Short abstract
Objective
Rapid eye movement (REM)-related obstructive sleep apnea (OSA) is characterized by respiratory events such as apnea and hypopnea predominately or exclusively during REM sleep. Several studies have revealed clinical predictors of adherence to the use of continuous positive airway pressure (CPAP). However, the effect of REM-related OSA on adherence to CPAP use remains unclear. Therefore, we investigated the effects of REM-related OSA on adherence to CPAP use 6 months after treatment initiation.
Methods
We enrolled 161 patients in this study and divided them into 3 groups: the good adherence, poor adherence, and dropout groups. We compared polysomnographic data and clinical findings, including those regarding morbidity of REM-related OSA, among the three groups to identify predictors of adherence to CPAP use.
Results
None of the 43 patients in the good adherence group had REM-related OSA. Multivariate logistic regression analysis of the good adherence and dropout groups indicated that REM-related OSA was the only factor associated with adherence to CPAP use (odds ratio, 41.984; 95% confidence interval, 2.257–781.007).
Conclusions
REM-related OSA is a reliable risk factor for dropout from CPAP therapy.
Keywords: REM-related obstructive sleep apnea, adherence, obstructive sleep apnea, continuous positive airway pressure, polysomnography, predictive factors
Introduction
Obstructive sleep apnea (OSA) is characterized by periodic complete or partial collapse of the upper airway during sleep and results in apnea and hypopnea despite ongoing breathing efforts. OSA affects 5% to 24% of men and 1% to 9% of women.1–3 Patients with untreated OSA have higher risks of developing hypertension,4 cardiovascular disease,5 cerebrovascular disease,6 and motor vehicle accidents due to daytime sleepiness.7
Continuous positive airway pressure (CPAP) therapy is currently the gold standard treatment for the management of OSA. Adequate and appropriate use of CPAP improves several comorbidities of OSA8–11 and reduces daytime sleepiness.12 CPAP treatment is a symptomatic rather than radical treatment; therefore, maintaining adherence is crucial to its beneficial effects. It is important to predict CPAP adherence prior to treatment initiation and use intensive interventions in patients who are unlikely to adhere to CPAP use.
Weaver et al.13 reported that 29% to 83% of patients with OSA are nonadherent, meaning that they use CPAP for a mean of ≤4 hours per night.14 Another study showed that patterns of CPAP use are established during the first week of treatment initiation.15 Many studies have shown that the severity of disease, degree of sleepiness before treatment, age, sex, and CPAP pressure at initiation are clinical predictors of adherence to CPAP use.16–18
Rapid eye movement (REM)-related OSA is a condition wherein respiratory events such as apnea and hypopnea occur predominantly or exclusively during REM sleep. This is usually treated by auto CPAP, which modifies the level of positive pressure applied during the night in the presence or absence of a respiratory event.19 However, no guidelines on management of this disease are currently available.20
In general, two sets of criteria are used to identify REM-related OSA:20,21 (i) an overall apnea–hypopnea index (AHI) of ≥5 and a ratio of the AHI during REM sleep to the AHI during non-REM (NREM) sleep of >2 and (ii) an overall AHI of ≥5, a ratio of the AHI during REM sleep to the AHI during NREM sleep of >2, and an AHI during NREM sleep of <15. NREM sleep is thought to comprise approximately 80% of total sleep in adults.22 Thus, patients with REM-related OSA treated with auto CPAP require a relatively lower pressure during a long portion of total sleep, but they also require a higher pressure during every REM sleep period. In addition, fluctuation in CPAP pressure is caused by arousal from sleep, which may disturb CPAP use. Whether patients with REM-related OSA tolerate CPAP therapy remains unclear.20 Consequently, we hypothesized that REM-related OSA may influence adherence to CPAP use.
The aim of this study was to determine whether REM-related OSA is a predictor of adherence to CPAP use 6 months after treatment initiation.
Patients and methods
Patient population and study design
This retrospective study was approved by the ethics committee of Aichi Medical University Hospital (AMUH). The need for informed consent was waived due to the retrospective nature of the study. We assessed patients who first visited the Sleep Disorder Center of AMUH and underwent nocturnal polysomnography (PSG) because of suspicion for OSA from January 2008 to May 2011. All clinical records and PSG data were made available in an anonymized format.
