Makhdoomi R, Ramzan A. Compressive myelopathy due to osteochondroma of the atlas and review of the literature. Pediatr Neurosurg. 2016;51:99–102. [DOI] [PubMed] [Google Scholar]
- 79. Tahasildar N, Sudesh P, Goni V, Tripathy SK. Giant osteochondroma of axis in a child with multiple hereditary exostoses: case report and review of literature. J Pediatr Orthop B. 2012;21:280–285. [DOI] [PubMed] [Google Scholar]
- 80. Teka A, Admassie D, Schneider J. Osteochondroma of the coccyx: a case report. Ethiop Med J. 2010;48:247–251. [PubMed] [Google Scholar]
- 81. Tian Y, Yuan W, Chen H, Shen X. Spinal cord compression secondary to a thoracic vertebral osteochondroma. J Neurosurg Spine. 2011;15:252–257. [DOI] [PubMed] [Google Scholar]
- 82. Tubbs RS, Maddox GE, Grabb PA, Oakes WJ, Cohen-Gadol AA. Cervical osteochondroma with postoperative recurrence: case report and review of the literature. Childs Nerv Syst. 2010;26:101–104. [DOI] [PubMed] [Google Scholar]
- 83. Wang V, Chou D. Anterior C1-2 osteochondroma presenting with dysphagia and sleep apnea. J Clin Neurosci. 2009;16:581–582. [DOI] [PubMed] [Google Scholar]
- 84. Wenyuan D, Baojun L, Yong S, Wei Z, Yingze Z. Osteochondroma arising from the thoracic transverse process. SAS J. 2009;3:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wong K, Bhagat S, Clibbon J, Rai AS. “Globus symptoms”: a rare case of giant osteochondroma of the axis treated with high cervical extrapharyngeal approach. Global Spine J. 2013;3:115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xu J, Xu CR, Wu H, Pan HL, Tian J. Osteochondroma in the lumbar intraspinal canal causing nerve root compression. Orthopedics. 2009;32:133. [PubMed] [Google Scholar]
- 87. Yagi M, Ninomiya K, Kihara M, Horiuchi Y. Symptomatic osteochondroma of the spine in elderly patients. Report of 3 cases. J Neurosurg Spine. 2009;11:64–70. [DOI] [PubMed] [Google Scholar]
- 88. Yoshida T, Matsuda H, Horiuchi C, et al. A case of osteochondroma of the atlas causing obstructive sleep apnea syndrome. Acta Otolaryngol. 2006;126:445–448. [DOI] [PubMed] [Google Scholar]
- 89. Zaijun L, Xinhai Y, Zhipeng W, et al. Outcome and prognosis of myelopathy and radiculopathy from osteochondroma in the mobile spine: a report on 14 patients. J Spinal Disord Tech. 2013;26:194–199. [DOI] [PubMed] [Google Scholar]
- 90. Zhang Y, Ilaslan H, Hussain MS, Bain M, Bauer TW. Solitary C1 spinal osteochondroma causing vertebral artery compression and acute cerebellar infarct. Skeletal Radiol. 2015;44:299–302. [DOI] [PubMed] [Google Scholar]
- 91. Zhao CQ, Jiang SD, Jiang LS, Dai LY. Horner syndrome due to a solitary osteochondroma of C7: a case report and review of the literature. Spine (Phila Pa 1976). 2007;32:E471–E474. [DOI] [PubMed] [Google Scholar]
- 92. Zinna SS, Khan A, Chow G. Solitary cervical osteochondroma in a 9-year-old child. Pediatr Neurol. 2013;49:218–219. [DOI] [PubMed] [Google Scholar]


