Short abstract
Objective
To investigate the effects of polaprezinc (PZ) on cyclophosphamide (CTX)- or cisplatin (DDP)-induced gastric mucosal injury and on a rat model of neurotransmitter-mediated vomiting.
Methods
Sprague–Dawley rats were divided at random into Control, CTX, DDP, PZ+CTX, and PZ+DDP groups. After 20 days, brain tissues and sera were analyzed for the levels of dopamine (DA), 5-hydroxytryptamine (5-HT), and nuclear factor kappa B (NF-κB). Hematoxylin and eosin-stained sections of stomach, intestine, and brain tissues were examined using light microscopy.
Results
The levels of DA, 5-HT, and NF-κB in brain and serum samples of rats treated with CTX or DDP were significantly increased compared with those of rats in the Control group. There was a significant decrease in these values in the PZ group. Moreover, PZ reduced damage to brain tissue caused by CTX or DDP.
Conclusions
PZ decreased the levels of DA, 5-HT, and NF-κB in blood and brain tissues caused by CTX or DDP and reduced the chemotherapy-induced damage to the small intestine, stomach, and brain. These findings can be translated to the clinic to enhance the efficacy and safety of chemotherapy.
Keywords: Polaprezinc, cancer chemotherapy, cisplatin, cyclophosphamide, 5-hydroxytryptamine, nuclear factor kappa B, dopamine
Introduction
Chemotherapy is one of the major components of cancer treatment. Although chemotherapeutic drugs can kill cancer cells, they often cause adverse effects such as nausea and vomiting. These problems frequently occur when patients are treated with drugs such as platinum and alkylating agents. Highly emetogenic chemotherapy without control of these symptoms often produces a series of related complications such as malnutrition, metabolic disorder, and dehydration.1 The development of anticancer drug therapy has promoted the development of antiemetic drugs that are an important component of anticancer therapy. For example, ondansetron is an effective antiemetic and the first 5-hydroxytryptamine-3 (5-HT3) receptor antagonist.2
Zinc-L-carnosine (polaprezinc, PZ) is a chelate that is in wide clinical use for the treatment of gastric ulcers in Japan.3 L-Carnosine increases granulation tissue and accelerates gastric ulcer healing, and it is an ideal Zn transporter that mediates metabolism in vivo. Zn protects the gastric mucosa against experimentally induced lesions. Because PZ is an antioxidant with anti-inflammatory effects,4 it ameliorates oral mucositis caused by chemotherapy.5 Moreover, Zn deficiency can significantly reduce learning and memory, and trace elements in Zn supplements can reduce the concentrations of dopamine (DA) and 5-HT in blood and telencephalon,6 indicating how Zn reduces vomiting of patients receiving chemotherapy. Here we used a rat model to explore the mechanism through which PZ reduces vomiting caused by cancer chemotherapy and provide an experimental basis for translating our findings to the clinic.
Materials and methods
Materials and reagents
PZ was provided by Jilin Broadwell Pharmaceutical Co. Ltd. (Changchun, China). Cisplatin (DDP) for injection (freeze-dried, batch number 1WA2A1408008A, 20 mg/vial) was purchased from Qilu Pharmaceutical Factory (Jinan, China). Cyclophosphamide (CTX) for injection (batch number 14091421, 200 mg/vial) was purchased from Jiangsu Hengrui Medicine Co., Ltd. (Lianyungang, Jiangsu Province, China). Standard bovine serum albumin was purchased from Sigma-Aldrich (St. Louis, MO, USA). DA, 5-HT, and nuclear factor kappa B (NF-κB) ELISA Assay Kits were purchased from Beijing Winter Song Boye (Biotechnology Co. Ltd., Beijing, China). Male Sprague–Dawley (SD) rats (laboratory animal quality certificate No. SCXK [Beijing] 2014-0013, 140–160 g body weight) were provided by the Chinese Institute of Food and Drug Testing (Beijing, China). A Super Clean Bench was purchased from Beijing New Town County Liang Jia Camp Purification Equipment Factory. An Ohaus electronic precision balance was purchased from OHAUS Corporation (Parsippany, NJ, USA). A Nikon inverted microscope, an Olympus/PM-6 microscope, a Thermo Fisher T16-40 centrifuge, and a TECAN Infinite F50 Microplate reader were purchased from Nikon Corp. Instruments Co. (Sendai, Japan), Olympus Imaging China Co. Ltd. (Beijing branch), Thermo Fisher Scientific Inc. (Rockford, IL, USA), and TECAN Group Lt (Männedorf, Switzerland). A CJ-2F medical purification table was purchased from the Suzhou Feng Group Laboratory Animal Equipment Co. Ltd. (Jaingsu, China), and its specifications are as follows: Clean-level 100 (federal standard 209E), average colony number ≤0.5 per dish, average air flow, 0.4 ± 20%, noise ≤62dB (A), and vibration ≤3 m3.