Patients were excluded from the study for the following reasons: age of <20 or >75 years, a history indicative of a diagnosis of any other sleep disorder, CPAP treatment with a machine other than the REMstar M Series Auto CPAP machine (Philips Respironics GK; Tokyo, Japan), and transfer to another hospital within 6 months of OSA diagnosis or CPAP initiation. Although several kinds of CPAP machines are generally prescribed, all patients included in this study used the REMstar M Series Auto CPAP machine with a nasal mask to exclude potential confounding due to the use of different algorithms in the CPAP machines. Patients who transferred to another hospital within 6 months of OSA diagnosis or CPAP initiation were excluded because they lived far from AMUH and were difficult to follow up.
The patients were divided into three groups: the good adherence, poor adherence, and dropout groups. The good adherence group comprised patients who used the CPAP machine for a mean of >4 hours per day and had a >70% rate of CPAP use for 6 months following treatment initiation.23 The poor adherence group comprised patients who used the CPAP machine for 6 months following treatment initiation but used the machine for <4 hours per day and had a <70% rate of CPAP use. The dropout group comprised patients who ceased CPAP machine use within 6 months of treatment initiation. We compared the PSG and clinical findings among the three groups. We specifically considered the morbidity of REM-related OSA to identify predictors of adherence to CPAP use.
Baseline characteristics and sleepiness assessment
The body mass index (BMI) and neck circumference (NC) of each patient were measured at the time of the first visit. Daytime sleepiness was evaluated using the Epworth Sleepiness Scale (ESS).24
Nocturnal sleep study
Overnight PSG was performed for all patients using the Alice4 system (Philips Respironics GK) over the course of their hospitalization. The following biological variables were continuously monitored during the overnight visit: submental and anterior tibial electromyography, electrocardiography, electroencephalography (F4-M1, C4-M1, O2-M1, F3-M2, C3-M2, and O1-M2), and electrooculography.
Inductance plethysmography belts were used to monitor chest and abdominal movements. A nasal cannula with a pressure transducer was used to measure airflow. Snoring was recorded using a microphone. Pulse oximetry was used to monitor arterial oxygen saturation.
The Rechtschaffen and Kales sleep staging criteria were used to score sleep stages and arousal.25 The AHI is defined as the mean number of apnea and hypopnea events per hour of sleep. The oxygen desaturation index (ODI) was calculated as the number of desaturations of >3% per hour of sleep. Obstructive apnea was defined as the absence of oral and nasal airflow for longer than 10 s, while obstructive hypopnea was defined as a ≥30% reduction of oral and nasal airflow for longer than 10 s that was associated with an electroencephalographic arousal or desaturation of >3%. Respiratory effort-related arousals (RERAs) were defined as increasing respiratory efforts terminating in arousals that do not meet the criteria for apnea or hypopnea. The RERA index was calculated as the number of RERAs per hour of sleep.
Respiratory events included apnea and hypopnea events. The cumulative percentage of time spent at saturations below 90% was calculated from the oximetry data. The limb movement index refers to the number of limb movements per hour of sleep regardless of periodic movement.
Data were manually scored by specialized clinical engineers according to the American Academy of Sleep Medicine 2007 scoring system.26 The patients were considered to have REM-related OSA if the ratio of AHI during REM sleep to that during NREM sleep was >2. They were considered to have non-stage-specific OSA if the ratio of AHI during REM sleep to that during NREM sleep was ≤2, as described previously.27
CPAP therapy
In accordance with the guidelines of the Japanese health insurance system, patients who were diagnosed with OSA with >20 AHI events per hour, as assessed using PSG, were recommended to start CPAP treatment. Patients who agreed to undergo CPAP treatment received adequate education regarding their disease and treatment by a special physician. Mask fitting was performed by specialized clinical engineers. The REMstar Auto M Series, which is an auto-titrating device, was used to perform CPAP titration.
Follow-up and CPAP adherence data
In accordance with the guidelines of the health insurance system in Japan, all patients undergoing CPAP treatment were required to visit the clinic or hospital once every month. The patients were required to bring the built-in memory chip from their CPAP device, which recorded CPAP usage data, to their monthly visits. The rates of CPAP usage and the mean daily usage of the CPAP machine (hours) were analyzed based on the recorded data. CPAP usage data were retrospectively analyzed for 6 months beginning at the time of CPAP treatment initiation.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median (interquartile range). Categorical variables are expressed as number (proportion). Comparisons between groups were performed using Fisher’s exact test for categorical variables and the unpaired t test or Mann–Whitney U test for continuous variables. For the multivariate logistic regression analysis, we first modeled the presence of REM-related OSA, followed by a stepwise approach to enter the significant covariates into the model. When the response variable had a low or high event rate, all responses had the same event status. One approach used to solve this problem is to use the penalized likelihood method proposed by Firth.28 Therefore, we calculated odds ratios (ORs) and 95% confidence intervals (CIs) using Firth logistic regressions. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Of 1,922 patients assessed during the study period, 443 were diagnosed with OSA and began CPAP treatment. Of these 443 patients, 161 were included in the present study. No patients had missing data. A study flow chart is shown in Figure 1. Most patients were male (85.1%). The mean age of all patients was 55.7 ± 12.7 years, mean NC was 39.6 ± 3.3 cm, mean BMI was 27.0 ± 4.4 kg/m2, and mean ESS score was 9.2 ± 5.0.
