Short abstract
Objective
Clinical sepsis-associated biomarkers were utilized in a cecal ligation and puncture (CLP) septic mouse model to provide a reference for investigating pathophysiological mechanisms and evaluating novel therapeutic interventions for sepsis.
Methods
Sepsis in mice was induced by CLP, and clinical biomarkers were evaluated (survival rate, blood physiological and biochemical indices, cytokines, hepatorenal function parameters, and blood coagulation).
Results
The mortality rate was >70%. The body temperature, blood pressure, and heart rate decreased within 48 h. Low lactic acid was found at 8 h. The CLP mice showed typical inflammatory symptoms with decreased white blood cells and procalcitonin and increased levels of soluble triggering receptor expressed on myeloid cells-1, interleukin (IL)-6, IL-10, tumor necrosis factor-α, macrophage inflammatory protein (MIP)-1α, MIP-1β, and MIP-2. The platelet count and activated partial thromboplastin time significantly decreased, and the prothrombin time and prothrombin time–international normalized ratio markedly increased. Phenotypes of multiple organ dysfunction were found in the CLP model, including increased liver alanine aminotransferase and aspartate transaminase; significantly reduced total protein, globulin, and serum albumin; increased blood urea nitrogen and creatinine; and decreased blood glucose.
Conclusion
The clinical features of the CLP mouse model were similar to those of human patients with sepsis.
Keywords: Sepsis, cecal ligation and puncture (CLP), biomarkers, clinical indicators, mouse model, diagnostic criteria
Introduction
Sepsis is a highly fatal systemic illness with major clinical manifestations such as pyrexia, hypotension, hyperlactacidemia, coagulopathy, excessive inflammation, and multiple organ dysfunction.1 Sepsis is a syndrome caused by the host’s response to infection. Due to the complexity of the pathogenesis and disease progression, the final report of the International Sepsis Definitions Conference systematically formulated the diagnostic criteria for sepsis in 2001.2 In 2016, several indicators, including those of renal and hepatic dysfunction, coagulopathy, and abnormalities involving various organ systems (respiratory, cardiovascular, and nervous), were taken into consideration in the Third International Consensus Definitions for Sepsis and Septic Shock to clinically characterize the extent of organ function or rate of organ failure in patients with sepsis (Table 1).3
Table 1.
Clinical diagnosis and laboratory tests
| Clinical diagnosis* | Laboratory test |
|---|---|
| General parameters:Fever or hypothermia; HR above the normal value for age; tachypnea; altered mental status; significant edema or positive fluid balance; hyperglycemia in the absence of diabetes | Survival, body temperature, HR, blood lactate, GLU |
| Inflammatory parameters: Leukocytosis; leukopenia; plasma CRP level above the normal value; plasma PCT above the normal value | WBC, CRP, PCT, sTREM-1, CD163, HIF-1α, IL-6, IL-10, TNF-α, MIP-1α, MIP-1β, MIP-2 |
| Hemodynamic parameters: Arterial hypotension or a decrease in SBP; mixed venous oxygen saturation of >70%; cardiac index of >3.5 L min−1 m−2 | SBP |
| Organ dysfunction parameters: Arterial hypoxemia; acute oliguria; CREA increase; coagulation abnormalities; ileus (absent bowel sounds); thrombocytopenia; hyperbilirubinemia | Hypoxemia: blood lactateKidney: BUN, CREALiver: ALT AST ALB TP GLOBCoagulation: PLT, PT, APTT |
| Tissue perfusion parameters: Hyperlactatemia; decreased capillary refill or mottling | Blood lactate |
*Some of the clinical diagnostic indicators listed in the table are interrelated with the indicators detected in this study; however, the table does not list all possible clinical features of sepsis.
HR, heart rate; GLU, glucose; CRP, C-reactive protein; PCT, procalcitonin; WBC, white blood cell; sTREM, soluble triggering receptor expressed on myeloid cells; HIF, hypoxia-inducible factor; IL, interleukin; TNF, tumor necrosis factor; MIP, macrophage inflammatory protein; SBP, systolic blood pressure; BUN, blood urea nitrogen; CREA, creatinine; ALT, alanine transaminase; AST, aspartate transaminase; ALB, albumin; TP, total protein; GLOB, globulin; PCT, procalcitonin; PT, prothrombin time; APTT, activated partial thromboplastin time.
