Short abstract
Objective
Delirium in critically ill patients is considered a risk factor for various long-term consequences. We evaluated delirium and associated long-term outcomes in patients with acute respiratory distress syndrome with non-H1N1 and H1N1- associated severe community-acquired pneumonia (sCAP) who had been recommended to take antiviral drugs associated with delirious symptoms as adverse effects.
Methods
Of 64 patients, 42 survivors (H1N1, 15; non-H1N1, 27) were analyzed regarding the relationship between medication and the duration of delirium in the intensive care unit. During follow-up (n = 23), we assessed cognitive abilities, post-traumatic stress disorder (PTSD), physical capacity, and health-related quality of life (HRQoL).
Results
The incidence of delirium was 88%. There was no difference in the incidence and duration of delirium between patients with H1N1 and non-H1N1 infection. The haloperidol and opioid doses were associated with a longer delirium duration. The delirium duration was correlated with reduced cognitive performance in motor skills, memory function, and learning efficiency. Patients with PTSD (16%) had a significantly longer delirium duration and low mental HRQoL.
Conclusions
H1N1 infection and corresponding antiviral medication had no impact on delirium. The duration of delirium in these patients was associated with impairments in various outcome parameters, illustrating the burden of sCAP.
Keywords: Community-acquired pneumonia, delirium, long-term outcome, post-traumatic stress disorder, health-related quality of life, analgesia
Introduction
Severe community-acquired pneumonia (sCAP) is an important cause of intensive care unit (ICU) admission. Diagnosis of sCAP is based on the presence of one of two major criteria (requirement for mechanical ventilation or need for vasopressors) and at least two of nine minor criteria (respiratory rate of ≥30 breaths/min, PaO2/FiO2 ratio of ≤250, multilobar disease, confusion/disorientation, blood urea nitrogen level of ≥20 mg/dL, leukopenia [white blood cell count of <4000 cells/mm³], thrombocytopenia [platelet count of <100 000 cells/mm³], hypothermia [core temperature of <36°C], and hypotension requiring aggressive fluid resuscitation).1,2 sCAP is associated with a risk of acute respiratory distress syndrome (ARDS). ARDS is characterized by bilateral inflammatory pulmonary infiltrates, impaired oxygenation, and acute onset and with an incidence of 13.5 to 58.7 cases per 100,000 person-years, making it a common lung disease requiring intensive care treatment.3–6
Regardless of the cause of ARDS (such as sCAP, nosocomial pneumonia, sepsis, or trauma), patients with ARDS are well known to have a long-term reduction in their health-related quality of life (HRQoL).7,8 Cognitive function, particularly memory performance, concentration, attention, and processing speed, remain affected even years after ARDS onset.9 Therefore, delirium in the ICU has been identified as a predictor of cognitive impairment in patients with ARDS patients.10,11 Delirium is a serious and common complication in up to 80% of patients with severe illness being treated in the ICU and is associated with a prolonged hospital stay and poor long-term outcome.12 However, the long-term outcome of CAP has not been well investigated, especially in patients with CAP in the ICU.13 In a cohort of patients with mild, moderate, and severe CAP, comorbidities other than CAP itself were speculated to have contributed to the observed reduction in HRQoL.14 HRQoL using quality-adjusted life years was recently investigated in elderly patients with and without CAP. The authors observed lower HRQoL in patients with than without CAP as measured with the 36-Item Short Form Health Survey and EQ-5D Index up to 12 months after discharge, and 9.7% of patients with radiologically confirmed CAP were admitted to the ICU.15
Furthermore, in a subgroup of patients with H1N1-associated ARDS, Luyt et al. observed psychological impairment, poorer HRQoL, and symptoms of anxiety, depression, and post-traumatic stress disorder (PTSD).16 Additionally, delirium and delirium-like symptoms have been reported as adverse effects of antiviral drugs such as the neuroaminase inhibitor oseltamivir, which was widely recommended for patients with H1N1 influenza to shorten the disease course.17 Other treatment-related factors that influence the development of delirium are exposure to opioids18,19 and psychoactive medications,20,21 which are administered to 98% of patients in the ICU.22 The pathophysiology of critical illness-associated delirium remains ambiguous and may vary from case to case; thus, the pharmacological approaches and their impact are still unclear. No clear causality between medication and delirium has been identified.
Delirium during treatment in the ICU and its associated risk factors have not been thoroughly investigated in survivors of severe CAP and ARDS. Therefore, in the present study, we focused on the association between medications and delirium in these patients. We examined the incidence and duration of delirium and considered the impact of oseltamivir, opioids, benzodiazepines, propofol, and antipsychotics during treatment of patients with sCAP and ARDS in the ICU. Moreover, we targeted patient-centered long-term outcomes and therefore performed a long-term follow-up of cognitive performance, physical capacity (6-minute walk test [6MWT]), development of PTSD, HRQoL, and the rate of return to work in these patients.
We hypothesized that oseltamivir used for treatment of H1N1 infection increases the incidence and duration of delirium; that the duration of delirium is associated with the administration of opioids, benzodiazepines, and propofol during ICU treatment; that the decreased cognitive performance and increased incidence of PTSD during follow-up are related to the duration of delirium; and that HRQoL is lower in patients with sCAP than in healthy controls.
This study was a secondary analysis and follow-up assessment of a dataset used to investigate the clinical courses of H1N1-associated and non-H1N1-associated infection (see Töpfer et al.).23
Materials and methods
Patient population
Patients with CAP and ARDS who were enrolled in a study of H1N1 vs. non-H1N1 infection from January 2009 to December 2010 and discharged alive from the hospital were included in this study and underwent comprehensive follow-up examinations. The follow-up examinations took place from November 2013 to April 2014 at our center 50 ± 6 months after discharge from the ICU. Age-matched healthy controls were also evaluated for cognitive testing using the Wortschatztest [WST] (a vocabulary test), Cambridge Neuropsychological Test Automated Battery (CANTAB), Visual Verbal Learning Test (VVLT), and Stroop Color and Word Test (SCWT).
