Abstract
Persistent elevation of thyroid stimulating hormone (TSH) is a common clinical problem in outpatient clinics treating patients with primary hypothyroidism. One challenge to practitioners involves predicaments where patients have an inadequate response to a seemingly appropriate dose of levothyroxine (L-T4). A patient’s self-assessed compliance to hormone replacement therapy or verification refill history at the patient’s pharmacy might not be a reliable form of confirmation of non-adherence to the drug by the patient, which has been referred to as “L-T4 pseudo-malabsorption.” A fast and inexpensive tool to rule out true LT4 malabsorption and thereby properly diagnosing and ultimately successfully treat LT4 pseudo-malabsorption is available in the outpatient setting. This allows clinicians to identify which patients for individual support in adhering to their prescribed therapy and may also reduce unnecessary referrals for sub-specialty care by endocrinologists.
Keywords: ambulatory, endocrinology, hypothyroidism, levothyroxine, levothyroxine absorption test, nonadherence
Introduction
Hypothyroidism is a common disorder that has been successfully treated with synthetic thyroxine (T4) since 1927.1 Patients who are athyreotic or essentially athyreotic require a dose of levothyroxine (L-T4) of 1.6–1.8 μg/kg daily to adequately treat their hypothyroidism and, as a result, normalize their thyroid stimulating hormone (TSH) concentrations.2,3 The absorption of oral L-T4 occurs primarily in the duodenum and jejunum. The L-T4 dose should be taken on an empty stomach 60 minutes prior to food ingestion.4,5 Foods such as large quantities of papaya, soy products, coffee or a fiber-rich diet can prevent the dose from being fully absorbed.6–8 The absorption of L-T4 may also be affected by some gastrointestinal disorders like Helicobacter pylori infection, celiac disease, lactose intolerance, pancreatic insufficiency and jejunoileal bypass surgery.4,9–12 Additionally, a number of commonly used medications like ferrous sulfate, calcium carbonate, aluminum hydroxide, sevelamer, sucrulfate and cholestyramine have been reported to alter the absorption of L-T4.13–19 In addition, some antiepileptic drugs such as carbamazepine or phenytoin increase the metabolism of L-T4, leading to higher dosing requirements.20,21
Nonadherence to medications is a major challenge in the management of any chronic disease. When patients do not take their L-T4 regularly, but do not report such behaviors, the clinician will often be left wondering if a persistently elevated TSH relates to gastrointestinal absorption issues.22 The term levothyroxine pseudo-malabsorption has been used to describe this specific situation – that is, nonadherence to thyroid hormone replacement therapy which is not fully endorsed by the patient and which mimics decreased gastrointestinal absorption of the drug. This term was first used by Ain and colleagues in 1991 after evaluating four cases of persistent hypothyroidism despite high oral doses of thyroid replacement therapy, eventually confirmed to be related to non-adherence.23
Case description
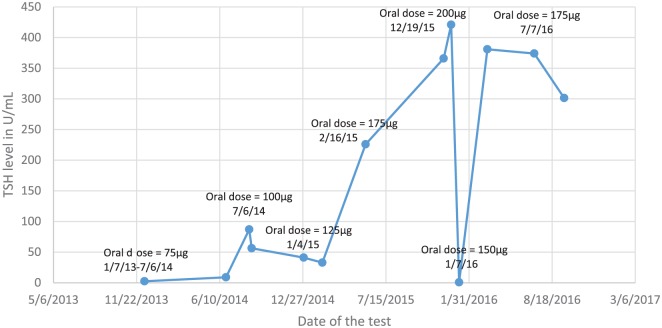
A 35-year-old, 49 kg female with a history of multinodular goiter status post thyroidectomy in 2004 and completion total thyroidectomy in 2014 presented with severe hypothyroidism despite being prescribed levothyroxine 200 μg (4.1 μg/kg) daily. She reported taking the levothyroxine regularly at 6 a.m. on an empty stomach, without any food or other medications for at least 2 h after administration. She took no medications with the potential to alter the absorption or metabolism of L-T4. TSH was markedly elevated at 421 μU/ml (normal, 0.30–4.20 μU/ml) (Figure 1), free T4 was reduced at 0.19 ng/dl (normal 0.80–1.80 ng/dl) and T3 total was at 39 ng/dL (normal 79–149 ng/dL) during a hospital admission in December 2015. During the hospitalization, the patient’s retail outpatient pharmacy was contacted. The pharmacy revealed that a 3-month supply of L-T4 was dispensed 4 months prior, suggesting some degree of non-adherence.