SD rat model
SD rats were randomly divided into five groups (n = 6 each) as follows: Control group; CTX group: CTX 40 mg/kg body weight, intraperitoneally once every 3 days, 7 times; DDP group: DDP 5 mg/kg body weight, intraperitoneally once every 3 days, 7 times; PZ+CTX group: PZ 100 mg/kg body weight, orally once daily, 19 times + CTX 40 mg/kg body weight, intraperitoneally once every 3 days, 7 times; PZ+DDP group: PZ 100 mg/kg body weight, orally once daily, 19 times + DDP 5 mg/kg body weight, intraperitoneally once every 3 days, 7 times. Animals were observed daily for 20 days. Approximately 24 hours after the last treatment, the rats were killed, and gastric, intestinal, and brain tissues were harvested. Tissues were fixed in 10% formalin, stained with hematoxylin and eosin, and observed using a light microscope. DA, 5-HT, and NF-κB from blood and brain tissue homogenates were detected using the ELISA kits described above. Rats were housed in an animal feeding chamber with a barrier system at room temperature (22°C–25°C, diurnal temperature difference ≤3°C), relative humidity 40%–60%, illumination for 12 hours, class-100 air quality, ammonia concentration ≤14 mg/m3, noise ≤60 dB, illumination 150–300 LX, and animal illumination, 100–200 LX.
The experiments were performed according to the provisions of Laboratory Animal Use Permit No. SYXK (Beijing) 2014-0003. The Animal Care and Welfare Committee of our institution approved the use of animals for experiments that were performed in accordance with the principles of laboratory animal care.
Detection of DA, 5-HT, and NF-κB
Samples frozen in sealed bags were removed and placed at room temperature for 20 min and then weighed. The samples (50 µL of different concentrations of DA, 5-HT, or NF-κB) were placed in the wells of a multiwell plate. Samples contained 10 µL of sample and 40 µL of diluent. Nothing was added to the wells that served as blanks. Except for the blank wells, 100 µL of horseradish peroxidase was added to label the antibody, the wells were sealed using a membrane cover, incubated at 37°C for 60 min in a water bath or incubator, and absorption was determined at 450 nm. The protein concentration of the brain tissue homogenate was measured to prepare a standard curve.
Statistical analyses
The results are expressed as the mean ± standard error. Statistical significance was evaluated using the t test and the data were subjected to two-sample variance analysis. P <0.05 represents statistical significance.
Results and discussion
Effects of PZ combined with CTX or DDP
Body weights after administration of CTX or DDP for 20 days significantly decreased compared with the Control group, whereas PZ suppressed this decrease. The body weights of rats treated with PZ combined with CTX or DDP did not significantly differ compared with treatment with CTX or DDP alone (Figure 1, Table 1).
Figure 1.
Influence of polaprezinc on the body weight of rats after chemotherapy.
Table 1.
Effects of polaprezinc on body weight of rats after chemotherapy
| Group | Dosage (mg/kg) | Number |
Administration |
Weight (g), mean ± SD |
P | |
|---|---|---|---|---|---|---|
| Frequency | Pre | Pro | ||||
| Control | DW | 6 | Oral, once daily, 19 times | 143.2±2.5 | 262.3±14.6 | |
| CTX | 40 | 6 | Intraperitoneal, once every 3 days, 7 times | 144.7±4.5 | 175.7±17.9 | *<0.01 |
| DDP | 5 | 6 | Intraperitoneal, once every 3 days, 7 times | 143.7±3.9 | 158.0±12.0 | *<0.01 |
| PZ | 100 | Oral, once daily, 19 times | ||||
| + | 6 | 144.3±4.3 | 192.2±16.7 | *<0.01 | ||
| CTX | 40 | Intraperitoneal, once every 3 days, 7 times | **<0.05 | |||
| PZ | 100 | Oral, once daily, 19 times | ||||
| + | 6 | 145.2±4.1 | 181.5±12.4 | *<0.01 | ||
| DDP | 5 | Intraperitoneal, once every 3 days, 7 times | ***<0.01 | |||
DW, Double-distilled water; CTX, cyclophosphamide; DDP, cisplatin; PZ, polaprezinc; SD, standard deviation.