Figure 1.
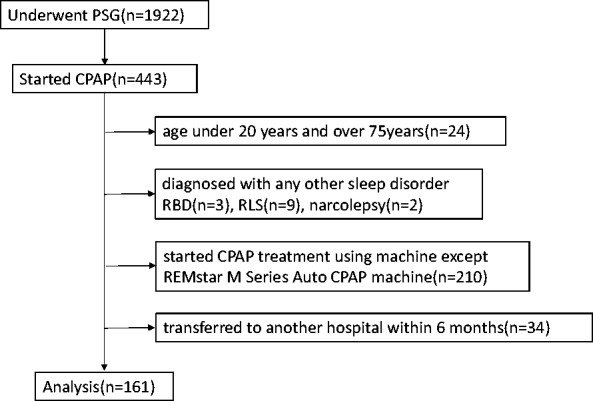
Flow diagram of the study.
PSG, polysomnography; CPAP, continuous positive airway pressure; RBD, rapid eye movement sleep behavior disorder; RLS, restless legs syndrome.
Table 1 shows the baseline characteristics and PSG variables of patients with non-stage-specific OSA and REM-related OSA. Of the 161 participants, 27 had REM-related OSA and 134 had non-stage-specific OSA. We found significant differences between patients with REM-related OSA and non-stage-specific OSA in the sex distribution (11.9% vs. 29.6% women, respectively; p = 0.03) and overall AHI (35.9 ± 12.1 vs. 28.4 ± 5.8, respectively; p = 0.002). Of the 161 participants, 43 had good adherence (26.7%), 83 had poor adherence (51.6%), and 35 dropped out (21.7%). None of the 43 patients in the good adherence group had REM-related OSA.
Table 1.
Baseline characteristics and polysomnographic variables in patients with non-stage-specific OSA and those with REM-related OSA
| Non-stage-specific OSA (n = 134) | REM-related OSA (n = 27) | p value | |
|---|---|---|---|
| Age (years) | 56.2 ± 12.2 | 53.2 ± 15.0 | 0.272 |
| Female sex (%) | 16 (11.9) | 8 (29.6) | 0.034* |
| ESS score | 9.3 ± 5.0 | 8.8 ± 5.26 | 0.653 |
| BMI (kg/m2) | 26.6 ± 4.0 | 28.6 ± 5.58 | 0.031* |
| NC (cm) | 39.6 ± 3.1 | 39.5 ± 4.39 | 0.866 |
| Sleep latency (min) | 6.5 (3–11.5) | 6.5 (3–10.5) | 0.563 |
| REM sleep latency (min) | 144.1 ± 85.2 | 101.3 ± 33.85 | 0.011* |
| TST (min) | 397.2 ± 61.5 | 406.6 ± 45.7 | 0.454 |
| Sleep efficiency (%) | 87.0 ± 10.8 | 88.9 ± 9.17 | 0.396 |
| REM sleep time/TST (%) | 15.7 ± 6.4 | 21.6 ± 5.7 | <0.0001* |
| NREM sleep time/TST (%) | 84.3 ± 6.4 | 78.4 ± 5.7 | <0.0001* |
| Stage 1 + 2 sleep time/TST (%) | 83.0 ± 7.2 | 74.9 ± 7.94 | <0.0001* |
| Stage 3 + 4 sleep time/TST (%) | 1.4 ± 2.7 | 3.6 ± 6.3 | 0.004* |
| AI (events/h) | 26.6 ± 14.1 | 16.2 ± 9.37 | <0.0001* |
| HI (events/h) | 9.5 ± 9.1 | 12.5 ± 9.19 | 0.121 |
| Respiratory event duration (%) | 35.9 ± 12.1 | 28.4 ± 5.81 | 0.002* |
| REM-AHI (events/h) | 35.8 ± 17.5 | 57.3 ± 11.74 | <0.0001* |
| NREM-AHI (events/h) | 36.5 ± 12.1 | 20.7 ± 6.59 | <0.0001* |
| Mean respiratory event duration (s) | 26.7 ± 6.7 | 22.9 ± 4.48 | 0.01* |
| Respiratory event duration (%) | 26.9 ± 10.6 | 17.7 ± 3.76 | <0.0001* |
| Minimum SpO2 (%) | 77.3 ± 10.3 | 74.8 ± 8.54 | 0.246 |
| ODI (events/h) | 32.1 ± 13.0 | 27.2 ± 6.51 | 0.058 |
| REM-ODI (events/h) | 34.4 ± 18.0 | 54.6 ± 14.0 | <0.0001* |
| NREM-ODI (events/h) | 31.8 ± 13.5 | 20.0 ± 6.7 | <0.0001* |
| CT90 (%) | 2.9 (0.7–5.6) | 3.2 (1.9–5.6) | 0.451 |
| RERA index (events/h) | 40.2 ± 13.0 | 27.4 ± 9.21 | <0.0001* |
| LMI (events/h) | 0 (0–8.6) | 0 (0–5.9) | 0.245 |
| Mean nadir SpO2 | 90.8 ± 3.3 | 90.1 ± 2.49 | 0.348 |
Continuous variables are expressed as mean ± standard deviation or median (25th–75th percentile), and categorical variables are expressed as number (proportion).