Figure 1.
TSH levels and prescribed levothyroxine oral daily doses.
The patient was seen in the endocrine clinic in January 2016. In retrospect, the patient had had difficulties in controlling hypothyroidism for several years. Her symptoms were mixed with some indicating hypothyroidism but other suggesting thyrotoxicosis. They included low energy, cold intolerance, hair loss and dry skin, ‘foggy’ vision, palpitations, shortness of breath and lower abdominal pain. She also reported episodic tremor, a 5 lb. weight loss and diaphoresis. Initial evaluation exploring for signs of generalized intestinal malabsorption proved negative, including testing for celiac disease (tissue transglutaminase IgA negative). All other laboratory tests, including hemoglobin, albumin and several vitamins, were within the normal range.
The patient received a dose of levothyroxine 50 μg IV, and an oral dose of levothyroxine 175 μg during the hospitalization in December 2015. These interventions and possible improvement in compliance to L-T4 administration brought the TSH level to 0.76 μU/ml (normal 0.30–4.20 μU/ml) and free T4 was elevated at 3.46 ng/dl (normal 0.80–1.80 ng/dl) by early January 2016. During a visit in March, however, the TSH was again elevated to 381 μU/ml, with free T4 reduced to 0.18 ng/dl. TSH remained elevated (374, 301 μU/ml) and free T4 low (0.23, 0.22 ng/dl) in July and September of the same year despite prescribed L-T4 doses of 150–175 μg/daily (3.06–3.57 μg/kg) respectively.
The patient was then referred to the pharmacist at the outpatient clinic for evaluation of adherence and the consideration of L-T4 absorption testing. The patient expressed frustration that the treatment had not been helping her condition. She subsequently agreed to undergo further testing. She underwent a levothyroxine (synthetic thyroxine) absorption testing, with arrival at 8:00 a.m. after having fasted for at least 8 h prior and not having taken her levothyroxine dose that day. Serum levels of TSH, T4 total, and T3 total were drawn at 0, 60 and 120 min after administration of 1000 μg of levothyroxine orally as five tablets of 200 μg each.
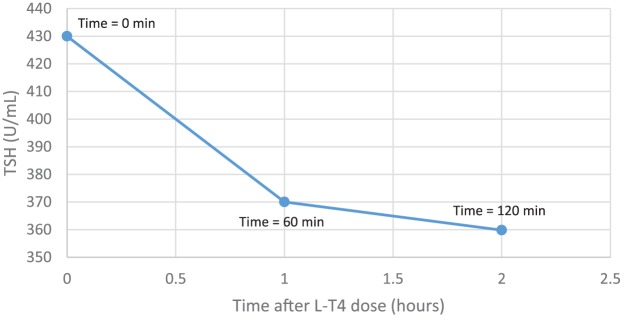
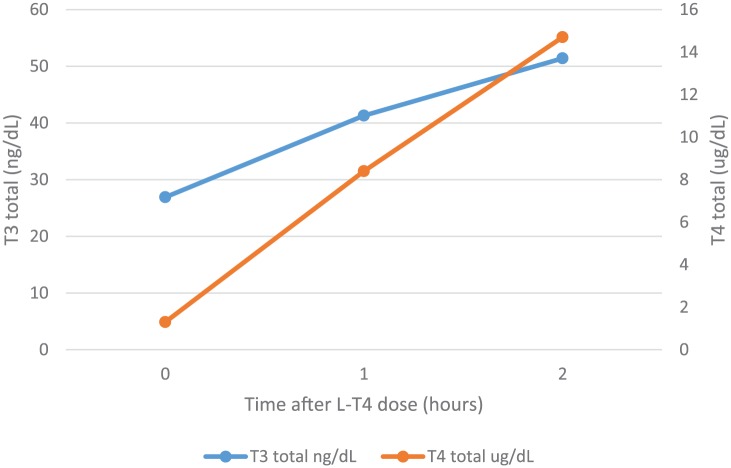
Both T4 and T3 increased substantially during the test and the TSH fell appropriately (Figures 2 and 3). Inadequate levothyroxine absorption was therefore excluded. As per recommendations from an endocrinology consultant, the dose of L-T4 was reduced to 1.6 μg/kg/day. Since the reinforcement of the importance of adherence had not worked in the past, the patient was offered ‘direct observation’ of administration of her L-T4 regimen at the outpatient clinic, supervised by a pharmacist. Her twice-weekly weight-based dose of L-T4 was calculated to be 288 μg. The patient therefore was scheduled to come into the outpatient clinic for twice-weekly administration of this L-T4 dose for 8 weeks. During this time, her TSH decreased to 9.87 μU/ml, with the T4 total level increasing to 5.7 μg/dl (normal 5.0–10.6 μg/dl), Free T4 to 1.66 ng/dL (normal 0.80-1.80 ng/dL), and her T3 climbing to 88.9 ng/dl (normal 79.0–149.0 ng/dl).