Cecal ligation and puncture (CLP) is a standard animal model for sepsis.4 It is commonly used to study the pathogenesis and therapeutic targets of sepsis. The continuous spread of cecal contents into the abdominal cavity causes bacteria to enter the bloodstream, which eventually leads to systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome. Therefore, the CLP mouse model is able to partially represent the progression of pathophysiological phenomena similar to those that occur in humans, such as the changes that occur in the early stage of inflammation, and to exhibit cytokine kinetics comparable with those in clinical sepsis.5 Ideally, animal models should mimic the pace and severity of human sepsis to help with the progression of pharmaceutical research and the development of clinically useful therapeutics. For example, the key hemodynamic and immunologic stages, host response, and organ dysfunction seen in humans should be simulated in animal models.6 The CLP model has been considered the gold standard for sepsis research by many investigators. Clinical sepsis can originate from different sources and can be accompanied by many complicating conditions, and the CLP model can recreate the immunological, hemodynamic, and metabolic phases of clinical sepsis only to a certain extent.7 However, whether this animal model can mimic human sepsis and thus assist physicians in their daily practice remains unknown. Serological biomarkers are important for the diagnosis and treatment of clinical sepsis, but the evaluation of biomarkers is complex and unclear. Differences exist between rodent CLP models and human sepsis. Compared with the diagnostic criteria for sepsis in humans, a comprehensive understanding of the basic parameters and indicators of the CLP model is lacking. This is one of the main reasons for failure of a large number of therapeutic targets and strategies in clinical trials.
In this present study, we used the 2001 sepsis diagnostic criteria in a CLP mouse model to investigate factors related to the clinical characteristics of severe sepsis, such as survival, body temperature, heart rate (HR), systolic blood pressure (SBP), fasting glucose (GLU) concentration, and blood bacteria load. In addition, we tested several biomarkers that can identify inflammation, hypoperfusion, hepatorenal dysfunction, and coagulopathy in mice with CLP. This study was performed to investigate whether the CLP mouse model can provide data useful for the treatment of human patients in the clinical setting.
Material and methods
Animals
One hundred sixty male C57BL/6J mice (9–10 weeks old; weight, 25–29 g) were obtained from the Medical Experimental Animal Center of Guangdong Province. The mice were housed in a specific pathogen-free facility with a 12-h light and dark cycle and free access to food and water. All animal experiments in this study were approved by the Institutional Animal Care and Use Committee of the Guangdong Laboratory Animals Monitoring Institute (accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)). All procedures were carried out in accordance with the AAALAC guidelines.
CLP procedure
CLP was performed as described previously.8 Briefly, the mice were anesthetized by inhalation of isoflurane with an isoflurane vaporizer (Matrx; Midmark Corp., Dayton, OH, USA). A 1-cm midline incision was made, and the cecum was carefully exposed to avoid damage to the blood vessels. The cecum was then tightly ligated with a 3/0 silk suture at the middle and punctured twice using a 21-gauge needle at about 0.5 cm from its distal end. A small amount of stool was extruded to ensure patency of the puncture sites. The cecum was placed back into its normal intra-abdominal position, and the abdomen was closed in two layers. Mice in the sham group underwent exactly the same procedures without CLP. All animals received a subcutaneous injection of 1 mL of 37°C normal saline for fluid resuscitation immediately after surgery. Tramadol hydrochloride analgesic (10 mL/kg; Grünenthal Co., Ltd., Aachen, Germany) was administered by hypodermic injection every 12 h for 48 h. Blood samples were collected before surgery (0 h) and at 8, 16, and 48 h after surgery.
Survival rate, SBP, and HR of mice in the CLP group
The mortality of mice within 12 days after CLP surgery was recorded by three independent experiments. Ten mice were used in each experiment. These experiments were used to verify the stability of the CLP operation and to determine the number of samples needed to detect a series of indicators. A blood pressure meter (Softron Beijing Biotechnology Co., Ltd., Beijing, China) was sheathed onto the tail of the mouse, and the blood flow signal was monitored by the pressure and the pressure release of the tail artery through inflation and deflation. The SBP and HR were obtained by the tail pressure method. The postoperative body temperature of the mice was measured with a thermometer (Braun, Kronberg, Germany) until the body temperature normalized.