*Compared with Control group; **Compared with CTX group; ***Compared with DDP group.
Effects of PZ combined with CTX or DDP on the levels of DA, 5-HT, and NF-κB in serum and brain
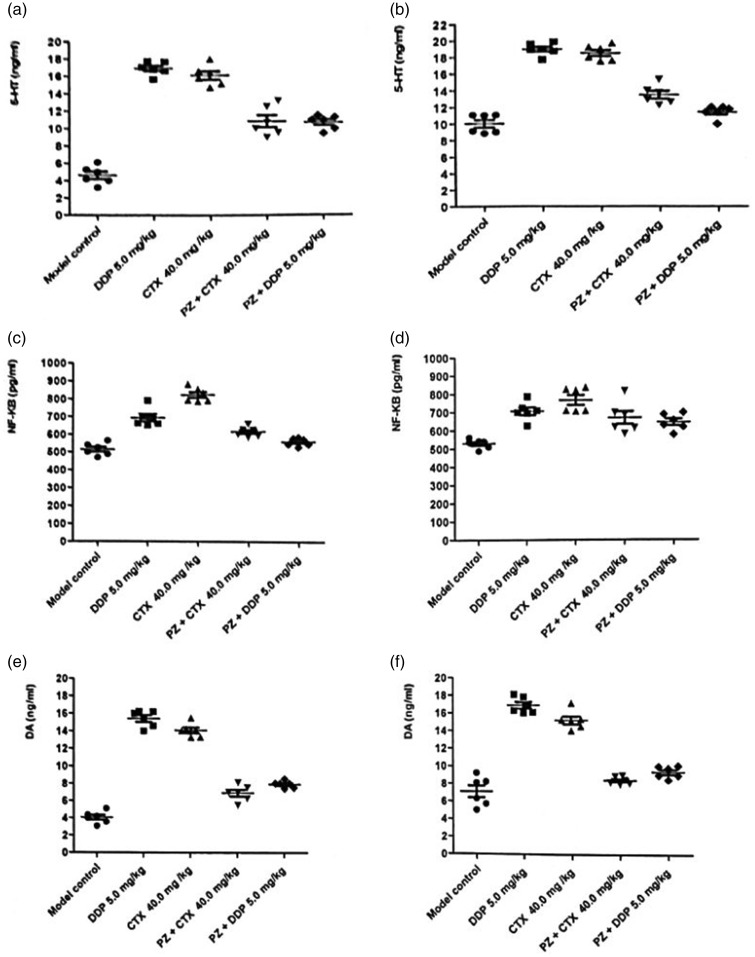
The levels of DA, 5-HT, and NF-κB significantly increased in rats treated with CTX or DDP alone. However, when these drugs were combined with PZ, there was a significant decrease in the levels of DA, 5-HT, and NF-κB (Figure 2).
Figure 2.
Neurotransmitter levels of rats administered polaprezinc combined with chemotherapy. (a) 5-hydroxytryptamine (5-HT) in serum assay. (b) 5-HT in brain tissue assay. (c) Nuclear factor kappa B (NF-κB) in serum assay. (d) NF-κB in brain tissue assay. (e) Dopamine (DA) in serum assay. (f) DA in brain tissue assay.
Effects of PX combined with CTX or DDP on the gastric mucosa
Microscopy revealed that PZ significantly reduced damage caused by CTX or DDP to the gastric mucosa, intestinal mucosa, and structure of the brain (Figure 3).
Figure 3.