Figure 2.
Two-hour levothyroxine absorption test results: TSH level after 1000 μg of oral levothyroxine.
Figure 3.
Two-hour levothyroxine absorption test results: T3 total and T4 total levels after oral administration of 1000 μg of levothyroxine.
After completion of the 8 weeks of observed administration of L-T4 at 288 μg twice weekly, the regimen was changed to home administration, also twice weekly with reminder phone calls. At follow-up visit after 7 weeks, the TSH had increased to 32, with T4 and T3 levels remaining within their normal ranges. The patient acknowledged missing about 2–3 doses during the 7-week period between lab tests. The patient’s dose was therefore increased to 300 μg twice weekly (1.74 μg/kg). Additionally, the patient was instructed to take any missed doses on the following day. The TSH, T4 and T3 levels subsequently normalized (TSH = 0.682 μU/ml, T4 total = 9.1 ng/dl, T4 free = 1.52 ng/dl, T3 = 140.4 ng/dl) and the patient reported resolution of prior hypothyroid as well as thyrotoxic symptoms (Table 1).
Table 1.
The dose of levothyroxine (L-T4), thyroid stimulating hormone (TSH), T4 and T3 levels during twice-weekly dosing regimen.
| Date | ||||
|---|---|---|---|---|
| 4 October 2016 | 6 December 2016 | 26 January 2017 | 27 February 2017 | |
| L-T4 twice-weekly dose (μg) | Prior starting, home daily dose (4.1 μg/kg/day) |
288 (1.68 μg/kg/day) |
Increased to 300 (1.75 μg/kg/day) |
300 (1.75 μg/kg/day) |
| TSH; reference range 0.30–4.20 (U/ml) | 430.40 | 9.87 | 32.62 | 0.68 (in range) |
| Total T4; reference range 5.0–10.6 (μg/dl) | 1.3 | 5.7 | 9.8 | 9.1 (in range) |
| Free T4; reference range 0.80–1.80 (ng/dl) | 1.94 | 1.00 | 1.66 | 1.52 (in range) |
| Total T3; reference range 79.0–149.0 (ng/dl) | 26.9 | 88.9 | 99.9 | 140.4 (in range) |
Psychiatric disorder and evaluation should be considered if intentional noncompliance is suspected.23–26
Discussion
We herein report a case of uncontrolled primary hypothyroidism due to L-T4 pseudo-malabsorption, documented by a normal L-T4 absorption test and by normalization of TSH following direct-observation L-T4 therapy. Nonadherence to LT4 daily administration is the most common reason for not responding to seemingly appropriate dose of LT4, ultimately leading to diagnosis of pseudo-malabsorption after proper testing (29) In contrast, a key characteristic of LT4 pseudo-malabsorption is the patient’s denial of poor adherence, leading to obvious challenges in diagnosis and management.25,26
The L-T4 absorption test is an important tool to consider for distinguishing between nonadherence and true intestinal malabsorption (after excluding gastrointestinal and liver diseases, medication and dietary interference), the latter of which is very rare.27 There is no gold-standard method for the L-T4 absorption test, with various protocols advocated in the literature. The time commitment from a patient ranges from 2 h to 5 days.28–30 We elected to utilize a rapid, 2-h test. Adequate absorption of L-T4 was demonstrated with a subsequent prominent spike in free T4 plasma levels and concomitant suppression of TSH. Moreover, her FT4 and TSH quickly normalized after direct-observation therapy, twice weekly. Twice-weekly administration rather than once-weekly administration (as advised by some28,31,32) was chosen to reduce the likelihood of fluctuations in T4 levels in the body.