Blood bacteria load of mice in the CLP group
From 100-µL blood samples, 50 µL was taken as an undiluted blood stock solution and the remaining 50 µL was diluted (1:9) in sterile phosphate-buffered saline. Diluent and blood stock solution were plated onto a trypsin blood plate (Guangzhou Detgerm Microbiological Technology Co., Ltd., Guangzhou, China), and each sample was plated in duplicate. The plates were incubated for 24 h at 37°C in a biochemical incubator (Blue Pard; Shanghai Yiheng Scientific Instruments Co., Ltd., Shanghai, China). Colonies were counted separately for each sample. The number of colonies on the plate should range from 30 to 300 to avoid counting error. The bacterial load (CFU/mL) was calculated as follows: mean value × dilution ratio × 20.
Cytokine assay
The levels of plasma cytokines (interleukin (IL)-6, IL-10, tumor necrosis factor (TNF)-α, macrophage inflammatory protein (MIP)-1α, MIP-1β, and MIP-2) were measured using Luminex kits (MAGPIX; Millipore, Darmstadt, Germany) and analyzed using the Luminex 200 system with xPONENT software (Millipore) according to the manufacturer’s instructions.
Routine blood testing and biochemical analysis
White blood cells (WBCs) and platelets (PLTs) were counted using a hematology analyzer (Sysmex, Kobe, Japan). Plasma was prepared for detection of alanine aminotransferase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), total protein (TP), serum albumin (ALB), globulin (GLOB), GLU, and serum creatinine (CREA) using a blood biochemical analyzer (Hitachi Co., Tokyo, Japan).
Blood coagulation factor assay
Blood samples were collected in a sodium citrate anticoagulation tube (1:9) from mice at 8, 16, and 48 h after surgery. The plasma was subsequently obtained by centrifuging (2500 × g) the blood sample at 4°C for 10 min. A 100-µL volume of plasma was used for the blood coagulation factor assay, which included measurement of the activated partial thromboplastic time (APTT), international normalized ratio–prothrombin time (PT-INR), and prothrombin time (PT) by a coagulation analyzer (STA Compact Max; Stago, Paris, France).
Detection of inflammatory markers
Serum was separated by centrifugation at 3000 × g for 10 min and stored at −80°C until use. The serum levels of C-reactive protein (CRP), soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), CD163, procalcitonin (PCT), and hypoxia-inducible factor (HIF-1α) were quantitated by enzyme-linked immunosorbent assay according to the manufacturer’s protocol (Cusabio, Wuhan, China).
Serum lactate level
Serum was separated by centrifugation at 3000 × g for 10 min and stored at −80°C until use. Serum lactate was estimated by a lactic acid assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. The test principle was based on the formation of a product due to oxidation of lactate by lactate dehydrogenase, which interacts with a probe to produce red dye. The intensity of the dye was determined at 530 nm by a microplate spectrophotometer (Multiskan; Thermo Fisher Scientific, Waltham, MA, USA), and the results are expressed as mmol/L.
Statistical analysis
The data are presented as mean ± standard error. The means between groups were compared for statistical significance using two-way analysis of variance in IBM SPSS Statistics, v.24.0 (IBM Corp., Armonk, NY, USA). A P value of <0.05 indicated statistical significance. All statistical analyses were conducted using GraphPad Prism v.6.0 (GraphPad Software, La Jolla, CA, USA) and SPSS v.24.0 (IBM Corp.).
Results
General clinical characteristics of mice in the CLP group
Most of the mice died within 6 days after CLP. The 12-day survival rate of CLP mice was about 30% (Figure 1(a)). Clinical manifestations of sepsis, including lethargy, shivering, ocular and nasal discharge, and fecal adhesions in the anus, were found in approximately 90% of the surviving mice during the observation period. Compared with the pre-CLP period, hypothermia was observed from 4 to 32 h after CLP (P < 0.05), and the body temperature returned to a normal level at 32 hours after surgery (Figure 1(b)). Similarly, decreases in the HR (Figure 1(c)) and SBP (Figure 1(d)) occurred within 40 h after CLP, which was the period of highest mortality. Because bacterial infection is a main feature of sepsis in the clinical setting and the blood bacteria load is closely associated with high mortality, the blood bacteria load was evaluated at 0, 8, 16, and 48 h after CLP. As seen in Figure 1(e), the blood bacteria load sharply increased after 8 h (P = 0.029) and remained high for 48 h.
Figure 1.