Methods
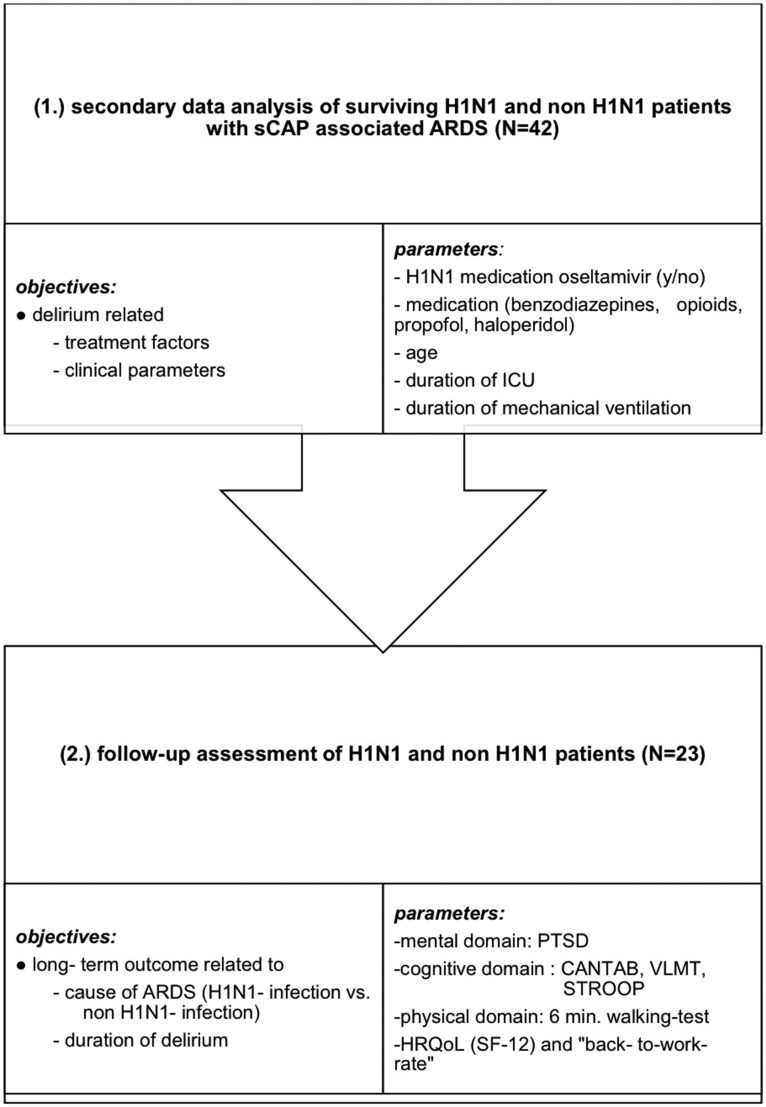
The study was approved by the ethics committee of the Charité-Universitätsmedizin Berlin (EA1/123/13) and registered in the German Clinical Trails Register (DRKS 00011913). Before follow-up enrollment, written informed consent was obtained from each survivor. The first part of the analysis focused on the clinical and treatment data in relation to the duration of delirium in surviving patients with H1N1 and non-H1N1 infection. The second part of the analysis focused on a follow-up assessment of the survivors with H1N1-infection with respect to their physical and mental health, cognitive performance, HRQoL, and rate of return to work (see Figure 1).
Figure 1.
Flowchart of study objective and analytical considerations. sCAP, severe community-acquired pneumonia; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; PTSD, post-traumatic stress disorder; CANTAB, Cambridge Neuropsychological Test Automated Battery; STROOP, Stroop Color and Word Test; HRQoL, health-related quality of life; SF-12, Medical Outcomes Study 12-Item Short Form; VVLT, Visual Verbal Learning Test.
Data concerning age, sex, cause of infection (H1N1/non-H1N1), ICU length of stay, duration of mechanical ventilation, daily scores for illness severity and organ dysfunction (Simplified Acute Physiology Score II [SAPS II], Sequential Organ Failure Assessment [SOFA], and Acute Physiology and Chronic Health Evaluation II [APACHE II]), analgosedation, delirium (Richmond Agitation-Sedation Scale [RASS] and Confusion Assessment Method for the ICU [CAM-ICU]), and daily mean and cumulative dose (in mg) of medications were extracted from the patient data management system (PDMS). Psychiatric lifelong and current diagnoses at the time of ICU admission (mood and anxiety disorders) were also extracted from the PDMS.
Medications
Benzodiazepine doses (diazepam equivalent) were calculated for diazepam, lorazepam, and midazolam. Opioid doses (morphine equivalent) were calculated for sufentanil, piritramide, and morphine. Propofol was mainly used for sedation, and haloperidol was mainly used as an antipsychotic medication for treatment of delirium. The ratio of the duration of haloperidol administration and duration of delirium was calculated to monitor the delirium treatment.
Delirium
The RASS was used to measure the patient´s agitation or sedation level.24,25 Daily screening for the presence and severity of delirium was conducted using the CAM-ICU and Delirium Detection Scale by trained nurses during all three shifts every day.26–29 Delirium was determined to be present in patients with a RASS score of +4 to −2, positive CAM-ICU result, and/or Delirium Detection Scale score of >7.
Several validated neuropsychological tests were applied to determine the essential aspects of cognitive performance. Intelligence before the onset of ARDS was determined to be the premorbid intelligence.
Premorbid intelligence and cognitive performance
Assessment of premorbid intelligence level
The WST is a vocabulary test that is widely used to estimate the premorbid intelligence level in the German language.30
Assessment of cognitive performance
The CANTAB is a computerized system for cognitive testing.31 The correct responses and reaction times were note for each subtest. The Motor Screening Task (MOT) measures speed and accuracy as an index of the subject’s motor skill. Pattern Recognition Memory (PRM) is a test of visual pattern recognition memory with immediate recognition (PRM 1) and 20-minute delayed recognition (PRM 2). Spatial recognition memory (SRM) was tested, and attention was assessed with choice reaction time tasks (CRT).
Assessment of learning efficiency and memory
The VVLT is based on Rey’s auditory recall of words and is used to examine learning efficiency and episodic declarative memory.32 A list of 15 words was learned in 3 sequential presentations at a fixed rate, and the patients were asked to recall as many words as possible. They were then asked to perform a fourth recall after a 20-minute delay. The number of errors and correctly recalled words were measured.
Assessment of executive function
The SCWT is a measure of executive function and contains three parts that measure selective attention, cognitive inhibition, and cognitive flexibility.33 First, the patients were asked to name a series of color words (“word task”). Second, they were asked to name the color of a bar (“color task”). Finally, they performed a combined “color–word task,” which was used to measure their mental flexibility and ability to inhibit a dominant response. The time and number of errors were recorded for all three parts of the SCWT. An interference measure was calculated according to Valentijn et al.34: Interference = Stroop III − [(Stroop I + Stroop II) / 2].
Assessment of PTSD
The German version of the Post-Traumatic Stress Syndrome 14-Questions Inventory (PTSS-14) was used to assess PTSD-related symptoms.35 The Post-traumatic Stress Diagnostic Scale (PDS) established by Foa et al.36 is a screening questionnaire for PTSD according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition. PTSD was diagnosed when both of the following criteria were met: the PTSS-14 score was ≥40 and PTSD was identified using the PDS.
A health status questionnaire was also used to ask patients about any current health problems such as neurological or mental disorders.