Histopathological examination of tissues of rats treated with polaprezinc (PZ) combined with cyclophosphamide (CTX) or cisplatin (DDP). (a) Intestine and (b) glandular stomach in the Control group, normal intestinal structure, neat cells, and complete villi. Hematoxylin and eosin (H.E.) ×400. (c) Intestine and (d) glandular stomach in the CTX group, wide small intestinal cell gap, wide cell gap, and cell atrophy. H.E. ×400. (e) Intestine and (f) glandular stomach in the DDP group, nuclear condensation, weak cell activity, atrophied villi. H.E. ×400. (g) Intestine and (h) glandular stomach in the PZ + CTX group, normal cell, clear structure, complete villi, neat glands. H.E. ×400. (i) Intestine and (j) glandular stomach in the PZ + DDP group, normal cell, complete villi, and neat glands. H.E. ×400.
The vomiting caused by chemotherapeutic drugs often reduces the quality of life of patients and diminishes the benefits of chemotherapy.7 The mechanism mainly involves drug-induced mucosal damage in vivo, particularly from the chromaffin cells of the stomach to the ileal mucosa that release DA, 5-HT, and activated DA. The 5-HT3 receptor expressed by cells in the intestinal mucosa activates the vagus nerve located in the fourth ventricle chemoreceptor trigger-zone area that expresses the 5-HT3 receptor, and the excited chemosensory region is the central cause of vomiting induced by the vomiting center.8,9 5-HT3 receptor antagonists such as ondansetron or palonosetron, which are widely used in the clinic, significantly inhibit chemotherapy-induced vomiting.10
PZ is a complex chelating agent consisting of doubly deprotonated β-alanyl-L histidine (L-carnosine) and Zn in a 1:1 molar ratio. PZ is widely used as a gastric-mucosal protective agent to treat gastric ulcers in Japan. The mechanisms of gastric mucosal protection are independent of prostaglandins as well as anti-inflammatory and antioxidant functions. These mechanisms play a significant role in the protection of the gastric mucosa and restore damage induced by alcohol,11 acetic acid,12,13 hydrochloric acid, and nonsteroidal anti-inflammatory drugs.14 Moreover, PZ ameliorates the complications induced by cancer chemotherapy and cancer radiotherapy. For example, PZ significantly reduces the incidence of severe esophagitis among patients with non-small cell lung cancer who undergo chemoradiotherapy,15 relieves bladder pain caused by CTX in mice,16 and resolves peripheral neuropathy caused by paclitaxel.17
Here we investigated the effects of PZ on gastric mucosal damage and vomiting-associated neurotransmitters in rats treated with CTX and DDP. The NF-κB system mainly contributes to the body's defensive response, tissue damage and stress, cell differentiation and apoptosis as well as the mediating signaling that inhibits tumor growth. After activation, NF-κB regulates the expression of genes such as those encoding the inflammatory cytokines TNF-α, IL-1, and IL-6 that can directly or indirectly affect microvascular endothelial cells or blood cells to mediate their interaction, which leads to the dysfunction of microcirculation, endothelial cell, and tissue injury.18
Ohata et al.,19 found that PZ protects against lipopolysaccharide-induced endotoxin shock via inhibition of NF-κB activation and subsequent induction of proinflammatory products including NO and TNF-α. In the present study, we show that the levels of NF-κB in the brain increased significantly after rats were administered CTX or DDP alone. In contrast, NF-κB levels significantly decreased significantly when with CTX or DDP after administered PZ was administered after CTX or DDP. The hippocampus controls advanced neural activity and serves as a logical unit of language learning, receiving and storing information. The weight of the hippocampus is approximately 1/80 of that of the entire human brain and stores approximately 1/6 of the total Zn content of the brain. Therefore, adequate Zn concentrations play a key role in the brain's flexibility and intelligence. For example, Zn deficiency increases the blood and telencephalon levels of DA and 5-HT in mice, and the increased concentrations of 5-HT and DA in the central and peripheral circulatory systems may be explained by the key role played by Zn in the metabolism of 5-HT and DA metabolism in these compartments. Further, Carlson et al.,20 found that administering high-doses of Zn reduces the stimulating effects of DA and 5-HT on weaned piglets.
In the present study, we evaluated the correlates of vomiting by measuring the levels of the neurotransmitters 5-HT and DA in the serum and brain tissue of rats administered CTX or DDP. Under these conditions, the concentrations of 5-HT and DA increased significantly. In striking contrast, the administration of PZ combined with CTX, or DDP significantly decreased the concentrations of these neurotransmitters, which is consistent with findings of others that PZ significantly improves the gastric mucosa and intestinal mucosal injury caused by nonsteroidal anti-inflammatory drugs.21 Watari et al.,22 used capsule endoscopy to follow patients who received low doses of aspirin for longer than 5 months and discovered that PZ significantly inhibits intestinal swelling, erosion, and ulcers.