(a) Survival curves of the mice within 12 days post-CLP. (b) The body temperature was obtained by measuring the ear temperature. (c, d) The HR and SBP were obtained by the tail pressure method. (e) The blood bacteria load was determined by incubating blood samples in plates for 24 h at 37°C. All data are presented as mean ± standard error. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with 0 h group (n = 8). CLP, cecal ligation and puncture; HR, heart rate; SBP, systolic blood pressure.
Typical inflammatory characteristics of CLP mouse model
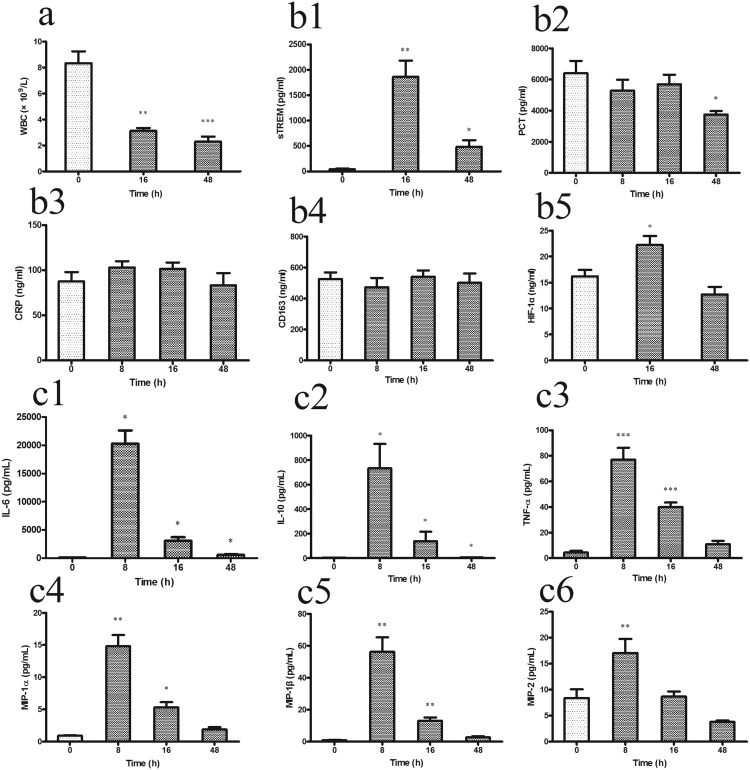
Immune system disorder is one of the main consequences of bacterial infection in patients with sepsis. In the early stage after CLP in the present study, severe leukopenia/lymphopenia occurred due to exhaustion of WBCs in their effort to eliminate the pathogenic bacteria. The complete blood count showed that the number of WBCs (Figure 2(a)) after CLP was significantly lower than that at 0 h (P < 0.01), and this low WBC count continued until the end of the observation period.
Figure 2.
The inflammatory parameters in the CLP mice were obtained by routine blood testing and cytokine assay at 0, 8, 16, and 48 h (n = 8 mice per group). (a) WBC; (b1) sTREM; (b2) PCT; (b3) CRP; (b4) CD163; (b5) HIF-1α; (c1) IL-6; (c2) IL-10; (c3) TNF-α; (c4) MIP-1α; (c5) MIP-1β; (c6) MIP-2. Data are presented as mean±standard error. *P<0.05, **P<0.01, and ***P<0.001 compared with 0 h group.CLP, cecal ligation and puncture; WBC, white blood cell; sTREM, soluble triggering receptor expressedon myeloid cells; PCT, procalcitonin; CRP, C-reactive protein; HIF, hypoxia-inducible factor; IL, interleukin; TNF, tumor necrosis factor; MIP, macrophage inflammatory protein. CLP, cecal ligation and puncture.
Immune activation causes changes in serum protein biomarkers and is an important aspect of sepsis diagnosis in clinical practice. Based on the clinical diagnostic criteria for sepsis, several biomarkers (sTREM-1, PCT, CD163, CRP, and HIF-1α) were evaluated in the present CLP mouse model. The serum sTREM-1 level (Figure 2(b1)) sharply increased at 16 h (P = 0.002) compared with that at 0 h, and the high sTREM-1 level was maintained for 48 h (P = 0.031). The PCT level (Figure 2(b2)) showed no significant change in the early stage of sepsis (8 and 16 h), but it significantly decreased at 48 h (P = 0.036) in the CLP mice. Unexpectedly, the serum CRP (Figure 2(b3)) and CD163 (Figure 2(b4)) levels showed no significant changes in the CLP mice. We found that the HIF-1α level (Figure 2(b5)) increased at 16 h (P = 0.030) after CLP compared with 0 h and returned to a normal level at 48 h.