Assessment of physical performance
The 6MWT is used to assess functional exercise capacity and was performed in accordance with the American Thoracic Society guidelines.37 The reference equations established by Enright and Sherrill38 were used to calculate the predicted 6MWT for each individual: female: (2.11 × height [cm]) − (2.29 × weight [kg]) − (5.78 × age [y]) + 667 and male: (7.57 × height [cm]) − (1.76 × weight [kg]) − (5.02 × age [y]) − 309.
Assessment of HRQoL
HRQoL was assessed using the Medical Outcomes Study 12-Item Short Form (SF-12) questionnaire, which includes two dimensions of quality of life.39 Six items reflect physical health, and six items reflect mental health. The total score ranges from 0 to 100; higher scores indicate a more favorable quality of life. We matched healthy controls by age and sex from the normal database for the German population.
Rate of return to work
A questionnaire of socioeconomic status was used to ask patients about their employment status at the time of follow-up: work/student; invalidity pension/sick; unemployment; or retirement.
Statistical analysis
Results are given as median and range or frequency with percentage.
In the first step of the statistical analysis, clinical and treatment-related variables that had been measured during the ICU stay were analyzed for all surviving patients. To compare patients from independent groups (H1N1 infection vs. non-H1N1 infection and follow-up vs. non-follow-up), the Mann–Whitney U test for nonparametric variables was used because the variables were not normally distributed. The chi-square test was used to compare frequencies between independent groups. Robust regression analysis with backward stepwise feature selection was applied to detect relationships between clinical parameters (length of ICU stay, duration of mechanical ventilation, age, and H1N1 infection) and the duration of delirium (first model) as well as between medications (cumulative dose of benzodiazepines, opioids, haloperidol, and propofol) and the duration of delirium (second model). Clinical and pharmacological factors selected from these two models were combined and included in a third model to estimate their impact on the duration of delirium. These selected clinical and pharmacological factors were found to have a significant association with the duration of delirium.
In the second step of the statistical analysis, all patients who underwent subsequent measurement of cognitive performance (CANTAB, VVLT, and SCWT), PTSD, HRQoL, and physical capacity (6MWT) were evaluated and compared with the non-followed-up patients and age-matched healthy controls. The Spearman correlation coefficient was applied to estimate relationships between the subdimensions of cognitive performance (visual and verbal memory, attention, executive function, and working speed) and the duration of delirium. A p-value of <0.05 was considered statistically significant. All tests were conducted as an exploratory data analysis. Therefore, no adjustments for multiple testing were made.
All calculations were performed using Microsoft Excel 2011 Version 14.0 (Microsoft Corp., Redmond, WA, USA), SPSS Version 22 (IBM Corp., Armonk, NY, USA), and R Version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients
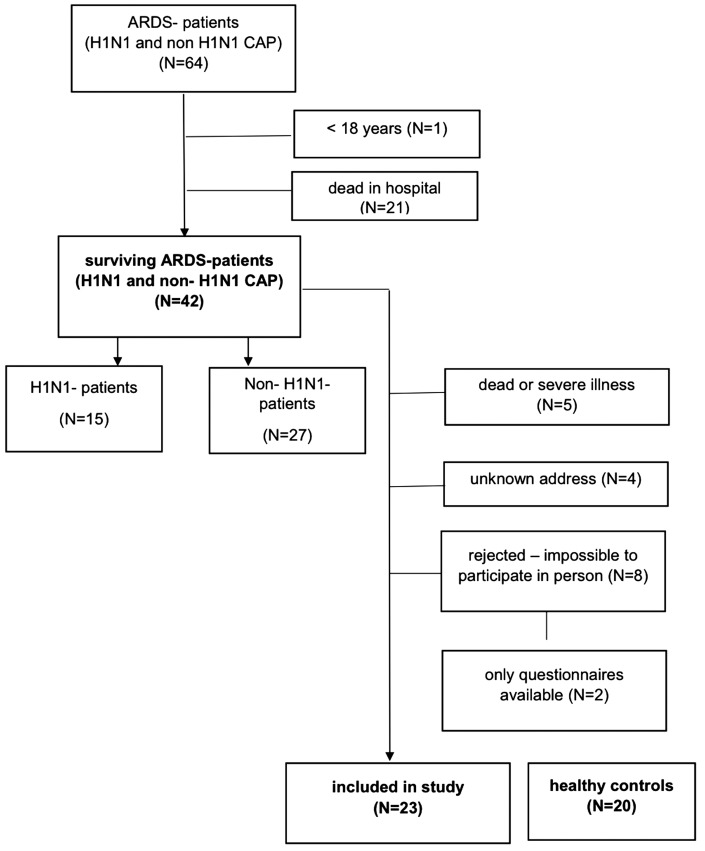
Of 64 patients with CAP and ARDS enrolled in the previous study of H1N1 vs. non-H1N1 infection from January 2009 to December 2010, 42 patients were discharged alive from the hospital. Of these 42 survivors (H1N1 infection, n = 15; non-H1N1 infection, n = 27), we recruited 23 patients (H1N1 infection, n = 8; non-H1N1 infection, n = 15) for comprehensive follow-up examinations. Nineteen patients were excluded (no address, n = 4; death after ICU discharge, n = 5; impossible to participate because of a severe handicap with the inability to undergo physical and cognitive examination, n = 8; and availability of only the questionnaire results with no physical or cognitive examination, n = 2) (see Figure 2). Twenty age-matched healthy controls were also assessed.
Figure 2.
CONSORT diagram of inclusion of patients with H1N1- and non-H1N1-associated ARDS. CONSORT, Consolidated Standards of Reporting Trials; ARDS, acute respiratory distress syndrome; CAP, community-acquired pneumonia.
Clinical and pharmacological characteristics in relation to duration of delirium
The patients with H1N1 infection and non-H1N1 infection showed no significant differences in sex, age, severity of illness (SAPS II, APACHE II), organ failure (SOFA), ICU length of stay, or duration of mechanical ventilation (see Table 1; see also Töpfer et al.23).
Table 1.
Demographic and clinical characteristics of surviving patients with H1N1 and non-H1N1 infection
| All patients(n = 42) | H1N1 infection(n = 15) | Non-H1N1 infection(n = 27) | p-value(H1N1 vs. non-H1N1) | |
|---|---|---|---|---|
| Male | 27 (64) | 8 (30) | 19 (70) | 0.325 |
| Female | 15 (36) | 7 (47) | 8 (53) | |
| Age, years | 42 (18–65) | 37 (21–65) | 45 (18–65) | 0.288 |
| APACHE II score | 18 (6–29) | 16 (6–28) | 20 (11–29) | 0.229 |
| SAPS II score | 37 (19–69) | 36 (19–69) | 38 (20–69) | 0.649 |
| SOFA score | 8 (3–16) | 9 (4–16) | 8 (3–13) | 0.806 |
| Time from discharge to investigation, months | 50 (37–60) | 48 (44–60) | 54 (37–58) | 0.382 |
| ICU length of stay, days | 32 (7–360) | 43 (14–161) | 29 (7–360) | 0.090 |
| Duration of mechanical ventilation, days | 22 (1–72) | 24 (1–72) | 20 (2–61) | 0.449 |
| ECLA/ECMO | 18 (45) | 6 (43) | 12 (46) | 1.00 |
| Duration of delirium, days | 5 (0–29) | 4 (0–11) | 7 (0–29) | 0.147 |
| Incidence of delirium | 37 (88) | 12 (87) | 25 (93) | 1.00 |
Data are presented as n (%) or median (range).