The absorption of PZ is pH-dependent and can be dissolved in acidic solutions such as gastric acid.23 PZ stabilizes membranes, has antioxidative activity, and can promote wound healing. L-carnosine promotes healing and regulates the immune response. PZ inhibits chemotherapy-induced oral mucositis by improving recovery from 5-fluorouracil–induced oral mucositis in hamsters,24 increases recovery from acute ischemic stroke in a rat model,25 and oral Zn supplementation is used to treat hepatic encephalopathy.26 Here we show that SD rats treated with CTX or DDP suffer injury to brain tissue, the intestinal mucosa, and the gastric mucosa. These serious adverse effects were ameliorated by the administration of PZ. In summary, PZ reduced the increased levels of DA, 5-HT, and NF-κB induced by CTX or DDP in blood and brain tissue. Further, PZ suppressed the damage to intestinal, stomach, and brain tissue caused by CTX or DDP. These findings provide a foundation for introducing PZ as an effective new antiemetic for patients who undergo chemotherapy.
Declaration of conflicting interest
The authors declares that there is no conflict of interest.
Funding
This study was funded by grants from Jilin Province Broadwell Pharmaceutical Co., Ltd., Changchun, China (Grant Number 2016-02-03) and from the Technology Innovation Fund for Enterprises (Grant Number 2017-001-FW).
References
- 1.Ikari Y, Ogata K, Nakashima Y, et al. Safety and pharmacokinetic evaluation of repeated intravenous administration of palonosetron 0.75 mg in patients receiving highly or moderately emetogenic chemotherapy. Support Care Cancer 2014; 22: 1959–1964. [DOI] [PubMed] [Google Scholar]
- 2.Craver C, Gayle J, Balu S, et al. Palonosetron versus other 5-HT3 receptor antagonists for prevention of chemotherapy-induced nausea and vomiting in patients with hematologic malignancies treated with melogenic chemotherapy in a hospital outpatient setting in the United States. J Med Econ 2011; 14: 341–349. [DOI] [PubMed] [Google Scholar]
- 3.Choi HS, Lim JY, Chun HJ, et al. The effect of polaprezinc on gastric mucosal protection in rats with ethanol-induced gastric mucosal damage: Comparison study with rebamipide. Life Sci 2013; 93: 69–77. [DOI] [PubMed] [Google Scholar]
- 4.Itagaki M, Saruta M, Saijo H, et al. Efficacy of zinc–carnosine chelate compound, Polaprezinc, enemas in patients with ulcerative colitis. Scand J Gastroenterol 2014; 49: 164–172. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe T, Ishihara M, Matsuura K, et al. Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. Int J Cancer 2010; 12: 1984–1990. [DOI] [PubMed] [Google Scholar]
- 6.Yan-qiang L, Shu-tian L, Dong-lan W, et al. Effect of Zinc deficiency on the concentrations of acetylcholine and serotonin in blood and brain of rats. China Public Health 2001; 17: 233–234. [Google Scholar]
- 7.Walsh D, Davis M, Ripamonti C, et al. 2016 Updated MASCC/ESMO consensus recommendations: Management of nausea and vomiting in advanced cancer. Support Care Cancer 2017; 25: 333–340. [DOI] [PubMed] [Google Scholar]
- 8.Gu L, Li J. The assessment and management of chemotherapy-induced nausea and vomiting among cancer patients in a chemotherapy ward: a best practice implementation project . JBI Database System Rev Implement Rep 2016; 14: 235–246. doi: 10.11124/JBISRIR-2016–2626. [DOI] [PubMed] [Google Scholar]
- 9.Johnston KD, Lu Z, Rudd JA. Looking beyond 5-HT (3) receptors: a review of the wider role of serotonin in the pharmacology of nausea and vomiting. Eur J Pharmacol 2014; 722: 13–25. [DOI] [PubMed] [Google Scholar]
- 10.Fiorino F, Severino B, Magli E, et al. 5-HT (1A) receptor: an old target as a new attractive tool in drug discovery from central nervous system to cancer. J Med Chem 2014; 7: 4407–4426. [DOI] [PubMed] [Google Scholar]