Plasma cytokines and chemokines were also evaluated in the CLP mice. The levels of several cytokines significantly increased at 8 h post-CLP: IL-6 (P = 0.014) (Figure 2(c1)), IL-10 (P = 0.039) (Figure 2(c2)), and TNF-α (P = 0.000) (Figure 2(c3)). The levels of these cytokines later decreased, but at 48 h they still remained higher than the levels at 0 h. A similar dynamic trend was observed in the plasma chemokines, including MIP-1α (Figure 2(c4)), MIP-1β (Figure 2(c5)), and MIP-2 (Figure 2(c6)). All cytokines and chemokines peaked at 8 h (P < 0.05), and the chemokine levels at 48 h returned to the 0-h levels.
Impairment of coagulation in CLP mice
Excessive coagulation activation is the main cause of high mortality caused by sepsis in clinical practice. Analysis of coagulation factors in the CLP mice showed that the PLT count significantly and continuously decreased throughout the observation period compared with the count at 0 h (P < 0.001) (Figure 3(a)). The APTT (Figure 3(b)) significantly increased after 16 h, and the PT-INR (Figure 3(c)) and PT (Figure 3(d)) increased after 48 h (P < 0.01).
Figure 3.
Coagulation parameters in the CLP mice were obtained by routine blood testing and blood coagulation factor assay at 0, 8, 16, and 48 h (n = 8 mice per group). (a) PLT, platelet; (b) APTT; (c)PT-INR; (d) PT. Data are presented as mean±standarderror. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with 0 h group.CLP, cecal ligation and puncture; PLT, platelet; APTT, activated partial thromboplastin time; PT-INR, prothrombin time -international normalized ratio; PT, prothrombin time.
Changes in hepatorenal function in CLP mice
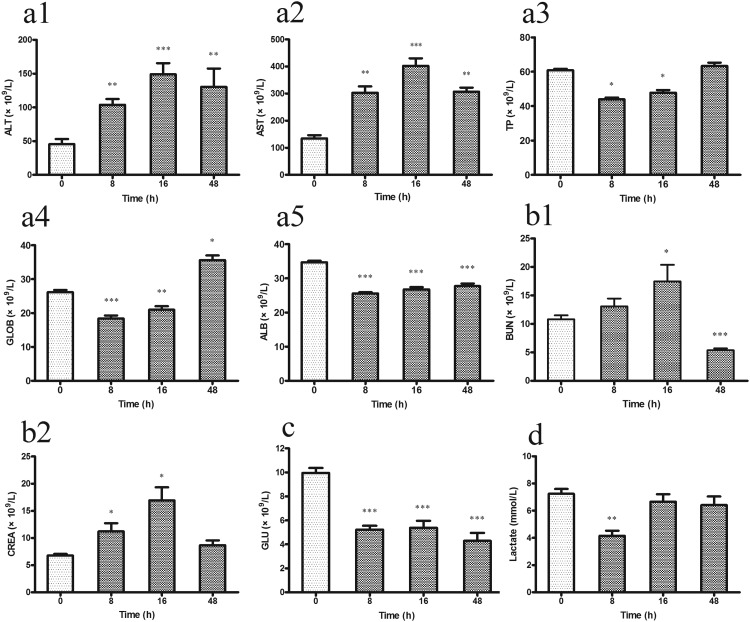
Hepatic and renal dysfunction is a subsequent pathological change caused by inflammatory activation after sepsis. In this study, blood biochemistry tests were performed to evaluate hepatic and renal function in the CLP mouse model. With respect to hepatic function, both ALT (P < 0.001) (Figure 4(a1)) and AST (P < 0.001) (Figure 4(a2)) significantly increased 8 h after CLP compared with their levels at 0 h, and these high levels were maintained throughout the observation period. The serum TP (Figure 4(a3)) and GLOB (Figure 4(a4)) levels markedly decreased 8 and 16 h after CLP compared with their levels at 0 h (P < 0.01). At 48 h after CLP, both levels were restored to the 0-h levels. In addition, the ALB level remained significantly low throughout the 48-h observation period (P < 0.001) (Figure 4(a5)).
Figure 4.