Mann–Whitney U test for comparison between patients with H1N1 infection and non-H1N1 infection. Chi-square test for comparison of frequencies of delirium and H1N1 and non-H1N1 infection. Significance was assumed at a two-tailed p-value of <0.05.
APACHE II, Acute Physiology and Chronic Health Evaluation II; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment; ICU, intensive care unit; ECLA, extracorporeal lung assist; ECMO, extracorporeal membrane oxygenation
Delirium
Thirty-seven patients (88%) developed delirium during their ICU stay. Two patients (5%) were not assessable due to deep sedation. The median duration of delirium was 5 days (range, 0–29 days). However, in all patients, antipsychotic medication was given for twice as long as delirium was detected, and it was given for significantly longer in patients with H1N1 infection than non-H1N1 infection (threefold longer in H1N1 infection vs. twofold longer in non-H1N1 infection, p = 0.019) (see Table 2). Patients with H1N1 infection and non-H1N1 infection received the same high daily dose of benzodiazepines, but those with H1N1 infection received a significantly higher cumulative dose of benzodiazepines (p = 0.001) (see Table 2).
Table 2.
Medications
| Drug name | All patients(n = 42) | H1N1 infection(n = 15) | Non-H1N1 infection(n = 27) | p-value (H1N1 vs. non-H1N1) |
|---|---|---|---|---|
| Benzodiazepine, mg | 171 (13–348) | 170 (84–348) | 168 (13–306) | 0.989 |
| Benzodiazepine cumulative dose, mg | 1009 (48–10,732) | 3069 (264–10,732) | 720 (48–8370) | 0.001* |
| Propofol, mg | 1164 (50–3396) | 1172 (50–2698) | 1152 (202–3396) | 0.753 |
| Propofol cumulative dose, mg | 8531 (50–192,442) | 9375 (50–140,286) | 8301 (330–192,442) | 0.862 |
| Opioids, mg | 17 (4–690) | 19 (4–530) | 15 (5–690) | 0.616 |
| Opioids cumulative dose, mg | 46 (4–2063) | 129 (4–647) | 39 (10–2063) | 0.977 |
| Haloperidol, mg | 3 (1–14) | 4 (1–14) | 3 (1–14) | 0.488 |
| Haloperidol cumulative dose, mg | 41 (2–349) | 50 (4–258) | 34 (2–349) | 0.163 |
| Duration of haloperidol to delirium, % | 200 (24–1600) | 342 (173–1600) | 175 (24–800) | 0.019* |
Data are presented as median (range).
Mann–Whitney U test between patients with H1N1 infection and non-H1N1 infection for comparison of daily mean dose and cumulative dose of medication.
Significance was assumed at a two-tailed p-value of < 0.05. *Significant difference.
All 15 patients with H1N1 infection received oseltamivir as an antiviral medication for a mean of 8 ± 5 days with a mean daily dose of 245 ± 82 mg and a mean cumulative dose of 1938 ± 1310 mg. There were no differences in the incidence or duration of delirium between patients with H1N1 and non-H1N1 infection (see Table 1). The association between the duration of delirium and drug dose (cumulative dose of opioids, benzodiazepines, haloperidol, and propofol) was analyzed with robust regression. This revealed that a higher cumulative dose of opioids (p = 0.00009) and haloperidol (p = 0.004) as well as a lower dose of propofol (p = 0.026) were significantly predictive of a longer duration of delirium in patients with sCAP with ARDS (see Table 3a). A second robust regression including H1N1 infection, the duration of mechanical ventilation, length of ICU stay, and age was conducted. Neither the H1N1-infection, duration of ICU, mechanical ventilation, nor age showed a significant impact on the duration of delirium (see Table 3b). Finally, in the selected setting (third model), a higher cumulative dose of opioids (p = 0.0051) and haloperidol (p = 0.011) were significantly associated with a longer duration of delirium (see Table 3c).
Table 3a.
Robust regression (selected model); multiple R2 = 0.288
| β | SE | p-value | |
|---|---|---|---|
| Opiate cumulative dose | 0.0118 | 0.0025 | 0.00009* |
| Propofol cumulative dose | −0.00008 | 0.000035 | 0.026* |
| Haloperidol cumulative dose | 0.0461 | 0.0148 | 0.004* |
Correlation between delirium duration (days) and medication (cumulative dose of benzodiazepine, opiate, propofol, and haloperidol (in mg)). *Significant difference.
SE, standard error.
Table 3b.
Robust regression (full model); multiple R2 = 0.1667
| β | SE | p-value | |
|---|---|---|---|
| Duration of mechanical ventilation | 0.063 | 0.042 | 0.148 |
| Duration of ICU stay | 0.065 | 0.063 | 0.305 |
| H1N1 infection/non-H1N1 infection | −4.83 | 2.503 | 0.061 |
| Age | −0.04 | 0.046 | 0.575 |
Correlation between delirium duration (days) and clinical variables (duration of mechanical ventilation (days), duration of ICU stay (days), age (years), and H1N1 infection (H1N1/non-H1N1)).
ICU, intensive care unit; SE, standard error.
Table 3c.
Robust regression (selected model); multiple R2 = 0.327
| β | SE | p-value | |
|---|---|---|---|
| H1N1/non-H1N1 infection | −2.985 | 2.086 | 0.165 |
| Opiate cumulative dose | 0.00976 | 0.00317 | 0.0051* |
| Propofol cumulative dose | 0.00006 | 0.000042 | 0.163 |
| Haloperidol cumulative dose | 0.045 | 0.0165 | 0.011* |
Correlation between delirium duration (days), opiate (cumulative dose in mg), propofol (cumulative dose in mg), and benzodiazepine (cumulative dose in mg). *Significant difference.
SE, standard error.
Long-term outcomes
We compared patients who were followed up (n = 23) and those who were unavailable for follow-up (n = 19) to identify potential confounders for sociodemographic and clinical characteristics between these two groups. Patients who were and were not followed up showed no significant differences in clinical characteristics, incidence of delirium (83% vs. 95%, respectively), or duration of delirium (see Table 4). The proportions of patients with H1N1 infection and non-H1N1 infection were equally distributed between the patients who were and were not followed up. Hence, the patients who were followed up were representative of the entire study population.