Poor adherence to any medical therapy is a well-recognized problem in chronic disease management. A frank discussion with patients of how current symptoms could be attributed to poor disease control due to irregular administration of vital medications often leads to improved adherence and, as a result, better symptom control. The predicament can be more of a challenge when the chronic disease is silent, as with hypertension and mild type 2 diabetes. An alternative to consider, in highly unreliable patients is supervised therapy at home, although often in the context of patients who otherwise qualify for visiting nurse activities. In the specific setting of medications with prolonged duration of action, such as oral L-T4, given twice-weekly, in-clinic supervised administration can be a reasonable strategy.23
In the setting of higher than expected L-T4 requirements, with TSH levels indicating continued inadequate control of primary hypothyroidism, the clinician should consider pseudo-malabsorption.
We describe a patient with this condition who was appropriately identified with a LT4 absorption test and, subsequently, with direct-observation therapy helped to normalize her TSH levels.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Grzegorz M. Rdzak, Pharmacy Department at Yale New Haven Hospital, BCPS Clinical Pharmacy Specialist Ambulatory Care, 55 Park Street, New Haven CT 06510, USA.
Laura M. Whitman, Yale Internal Medicine, Yale University School of Medicine, New Haven, CT, USA
Silvio E. Inzucchi, Yale Section of Endocrinology, Yale University School of Medicine, New Haven, CT, USA
References
- 1. Harington CR, Barger G. Chemistry of thyroxine: constitution and synthesis of thyroxine. Biochem J 1927; 21: 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician 2012; 86: 244–251. [PubMed] [Google Scholar]
- 3. Del Duca SC, Santaguida MG, Brusca N, et al. Individually-tailored thyroxine requirement in the same patients before and after thyroidectomy: a longitudinal study. Eur J Endocrinol 2015; 173: 351–357. [DOI] [PubMed] [Google Scholar]
- 4. Benvenga S, Bartolone L, Squadrito S, et al. Delayed intestinal absorption of levothyroxine. Thyroid 1995; 5: 249–253. [DOI] [PubMed] [Google Scholar]
- 5. Thien-Giang B, Nayak B, Loh J, et al. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metab 2009; 94: 3905–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deiana L, Marini S, Mariotti S. Ingestion of large amounts of papaya fruit and impaired effectiveness of levothyroxine therapy. Endocr Pract 2012; 18: 97–100. [DOI] [PubMed] [Google Scholar]
- 7. Liel Y, Harman-Boehm I, Shan S. Evidence for a clinically important adverse effect of fiber-enriched diet on the bioavailability of levothyroxine in adult hypothyroid patients. J Clin Endocrinol Metab 1996; 81: 857–859. [DOI] [PubMed] [Google Scholar]
- 8. Benvenga S, Bartolone L, Pappalardo MA, et al. Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid 2008; 18: 293–301. [DOI] [PubMed] [Google Scholar]
- 9. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med 1979; 90: 941–942. [DOI] [PubMed] [Google Scholar]
- 10. Lahner E, Virili C, Santaguida M, et al. Helicobacter pylori infection and drugs malabsorption. World J Gastroenterol 2014; 20: 10331–10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cellini M, Santaguida M, Gatto I, et al. Systematic appraisal of lactose intolerance as causes of increased need for oral thyroxine. J Clin Endocrinol Metab 2014; 99: 1454–1458. [DOI] [PubMed] [Google Scholar]
- 12. Centanni M. Thyroxine treatment: absorption, malabsorption, and novel therapeutic approaches. Endocrine 2013; 43: 8–9. [DOI] [PubMed] [Google Scholar]