(a1–a5) Hepatic and (b1, b2) renal function was estimated by blood biochemical analysis. (c) The fasting GLU level was detected in blood. (d) Serum lactate was estimated by lactic acid assay in the CLP mice at 0, 8, 16, and 48 h (n = 8 mice per group). Data are presented as mean ± standard error. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with 0 h group. CLP, cecal ligation and puncture; ALT, alanine transaminase; AST, aspartate transaminase; TP, total protein; GLOB, globulin; ALB, albumin; BUN, blood urea nitrogen; CREA, creatinine; GLU, fasting glucose.
With respect to renal function, the changes in the serum CREA and BUN levels are mainly determined by the glomerular filtration rate. Renal dysfunction resulted in increased CREA and BUN levels. No surprisingly, the BUN (Figure 4(b1)) and CREA (Figure 4(b2)) levels were increased in the serum of mice at 8 h after CLP. At 16 h, the CREA and BUN levels were significantly higher than the levels at 0 h (P < 0.05). Both CREA and BUN had gradually returned to normal by 48 h after CLP.
Biochemical analysis showed that the serum GLU level (Figure 4(c)) was significantly decreased 8 h after CLP (P < 0.001), and this low level was maintained throughout the observation period. The serum lactate level (Figure 4(d)) increased due to the metabolic changes caused by hypoxia. Interestingly, the serum lactate level had decreased at 8 h after CLP (P = 0.002), and it showed no significant difference at 16 or 48 h compared with that at 0 h.
Discussion
In this study, we found that some diagnostic biomarkers in the CLP mouse model were correlated with those in human sepsis. It is possible to evaluate indices of human sepsis in CLP mouse models. We found that the established CLP sepsis model in the C57 wild-type mice of the present study exhibited obvious characteristics of sepsis, including hypothermia, hypotension, a slow HR, and decreased blood lactate and GLU levels. The mortality rate was >70%. We also observed bacteremia, liver and kidney dysfunction, an inflammatory response, inflammatory metabolic-coagulation disorders, hypoxia and hypoperfusion, and other derangements. These changes were present within 48 h after surgery. In summary, the established CLP mouse model mimicked the general clinical characteristics of sepsis.
Decreases in the body temperature, blood pressure, and HR are important clinical features of human sepsis. In the CLP model, these features were found in the early stage of sepsis. A low body temperature, low blood pressure, and slow HR reduce the metabolic rate and oxygen supply, thereby inducing hypoxia in the body.9 In the early stage of sepsis, the HR is too low to maintain a normal blood pressure. This explains the low SBP in the mice of the present study. In the late stage of sepsis, however, we observed that the blood pressure and HR returned to normal to maintain hemodynamic stability and improve tissue perfusion. Blood lactate is a metabolite of glycolysis. In sepsis, the blood lactate level reflects the stage of cell hypoxia and the severity of hypoperfusion.10 Therefore, blood lactate is an important indicator of mortality and prognosis in the clinical diagnosis of sepsis. Previous studies have shown that the blood lactate increases in the early stage and then decreases in animal models and human patients.11–13 These studies revealed that in CLP models, this process might occur within 8 h after CLP, after which time the lactic acid level gradually returns to normal. In the present study, the changes in the blood lactate level were consistent with the changes in the body temperature, HR, and SBP, indicating that the CLP model induced ischemia and hypoxia that probably occurred within 8 h after surgery. In addition, in the early stage of sepsis, ischemia and hypoxia can activate the glycolytic pathway, which results in marked GLU consumption and eventually a low blood GLU level.
Serum biomarkers associated with sepsis include CRP, sTREM-1, CD163, PCT, and HIF-1α. The transcription factor HIF-1α is induced by bacterial infection and regulates the production of key immune effector molecules, including antimicrobial peptides, nitric oxide, and TNF-α.14 Mice lacking HIF-1α in their myeloid cell lineage show decreased bactericidal activity and fail to restrict the systemic spread of infection.15 Therefore, HIF-1α is related to regulation of the inflammatory response under ischemic and hypoxic conditions. Similarly, TREM-1 expression on the surface of monocytes and macrophages has been shown to be markedly upregulated in various inflammatory diseases as well as in bacterial sepsis. sTREM-1 appears after TREM-1 cleavage from the cell surface, which can be a useful biomarker of sepsis.16 sTREM-1 has higher sensitivity and specificity than CRP and PCT.17,18 Additionally, many studies have indicated that PCT is weakly correlated with the severity of sepsis19,20 because it is influenced by many noninfectious factors such as myocardial infarction, corticosteroids, and acute bleeding.21 Therefore, high expression of HIF-1α and sTREM-1 indicate that the host has enhanced its sensitivity to the pathogen and the activity of phagocytes (or monocytes) to prevent more bacteria from entering the bloodstream and decrease the bacteria-induced release of inflammatory mediators, thus limiting inflammation. However, the host also limits the activation of phagocytes and promotes apoptosis of monocytes to prevent a cascade of inflammatory mediators and cytokines. In the present study, the changes in the levels of protein biomarkers were consistent with the initial increase and then decrease in the levels of cytokines (HIF-1α, sTREM-1, IL-6, IL-10, TNF-α, MIP-1α, MIP-1β, and MIP-2). In sepsis, the release of a variety of inflammatory substances and cytokines initiates a cascade that promotes the formation of their “waterfall” release. Similarly, an acute inflammatory response was observed in the present CLP model. The transient increases in the IL-6, IL-10, TNF-α, MIP-1α, MIP-1β, and MIP-2 levels and the cytokine storm prior to death of the mice were consistent with the excessive inflammation that occurs in human sepsis.