- 11.Morgan RJ, Jr, Armstrong DK, Alvarez RD, et al. Ovarian cancer, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 9: 1134–1163. [DOI] [PubMed] [Google Scholar]
- 12.Xiao-ou Y, Jia-ming Q, Qiang C. Protection of polaprezinc induced HSP70 in rats gastric mucosal injury. Chin J Gastroenterol Hepatol 2014; 23: 50–53. [Google Scholar]
- 13.Opoka W, Adamek D, Plonka M, et al. Importance of luminal and mucosal zinc in the mechanism of experimental gastric ulcer healing. J Physiol Pharmacol 2010; 61: 581–591. [PubMed] [Google Scholar]
- 14.Wada I, Otaka M, Jin M, et al. Expression of HSP72 in the gastric mucosa is regulated by gastric acid in rats-correlation of HSP72 expression with mucosal protection. Biochem Biophys Res Commun 2006; 349: 611–618. [DOI] [PubMed] [Google Scholar]
- 15.Naito Y, Yoshikawa T, Yagi N, et al. Effects of polaprezinc on lipid peroxidation, neutrophil accumulation, and TNF-alpha expression in rats with aspirin-induced gastric mucosal injury. Dig Dis Sci 2001; 46: 845–851. [DOI] [PubMed] [Google Scholar]
- 16.Yanase K, Funaguchi N, Iihara H, et al. Prevention of radiation esophagitis by polaprezinc (zinc L-carnosine) in patients with non-small cell lung cancer who received chemo-radiotherapy. Int J Clin Exp Med 2015; 8: 16215–16222. [PMC free article] [PubMed] [Google Scholar]
- 17.Murakami-Nakayama M, Tsubota M, Hiruma S, et al. Polaprezinc attenuates cyclophosphamide- induced cystitis and related bladder pain in mice. J Pharmacol Sci 2015; 127: 223–228. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsumi K, Kaname T, Shiraishi H, et al. Polaprezinc reduces paclitaxel-induced peripheral neuropathy in rats without affecting anti-tumor activity. J Pharmacol Sci 2016; 131: 1– 4. [DOI] [PubMed] [Google Scholar]
- 19.Ohata S, Moriyama C, Yamashita A, et al. Polaprezinc Protects Mice against Endotoxin Shock. J Clin Biochem Nutr 2010; 46: 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlson D, Poulsen HD, Sehested J. Influence of weaning and effect of post weaning dietary zinc and copper on electrophysiological response to glucose, theophylline and 5-HT in piglet small intestinal mucosa. Comp Biochem Physiol A Mol Integr Physiol 2004; 137: 757–765. [DOI] [PubMed] [Google Scholar]
- 21.Omatsu T, Naito Y, Handa O, et al. Reactive oxygen species-quenching and anti-apoptotic effect of polaprezinc on indomethacin-induced small intestinal epithelial cell injury. J Gastroenterol 2010; 45: 692–702. [DOI] [PubMed] [Google Scholar]
- 22.Watari I, Oka S, Tanaka S, et al. Effectiveness of polaprezinc for low-dose aspirin-induced small-bowel mucosal injuries as evaluated by capsule endoscopy: a pilot randomized controlled study. BMC Gastroenterology 2013; 13: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsukura T, Takahashi T, Nishimura Y, et al. Characterization of crystalline L-carnosine Zn(II) complex (Z-103), a novel anti-gastric ulcer agent: tautomeric change of imidazole moiety upon complexation. Chem Pharm Bull (Tokyo) 1990; 38: 3140–3146. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe T, Ishihara M, Matsuura K, et al. Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. Int J Cancer 2010; 127: 1984–1990. [DOI] [PubMed] [Google Scholar]
- 25.Oh MK, Yoon KJ, Lee YT, et al. Effect of zolpidem on functional recovery in a rat model of ischemic stroke. J Int Med Res 2018; 46: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chavez-Tapia NC, Cesar-Arce A, Barrientos-Gutiérrez T, et al. A systematic review and meta-analysis of the use of oral zinc in the treatment of hepatic encephalopathy. Nutr J 2013; 12: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]