Table 4.
Demographic and clinical characteristics of followed-up and non-followed-up patients
| Patients | Followed up (n = 23) | Not followed up (n = 19) | p-value |
|---|---|---|---|
| Male | 13 (44) | 14 (74) | 0.337 |
| Female | 10 (56) | 5 (26) | |
| Age, years | 42 (19–65) | 46 (18–60) | 0.807 |
| APACHE II score | 19 (6–28) | 18 (9–29) | 0.625 |
| SAPS II score | 35 (20–69) | 40 (19–69) | 0.139 |
| SOFA score | 8 (3–16) | 9 (4–13) | 0.304 |
| Time between discharge and investigation, months | 50 (37–60) | ||
| ICU length of stay, days | 29 (7–75) | 43 (20–360) | 0.078 |
| Duration of mechanical ventilation, days | 21 (1–68) | 24 (2–72) | 0.334 |
| Delirium | 19 (83) | 18 (95) | 1.00 |
| Duration of delirium, days (n = 38) | 4 (1–29) | 7 (1–29) | 0.051 |
| Post-traumatic stress disorder (n = 25) | 4 (16) | ||
| Cause of admission | |||
| H1N1 Infection | 8 (35) | 7 (37) | 1.00 |
| non-H1N1 infection | 15 (65) | 12 (63) | |
| Employment status | |||
| Work/student | 8 (34) | ||
| Invalidity pension/sick | 4 (17) | ||
| Unemployment | 4 (17) | ||
| Retirement | 5 (22) |
Data are presented as n (%) or median (range).
Mann–Whitney U test for comparison between followed-up and non-followed-up patients (patients with H1N1 and non-H1N1 infection); Chi-square test for comparison of frequencies of H1N1 and non-H1N1 infection. Significance was assumed at a two-tailed p-value of <0.05.
APACHE II, Acute Physiology and Chronic Health Evaluation II; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment; ICU, intensive care unit
Cognitive performance
The estimated premorbid intelligence level, measured with the WST-A, was within the normal range (intelligence quotient [IQ], 100 ± 15) in patients who were followed up (IQ, 92 ± 10) and in healthy controls (IQ, 107 ± 12). Psychomotor speed measured by MOT latency and verbal processing speed detected with the SCWT1 were significantly lower in patients than in healthy controls (p = 0.007 and p = 0.010, respectively). Furthermore, the performance of visual short-term memory measured by PRM and verbal short-term memory/working memory measured by VVLT 1 were significantly lower in patients than in healthy controls (p = 0.004 and p = 0.028, respectively) (see Table 5).
Table 5.
Comparison of CANTAB between patients and healthy controls (Patients: correlation between delirium duration and CANTAB)
|
Patients(n = 23) |
Healthy controls(n = 20) |
Mann–Whitney U test (patients vs. healthy controls) |
Correlation between delirium duration and CANTAB |
||
|---|---|---|---|---|---|
| Cognitive tests | Median (range) | Median (range) | p-value | r | p-value |
| MOT mean error, % | 13 (9–18) | 12 (8–20) | 0.147 | −0.10 | 0.641 |
| MOT mean latency, ms | 966 (548–1384) | 701 (533–1730) | 0.007* | 0.54 | 0.012* |
| PRM 1 correct, % | 92 (67–100) | 100 (67–100) | 0.004* | 0.08 | 0.713 |
| PRM 1 correct latency, ms | 2804 (1283–7702) | 2480 (1238–3203) | 0.140 | 0.26 | 0.257 |
| SRM mean correct, % | 70 (30–85) | 70 (40–90) | 0.822 | −0.66 | 0.001* |
| SRM mean latency, ms | 2181 (1442–5603) | 2286 (690–3641) | 0.871 | 0.18 | 0.446 |
| CRT mean correct, % | 100 (98–100) | 100 (98–100) | 0.699 | −0.32 | 0.149 |
| CRT mean latency, ms | 388 (282–1043) | 348 (264–572) | 0.147 | 0.04 | 0.859 |
| PRM 2 mean correct, % | 75 (42–100) | 83 (25–100) | 0.310 | −0.30 | 0.180 |
| PRM 2 mean latency, ms | 2316 (1264–10,733) | 2577 (876–3640) | 0.947 | −0.03 | 0.883 |
| VVLT 1 correct, n | 7 (3–11) | 8 (5–13) | 0.028* | −0.49 | 0.023* |
| VVLT 2 correct, n | 10 (5–13) | 11 (5–15) | 0.173 | −0.55 | 0.01* |
| VVLT 3 correct, n | 12 (6–15) | 13 (7–15) | 0.192 | −0.41 | 0.065 |
| VVLT 1–3(∑correct − ∑incorrect) | 29 (16–38) | 32 (18–43) | 0.091 | −0.519 | 0.016* |
| VVLT 4 correct, n | 8 (2–13) | 10 (3–15) | 0.203 | −0.37 | 0.097 |
| SCWT 1 error, n | 0 (0–1) | 0 (0–0) | 1.0 | ||
| SCWT 1 time, s | 21 (13–29) | 16 (12–32) | 0.010* | 0.27 | 0.228 |
| SCWT 2 error, n | 0 (0–2) | 0 (0–2) | 0.802 | −0.15 | 0.498 |
| SCWT 2 time, s | 25 (17–47) | 23 (16–31) | 0.068 | 0.09 | 0.689 |
| SCWT 3 error, n | 1 (0–11) | 0 (0–5) | 0.293 | 0.13 | 0.571 |
| SCWT 3 time, s | 42 (28–102) | 42 (27–80) | 0.273 | 0.28 | 0.221 |
| SCWT – interference score | 30 (18–78) | 30 (17–64) | 0.394 | 0.31 | 0.173 |
*Significant difference.
Mann–Whitney U test for comparison of cognitive performance between patients and healthy controls: CANTAB subdimensions, VVLT, and SCWT; For patients: Spearman correlation between delirium duration (days) and subdimensions of cognitive performance. Subdimensions of CANTAB: MOT, PRM 1/PRM 2, SRM, CRT, VVLT, and SCWT (parts 1–3).
CANTAB, Cambridge Neuropsychological Test Automated Battery; SRM, spatial recognition memory; CRT, choice reaction time; VVLT, Visual Verbal Learning Test; SCWT, Stroop Color Word Test; MOT, motor screening; PRM, Pattern Recognition Memory (PRM 1 = immediate, PRM 2 = 20-minute delayed recognition).