CD163 inhibits the proliferation and activation of T lymphocytes and has anti-inflammatory and immune regulatory effects. Detection of the CD163 level in the blood can contribute to early diagnosis of severe infection and sepsis.22 Previous studies have shown that sCDl63 in the blood is shed by monocytes and macrophages from mCDl63.23 In the present CLP mouse model, the sCD163 concentration showed no significant changes. This might have been due to the inhibition of CD163 transcriptional translation,24 which resulted in no statistical changes in CD163.
Severe sepsis often results in multiple organ dysfunction. Coagulation disorder is one of the main causes of multiple organ failure. The PT and APTT are detected in clinical coagulation tests. In our study, the APTT and PT were significantly elevated 48 h after CLP. Severe sepsis is always accompanied by disseminated intravascular coagulation.25 Previous research has shown that sepsis results in concurrent activation of the inflammatory and procoagulant pathways, thus leading to a high APTT and PT.26 PT is a sensitive indicator of the degree of hepatocellular damage or the prognosis. PT is extended in the presence of liver cell necrosis or liver cirrhosis.27 Liver and kidney dysfunction is an important pathological phenotype induced by sepsis. In clinical practice, acute kidney injury is mainly diagnosed by the CREA concentration, which can partly reflect the glomerular filtration rate as a function of solute elimination by the kidney. BUN and CREA are also used to detect acute kidney injury.6 High levels of CREA can partly indicate damage to the renal parenchyma. In the present study, CLP mice had acute kidney injury as indicated by the abnormal levels of BUN and CREA, but the levels had decreased again by 48 hours after surgery. Aminotransferases are the most common indices of hepatocellular damage. In the clinical setting, a high ALT level indicates acute hepatocellular damage, and AST reflects the degree of liver cell injury.6 The change in the ALB level is similar to the change in the TP level when the liver is damaged because ALB is a part of TP. GLOB is related to the synthetic function of the liver. In clinical practice, these proteins are measured to assist in the diagnosis or treatment of hepatic diseases. Therefore, these biomarkers were examined in the present study. The results indicated the presence of acute hepatic injury in CLP mice.
Conclusion
CLP induced sepsis in mice by destroying the gut barrier, leading to persistent diffusion of intestinal flora in the peritoneal cavity with entrance into the bloodstream, triggering SIRS and multiple organ dysfunction. The multisystem syndrome of sepsis in mice includes liver and kidney dysfunction, bacteremia, an inflammatory response, inflammatory metabolic-coagulation disorders, hypoxia, and hypoperfusion. Investigation of biomarkers indicates that the CLP model is highly clinically relevant, exhibiting hypersensitivity reactions and immune-related multiple tissue damage. The transition from SIRS to compensatory anti-inflammatory response syndrome in the early stage of the immunologic response can be seen in this animal model. The septic CLP mouse model has the characteristics of a simple operation with high stability and reproducibility and is an ideal tool for studying sepsis in humans and testing anti-inflammatory drugs.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This work was supported by the National Natural Science Foundation of China (grants 31472061 and 31702074) and the Science and Technology Planning Project of Guangdong Province, China (grants 2016A030313790, 2015A030302034, and 2013B060300025).