- 13. Benavenga S, Di Bari F, Vita R. Undertreated hypothyroidism due to calcium or iron supplementation corrected by oral liquid levothyroxine. Endocrine 2017; 56: 138–145. [DOI] [PubMed] [Google Scholar]
- 14. Singh N, Weisler SL, Hershman JM. The acute effect of calcium carbonate on the intestinal obsorption of levothyroxine. Thyroid 2001; 11: 967–971. [DOI] [PubMed] [Google Scholar]
- 15. Singh N, Singh PN, Hershman JM. Effect of calcium carbonate on the absorption of levothyroxine. JAMA 2000; 283: 2822–2825. [DOI] [PubMed] [Google Scholar]
- 16. John-Kalarickal J, Pearlman G, Carlson H. New medications which decrease levothyroxine absorption. Thyroid 2007; 17: 763–765. [DOI] [PubMed] [Google Scholar]
- 17. Northcutt RC, Stiel JN, Hollifield JW, et al. The influence of cholestyramine on thyroxine absorption. JAMA 1969; 208: 1857–1861. [PubMed] [Google Scholar]
- 18. Campbell N, Hasinoff B, Stalts H, et al. Ferrous sulfate reduces tyroxine efficacy in patients with hypothyroidism. Ann Intern Med 1992; 117: 1010–1013. [DOI] [PubMed] [Google Scholar]
- 19. Liwanpo L, Hershman JM. Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab 2009; 23: 789–792. [DOI] [PubMed] [Google Scholar]
- 20. Yin-Xi Z, Chun-Hong S, Qi-Lun L, et al. Effects of antiepileptic drug on thyroid hormones in patients with epilepsy: a meta-analysis. Seizure 2016; 35: 72–79. [DOI] [PubMed] [Google Scholar]
- 21. Sherifa A. The effect of antiepileptic drugs on thyroid hormonal function: causes and implications. Expert Rev Clin Pharmacol 2015; 8: 741–750. [DOI] [PubMed] [Google Scholar]
- 22. Centanni M, Benvenga S, Sachmechi I. Diagnosis and management of treatment-refractory hypothyroidism: an expert consensus report. J Endocrinol Invest 2017; 40: 1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ain KB, Refetoff S, Fein HG, et al. Pseudomalabsorption of levothyroxine. JAMA 1991; 266: 2118–2120. [PubMed] [Google Scholar]
- 24. Van Wilder N, Bravenboer B, Herremans S, et al. Pseudomalabsorption of levothyroxine: a challenge for the endocrinologist in the treatment of hypothyroidism. Eur Thyroid J 2017; 6: 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Livadariu E, Valdes-Socin H, Murlacu C, et al. Pseudomalabsorption of thyroid hormones: case report and review of the literature. Ann Endoclinol 2007; 68: 460–463. [DOI] [PubMed] [Google Scholar]
- 26. Molines L, Fromont I, Morlet-Berla N, et al. L-thyroxine pesudomalabsorption: a factitious disease. Press Med 2007; 36: 1390–1394. [DOI] [PubMed] [Google Scholar]
- 27. Lips DJ, van Reisen MT, Voigt V, et al. Diagnosis and treatment of levothyroxine peseudomalabsorption. Neth J Med 2004; 62: 114–118. [PubMed] [Google Scholar]
- 28. Balla M, Jhingan R, Rubin D. Rapid levothyroxine absorption testing: a case series of nonadherent patients. Int J Endocrinol Metab 2015; 13: e31051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Butt M, Gupta N, Tan H, et al. Clinical application of the levothyroxine absorption test in the diagnosis of pseudo-malabsorption. Am J Case Rep 2014; 2: 253–255. [Google Scholar]
- 30. Srinivas V, Oyibo S. Levothyroxine pseudomalabsorption and thyroxine absorption testing with use of high-dose levothyroxine: case report and discussion. Endoct Pract 2010; 16: 1012–1015. [DOI] [PubMed] [Google Scholar]
- 31. Hannoush Z, Weiss R. Thyroid hormone replacement in patients following thyroidectomy for thyroid cancer. Rambam Maimonides Med J 2016; 7: e0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor J, Williams BO, Frater J, et al. Twice-weekly dosing for thyroxine replacement in elderly patients with primary hypothyroidism. J Int Med Res 1994; 22: 273–277. [DOI] [PubMed] [Google Scholar]