A relationship between the duration of delirium and cognitive performance was demonstrated in several subdimensions of cognition. Spearman’s correlation coefficient revealed a significant relationship between the duration of delirium and diminished MOT latency (speed of motor skills) (p = 0.012), spatial recognition memory correct (visual memory performance) (p = 0.001), VVLT 1 correct (p = 0.023), VVLT 2 correct (p = 0.01), and overall performance of ∑VVLT 1–3 correct (learning efficiency and episodic declarative memory) (p = 0.016) (Table 5). There were no group differences in cognitive performance measured with the CANTAB in any subtests, VVLT, or SCWT between patients with H1N1 infection and non-H1N1 infection.
Mental impairment
According to the PTSS-14 and PDS results, 16% of patients in the follow-up assessment were diagnosed with PTSD. Patients with PTSD had a significantly longer duration of delirium (median, 7 days; range, 6–8 days) than those without PTSD (median, 2 days; range, 0–29 days) (Z = −2.215, p < 0.027).
Analysis of the lifelong diagnosis at the time of hospital admission extracted from the PDMS showed that two patients (9%) had previous depression and one patient (4%) had phobia. Neither of these patients showed high PTSD scores. Furthermore, at follow-up, the two patients (9%) who reported depression in the questionnaire of current health status were not in the PTSD group.
HRQoL and physical performance
The physical dimension of HRQoL as measured with the SF-12 was significantly lower in patients than in age- and sex-matched healthy controls (p < 0.0001). In a comparison of patients with H1N1 and non-H1N1 infection versus healthy controls, only patients with non-H1N1 infection showed a significantly lower physical dimension than controls (p < 0.0001). The mental dimension was not significantly different between these groups (see Table 6).
Table 6.
Health-related quality of life (SF-12) of all patients (H1N1 and non-H1N1) and healthy controls
| SF-12 | Healthy controls(n = 20) | All patients(n = 23) | H1N1 infection(n = 8) | Non-H1N1 infection(n = 15) | p-value |
|---|---|---|---|---|---|
| Physical dimension | 47 (12–62) | 34 (19–59) | 46 (26–59) | 34 (19–56) | <0.0001 a,b |
| Mental dimension | 52 (13–72) | 50 (32–64) | 50 (32–64) | 51 (41–64) | n.s. |
SF-12 scores are presented as median (range). Mann–Whitney U test for comparison among all patients, patients with H1N1 infection, patients with non-H1N1 infection, and healthy controls.
aSignificant difference between patients and healthy controls
bSignificant difference between healthy controls and patients with non-H1N1 infection
Additionally, the 6MWT as a measurement of functional status demonstrated a significantly shorter distance for all patients (median, 425 m; range, 200–570 m) than the inter-individual norms (median, 651 m; range, 303–820 m) (Z = −4.19, p < 0.0001) according to Enright and Sherrill.38 There was no difference in functional capacity between patients with H1N1 infection (median, 458 m; range, 200–570 m) and those with non-H1N1 infection (median, 410 m; range, 200–550 m). None of the patients reported previous impairments in physical abilities. Five patients (22%) had performed sports on a regular basis before hospital admission.
Moreover, patients with PTSD showed a significantly diminished mental dimension of HRQoL (median, 44; range, 32–49) compared with patients without PTSD (median, 52; range, 33–64) (Z = −2.27, p ≤ 0.021) and healthy controls (median, 52; range, 13–72) (Z = −3.23, p < 0.0001).
At the time of follow-up, 34% of patients were employees or students. However, 17% of patients received an invalidity pension, 17% were unemployed, and 22% were retired (see Table 4).
Discussion
In this study, we assessed the association of PTSD and cognitive performance with the duration of delirium. Moreover, we considered than an increased duration of delirium was influenced by analgosedative medication. However, we found that young survivors of sCAP and ARDS had a relatively high incidence of cognitive dysfunction, reduced functional capacity, and high incidence of PTSD that were associated with diminished HRQoL and a decreased rate of return to work.
Because the clinical and sociodemographic data were not significantly different between the 23 followed-up patients and 19 non-followed-up patients, we assume that the follow-up results could be transferred to the entire study population.
The results of this study demonstrated that independent of the primary diagnosis, most of the patients in our sample developed delirium (88%). This incidence is slightly above the normal range12 and might be related to the high severity of illness in our population. Moreover, this investigation focused on comparison of the incidence of delirium between patients with H1N1 and non-H1N1 infection. In contrast to the hypothesis that delirium and delirium-like symptoms are common severe adverse effects of oseltamivir,17 we did not find a higher rate of delirium in the H1N1 group. Other factors suggested to have an important impact on the development of delirium are the ICU environment, treatment procedures, and medications.12,18–21 In the first regression model that considered the selected medication, the propofol dose was negatively associated with the duration of delirium. One meta-analysis revealed evidence of a positive effect of propofol on delirium-associated risk factors such as the duration of mechanical ventilation and length of stay, which might explain the shorter duration of delirium with longer propofol application.40 In a relatively high proportion of patients, mechanical ventilation is only tolerated with concurrent medication. A longer duration of propofol administration is helpful to allow for temporary discontinuation of benzodiazepines (e.g., to increase tube tolerance).41
In contrast to the literature, our second model showed no association between the duration of delirium and age, length of mechanical ventilation, or length of ICU stay. In the final third regression model, which considered the selected medication and significant clinical parameters, we found that opioids and antipsychotic medications had an increasing impact on the duration of delirium independent of the underlying disease. This is in line with the results reported by Pisani et al.,42 who showed an association of higher opioid doses and haloperidol use with persistent delirium. Both the incidence and severity of delirium appear to be associated with opiates.43
Although several studies have identified benzodiazepines as a risk factor for delirium, the effect of these medications remains ambiguous.20,21,42 In our population, we could not prove that the cumulative dose of benzodiazepines had an effect on the duration of delirium. Moreover, a meta-analysis of randomized trials on the effects of benzodiazepine-based versus non-benzodiazepine-based sedation in mechanically ventilated critically ill patients demonstrated a similar prevalence of delirium for both sedation strategies.40 Furthermore, the prevalence of delirium in numerous previous studies and the duration of delirium in the present study might be affected by different moderating variables.