References
- 1.Yang HL, Pan JX, Wu WK. Advanced pathophysiology. Beijing: Beijing Science Press, 2006. [Google Scholar]
- 2.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS Internatio-nal Sepsis Definitions Conference. Intensive Care Med 2003; 29: 530–538. [DOI] [PubMed] [Google Scholar]
- 3.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rittirsch D, Huber-Lang MS, Flierl MA, et al. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 2009; 4: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng D, Li X, Chao L, et al. Systematic investigation on the turning point of over-inflammation to immunosuppression in CLP mice model and their characteristics. Int Immunopharmacol 2017; 42: 49–58. [DOI] [PubMed] [Google Scholar]
- 6.Doi K, Leelahavanichkul A, Yuen PST, et al. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest 2009; 119: 2868–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Poll T. Preclinical sepsis models. Surg Infect (Larchmt) 2012; 13: 287–292. [DOI] [PubMed] [Google Scholar]
- 8.Rittirsch D, Huberlang MS, Flierl MA, et al. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 2009; 4: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao W, Deyo DJ, Traber DL, et al. Hemodynamic and cardiac contractile function during sepsis caused by cecal ligation and puncture in mice. Shock 2004; 21: 31–7. [DOI] [PubMed] [Google Scholar]
- 10.Wang HH. Prognosis of severe sepsis patients assessed by lactate clearance rate. Contemporary Medicine Forum 2014; 12: 105–106. [Google Scholar]
- 11.Soller B, Zou F, Prince MD, et al. Comparison of noninvasive pH and blood lactate as predictors of mortality in a swine hemorrhagic shock with restricted volume resuscitation model. Shock 2014; 44(Suppl 1): 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen F, Shou ST. Monitoring arterial blood lactate for risk stratification of sepsis. Chinese General Practice 2012; 15: 580–583. [Google Scholar]
- 13.Liu DH. Evaluation of the effect of esmolol on myocardial cells in rats with sepsis by multi paramter. Guangzhou: Southern Medical University, 2011. [Google Scholar]
- 14.Semenza GL. Life with oxygen. Science 2007; 318: 62–64. [DOI] [PubMed] [Google Scholar]
- 15.Walmsley SR, Print C, Farahi N, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α–dependent NF-κB activity. J Exp Med 2005; 201: 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmiere C, Bardy D, Mangin P, et al. Value of sTREM-1, procalcitonin and CRP as laboratory parameters for postmortem diagnosis of sepsis. J Infect 2013; 67: 545–555. [DOI] [PubMed] [Google Scholar]
- 17.Gibot S, Cravoisy A, Kolopp-Sarda MN, et al. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit Care Med 2005; 33: 792–796. [DOI] [PubMed] [Google Scholar]
- 18.Gibot S. Clinical review: Role of triggering receptor expressed on myeloid cells-1 during sepsis. Crit Care 2005; 9: 485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunkhorst FM, Wegscheider K, Forycki ZF, et al. Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis and septic shock. Crit Care 2000; 26(Suppl 2): 148–152. [DOI] [PubMed] [Google Scholar]
- 20.Endo S, Aikawa N, Fujishima S, et al. Usefulness of procalcitonin serum level for the discrimination of severe sepsis from sepsis: a multicenter prospective study. J Infect Chemother 2008; 14: 244–249. [DOI] [PubMed] [Google Scholar]
- 21.Şirinoğlu M, Soysal A, Karaaslan A, et al. The diagnostic value of soluble urokinase plasminogen activator receptor (suPAR) compared to C-reactive protein (CRP) and procalcitonin (PCT) in children with systemic inflammatory response syndrome (SIRS). J Infect Chemother 2017; 23: 17–22. [DOI] [PubMed] [Google Scholar]
- 22.Cui Y. The function of soluble form of CD163 in severe infection and sepsis. Chinese Pediatric Emergency Medicine 2011; 18: 175–177. [Google Scholar]
- 23.Hintz KA, Rassias AJ, Wardwell K, et al. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol 2002; 72: 711–717. [PubMed] [Google Scholar]
- 24.Gaini S, Koldkjaer OG, Pedersen SS, et al. Soluble haemoglobin scavenger receptor (sCD163) in patients with suspected community-acquired infections. APMIS 2006; 114: 103–111. [DOI] [PubMed] [Google Scholar]
- 25.Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med 2010; 38: S26–S34. [DOI] [PubMed] [Google Scholar]
- 26.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 2009; 4: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li WY. Thrombocytopenia in HBV related acute on chronic liver failure patients. Guangzhou: Southern Medical University, 2015. [Google Scholar]