In the present follow-up study, the prevalence of PTSD was 16%. This confirms previous epidemiological data.44–46 We also proved that patients with PTSD have a significantly longer duration of delirium. The duration of delirium appears to be significantly associated with subsequent PTSD symptoms. This supported previous studies that identified postoperative delirium as a risk factor for acute PTSD.47,48 In their cognitive model, Ehlers and Clark49 postulated that PTSD is characterized by a disturbance of autobiographical memory that manifests as poor elaboration and inadequate integration into autobiographical memories. Delirium is associated with fragmental memories or delusions and therefore seems to affect episodic memory.50 Consequently, delirium might inhibit encoding of factual ICU events und decrease impairment of autobiographic memory, which is associated with PTSD symptoms. In addition, a high level of fear during a traumatic event is a relevant factor in the etiology of PTSD and leads to a fear network according to the model described by Foa et al.51 Acute ICU stress and ICU mood were associated with delirious symptoms such as nightmares, agitation, and hallucination.47
Although the evidence regarding the efficacy of haloperidol in the treatment of delirium is controversial, this drug is still widely used.52 Our results suggest that antipsychotic medication during treatment in the ICU prolongs delirium and that delirium-related symptoms presumably trigger future anxiety disorders. Consequently, PTSD affects patients’ mental well-being and mental HRQoL. The hypothesis that patients with H1N1 infection treated with oseltamivir would show a higher risk of PTSD or traumatic memories was not confirmed. Contrary to the preceding results in our study population, none of the patients with PTSD had suffered from previous depression or anxiety disorders according to their lifelong diagnosis at the time of hospital admission extracted from the PDMS. Furthermore, none of these patients reported depression or anxiety disorders in the questionnaire of their current health status at follow-up. This might have been due to the prominent symptoms of PTSD.
In addition, among survivors of sCAP and ARDS, impairments in objective measurement of exercise capacity (6MWT) corresponded with the patients’ perspective of their limitations in physical functioning as measured by the SF-12. Patients described tiredness and fatigue that significantly affected their activities of daily living.
Moreover, at an average of 4 years after discharge, the patients had various markedly severe cognitive dysfunctions, and our interviews indicated that these dysfunctions might become a future career limitation. The duration of delirium was correlated with diminished cognitive performance, particularly the processing speed of motor skills, which is associated with motor impairments and visuomotor coordination. Disproportionate slowing of the information processing speed is presumably related to cognitive limitations.53 Difficulties in spatial recognition memory as well as short-term or working memory are correlated with the duration of delirium. Because working memory is responsible for planning and carrying out behavior, a decline in working memory leads to difficulties in problem solving and planning. These results confirm previous investigations showing that after hospital discharge, patients experienced a decreased speed of mental performance10,54 and deficits in episodic declarative memory, selective attention, cognitive inhibition, and cognitive flexibility as a function of the duration of delirium. Girard et al.45 discovered cognitive impairments in conjunction with the duration of delirium.
Several limitations of our study should be noted. First, the sample size of this study was small and limited by the survival rate and difficulties in participation due to the patients’ existing severe physical impairments. Second, because a main symptom of PTSD is the avoidance of anxiety-inducing or trauma-confronting situations, patients with PTSD avoid frequent re-examination or questioning and therefore also rarely participate in follow-up examinations. Third, because of the weak goodness-of-fit of the regression models, the results must be interpreted cautiously with regard to the influence of the medication on the duration of delirium.
In summary, in this group of young patients with sCAP, the duration of delirium was associated with long-term mental health and cognitive performance, which in some patients might become a future limitation to career development. Despite the small study group, our findings might be indicative of the disease burden in such patients.55 In addition to medication, nonpharmacological approaches such as nursing, general care provided by medical staff and family caregivers, and a healing environment in the ICU might provide cognitive and emotional support to strengthen any retained adaptive cognitive functioning that the patient possesses.
Abbreviations
APACHE II, Acute Physiology and Chronic Health Evaluation II; ARDS, acute respiratory distress syndrome; CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; CANTAB, Cambridge Neuropsychological Test Automated Battery; CAP, community-acquired pneumonia; HRQoL, health-related quality of life; ICU, intensive care unit; IQ, intelligence quotient; MOT, Motor Screening; PDMS, patient data management system; PDS, Post-traumatic Stress Diagnostic Scale; PRM, Pattern Recognition Memory; PRM 1, immediate Pattern Recognition Memory; PRM 2, 20-minute delayed Pattern Recognition Memory; PTSD, post-traumatic stress disorder; PTSS-14, Post-Traumatic Stress Syndrome 14-Questions Inventory; RASS, Richmond Agitation-Sedation Scale; SAPS II, Simplified Acute Physiology Score II; sCAP, severe community-acquired pneumonia; SCWT, Stoop Color Word Test; SF-12, Medical Outcomes Study 12-Item Short Form; SOFA, Sequential Organ Failure Assessment; WST, Wortschatztest (a vocabulary test); VVLT, Visual Verbal Learning Test; 6MWT, 6-Minute Walk Test
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2): S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ewig S, Hoffken G, Kern WV, et al. [Management of adult community-acquired pneumonia and prevention - update 2016]. Pneumologie 2016; 70: 151–200 [in German, English Abstract]. [DOI] [PubMed] [Google Scholar]
- 3.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 4.Luhr OR, Antonsen K, Karlsson M, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF study group. Am J Respir Crit Care Med 1999; 159: 1849–1861. [DOI] [PubMed] [Google Scholar]
- 5.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353: 1685–1693. [DOI] [PubMed] [Google Scholar]
- 6.Bersten AD, Edibam C, Hunt T, et al. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med 2002; 165: 443–448. [DOI] [PubMed] [Google Scholar]
- 7.Herridge MS. Recovery and long-term outcome in acute respiratory distress syndrome. Crit Care Clin 2011; 27: 685–704. [DOI] [PubMed] [Google Scholar]
- 8.Deja M, Denke C, Weber-Carstens S, et al. Social support during intensive care unit stay might improve mental impairment and consequently health-related quality of life in survivors of severe acute respiratory distress syndrome. Crit Care 2006; 10: R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury . Am J Respir Crit Care Med 2012; 185: 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins RO, Weaver LK, Pope D, et al. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med 1999; 160: 50–56. [DOI] [PubMed] [Google Scholar]
- 11.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med 2010; 38: 1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girard TD, Pandharipande PP, Ely EW. Delirium in the intensive care unit. Crit Care. 2008; 12(Suppl 3): S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob C, Mittendorf T, Graf von der Schulenburg JM. [ Costs of illness and health-related quality of life for community-acquired pneumonia–a systematic review]. Pneumologie 2011; 65: 498–502 [in German, English Abstract]. [DOI] [PubMed] [Google Scholar]
- 14.El Moussaoui R, Opmeer BC, de Borgie CA, et al. Long-term symptom recovery and health-related quality of life in patients with mild-to-moderate-severe community-acquired pneumonia. Chest 2006; 130: 1165–1172. [DOI] [PubMed] [Google Scholar]
- 15.Mangen MJ, Huijts SM, Bonten MJ, de Wit GA. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect Dis 2017; 17: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luyt CE, Combes A, Becquemin MH, et al. Long-term outcomes of pandemic 2009 influenza A(H1N1)-associated severe ARDS. Chest 2012; 142: 583–592. [DOI] [PubMed] [Google Scholar]
- 17.Toovey S, Rayner C, Prinssen E, et al. Assessment of neuropsychiatric adverse events in influenza patients treated with oseltamivir: a comprehensive review . Drug Saf 2008; 31: 1097–1114. [DOI] [PubMed] [Google Scholar]
- 18.Dubois MJ, Bergeron N, Dumont M, et al. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med 2001; 27: 1297–1304. [DOI] [PubMed] [Google Scholar]
- 19.Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA 1994; 272: 1518–1522. [PubMed] [Google Scholar]
- 20.Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Semin Respir Crit Care Med 2001; 22: 115–126. [DOI] [PubMed] [Google Scholar]
- 21.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma 2008; 65: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandharipande P, Ely EW. Sedative and analgesic medications: risk factors for delirium and sleep disturbances in the critically ill. Crit Care Clin 2006; 22: 313–327, vii. [DOI] [PubMed] [Google Scholar]
- 23.Töpfer L, Menk M, Weber-Carstens S, et al. Influenza A (H1N1) vs non-H1N1 ARDS: analysis of clinical course. J Crit Care 2014; 29: 340–346. [DOI] [PubMed] [Google Scholar]
- 24.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 166: 1338–1344. [DOI] [PubMed] [Google Scholar]
- 25.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 2003; 289: 2983–2991. [DOI] [PubMed] [Google Scholar]
- 26.Otter H, Martin J, Basell K, et al. Validity and reliability of the DDS for severity of delirium in the ICU. Neurocrit Care 2005; 2: 150–158. [DOI] [PubMed] [Google Scholar]
- 27.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001; 286: 2703–2710. [DOI] [PubMed] [Google Scholar]
- 28.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 2001; 29: 1370–1379. [DOI] [PubMed] [Google Scholar]
- 29.Guenther U, Popp J, Koecher L, et al. Validity and reliability of the CAM-ICU Flowsheet to diagnose delirium in surgical ICU patients. J Crit Care 2010; 25: 144–151. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt K-H, Metzler P. Wortschatztest [Vocabulary test (WST)]. 1992; Weinheim: Beltz Test GmbH.
- 31.Robbins TW, James M, Owen AM, et al. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 1994; 5: 266–281. [DOI] [PubMed] [Google Scholar]
- 32.Brand N, Jolles J. Learning and retrieval rate of words presented auditorily and visually. J Gen Psychol 1985; 112: 201–210. [DOI] [PubMed] [Google Scholar]
- 33.Bohnen N, Twijnstra A, Jolles J. Performance in the Stroop color word test in relationship to the persistence of symptoms following mild head injury. Acta Neurol Scand 1992; 85: 116–121. [DOI] [PubMed] [Google Scholar]
- 34.Valentijn SA, van Boxtel MP, van Hooren SA, et al. Change in sensory functioning predicts change in cognitive functioning: results from a 6-year follow-up in the maastricht aging study. J Am Geriatr Soc 2005; 53: 374–380. [DOI] [PubMed] [Google Scholar]
- 35.Radtke FM, Franck M, Drews T, et al. [The post-traumatic stress Syndrome 14-Questions Inventory (PTSS-14) - Translation of the UK-PTSS-14 and validation of the German version]. Anasthesiol Intensivmed Notfallmed Schmerzther 2010; 45: 688–695 [in German, English Abstract]. [DOI] [PubMed] [Google Scholar]
- 36.Foa EB, Cashman L, Jaycox L, et al. The validation of a self-report measure of posttraumatic stress disorder: the posttraumatic diagnostic scale. Psychol Assess 1997; 9: 445–451. [Google Scholar]
- 37. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. DOI: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 38.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998; 158: 1384–1387. [DOI] [PubMed] [Google Scholar]
- 39.Bullinger M. German translation and psychometric testing of the SF-36 Health Survey: preliminary results from the IQOLA Project. International Quality of Life Assessment. Soc Sci Med 1995; 41: 1359–1366. [DOI] [PubMed] [Google Scholar]
- 40.Fraser GL, Devlin JW, Worby CP, et al. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: a systematic review and meta-analysis of randomized trials. Crit Care Med 2013; 41: S30–S38. [DOI] [PubMed] [Google Scholar]
- 41.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013; 41: 263–306. [DOI] [PubMed] [Google Scholar]
- 42.Pisani MA, Murphy TE, Araujo KL, et al. Factors associated with persistent delirium after intensive care unit admission in an older medical patient population. J Crit Care 2010; 25: 540 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granberg Axell AI, Malmros CW, Bergbom IL, et al. Intensive care unit syndrome/delirium is associated with anemia, drug therapy and duration of ventilation treatment. Acta Anaesthesiol Scand 2002; 46: 726–731. [DOI] [PubMed] [Google Scholar]
- 44.Jones C, Griffiths RD, Humphris G, Skirrow PM. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med 2001; 29: 573–580. [DOI] [PubMed] [Google Scholar]
- 45.Girard TD, Shintani AK, Jackson JC, et al. Risk factors for post-traumatic stress disorder symptoms following critical illness requiring mechanical ventilation: a prospective cohort study. Crit Care 2007; 11: R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davydow DS, Gifford JM, Desai SV, et al. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008; 30: 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wade DM, Howell DC, Weinman JA, et al. Investigating risk factors for psychological morbidity three months after intensive care: a prospective cohort study. Crit Care. 2012; 16: R192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drews T, Franck M, Radtke FM, et al. Postoperative delirium is an independent risk factor for posttraumatic stress disorder in the elderly patient: a prospective observational study. Eur J Anaesthesiol 2015; 32: 147–151. [DOI] [PubMed] [Google Scholar]
- 49.Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behav Res Ther 2000; 38: 319–345. [DOI] [PubMed] [Google Scholar]
- 50.Roberts BL, Rickard CM, Rajbhandari D, et al. Factual memories of ICU: recall at two years post-discharge and comparison with delirium status during ICU admission–a multicentre cohort study. J Clin Nurs 2007; 16: 1669–1677. [DOI] [PubMed] [Google Scholar]
- 51.Foa EB, Steketee G, Rothbaum BO. Behavioural/cognitive conceptualisations of post-traumatic stress disorder. Behav Ther 1989; 20: 155–176. [Google Scholar]
- 52.Schrijver EJ, de Graaf K, de Vries OJ, et al. Efficacy and safety of haloperidol for in-hospital delirium prevention and treatment: A systematic review of current evidence. Eur J Intern Med 2016; 27: 14–23. [DOI] [PubMed] [Google Scholar]
- 53.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev 1996; 103: 403–428. [DOI] [PubMed] [Google Scholar]
- 54.Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med 2005; 171: 340–347. [DOI] [PubMed] [Google Scholar]
- 55.Cassini A, Plachouras D, Eckmanns T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med 2016; 13: e1002150. [DOI] [PMC free article] [PubMed] [Google Scholar]