OSA, obstructive sleep apnea; ESS, Epworth sleepiness scale; BMI, body mass index; NC, neck circumference; REM, rapid eye movement; NREM, non-rapid eye movement; TST, total sleep time; AI, apnea index; HI, hypopnea index; AHI, apnea–hypopnea index; ODI, oxygen desaturation index; CT90, cumulative percentage of time spent at saturation below 90%; RERA, respiratory event-related arousal; LMI, limb movement index; SpO2, peripheral capillary oxygen saturation. *p < 0.05 when comparing non-stage-specific OSA with REM-related OSA.
Table 2 shows the baseline characteristics and PSG variables of the patients with good adherence, those with poor adherence, and those who dropped out. Patients in the poor adherence group had significantly higher sleep efficiency than those in the good adherence group (84.5% ± 12.9% vs. 89.6% ± 8.7%, respectively; p = 0.010). In addition, a higher percentage of patients had REM-related OSA in the poor adherence group than in the good adherence group (0.0% vs. 16.9%, respectively; p = 0.005).
Table 2.
Baseline characteristics and polysomnographic variables among good adherence, poor adherence, and dropout groups
| Good (n = 43) | Poor (n = 83) | Dropout (n = 35) |
p value |
|||
|---|---|---|---|---|---|---|
| Good vs. Poor | Good vs. Dropout | Poor vs. Dropout | ||||
| Age (years) | 59.2 ± 10.4 | 52.4 ± 13.6 | 59.1 ± 11.1 | 0.005* | 0.958 | 0.013* |
| Male sex (%) | 39 (90.7) | 69 (83.1) | 29 (82.9) | 0.295 | 0.330 | 1.000 |
| ESS score | 9.7 ± 5.0 | 8.8 ± 4.8 | 9.71 ± 5.47 | 0.339 | 0.958 | 0.353 |
| BMI (kg/m2) | 26.7 ± 3.3 | 27.4 ± 5.1 | 26.29 ± 3.58 | 0.453 | 0.592 | 0.265 |
| NC (cm) | 40.2 ± 2.7 | 39.5 ± 3.8 | 38.94 ± 3.01 | 0.281 | 0.055 | 0.439 |
| Sleep latency (min) | 8.5 (4.0–14) | 5.5 (2.5–10.5) | 7.0 (4.5–11) | 0.042* | 0.355 | 0.365 |
| REM sleep latency (min) | 154.8 ± 83.9 | 128.9 ± 74.8 | 133.0 ± 87.0 | 0.082 | 0.265 | 0.798 |
| TST (min) | 386.1 ± 66.4 | 407.7 ± 58.4 | 393.3 ± 48.4 | 0.063 | 0.594 | 0.202 |
| Sleep efficiency (%) | 84.5 ± 12.9 | 89.6 ± 8.7 | 85.6 ± 10.6 | 0.01* | 0.685 | 0.036* |
| REM sleep time/TST (%) | 16.2 ± 6.4 | 16.6 ± 7.0 | 17.3 ± 6.0 | 0.713 | 0.420 | 0.618 |
| NREM sleep time/TST (%) | 83.9 ± 6.4 | 83.4 ± 7.0 | 82.7 ± 6.0 | 0.713 | 0.420 | 0.618 |
| Stage 1 + 2 sleep time/TST (%) | 82.6 ± 6.4 | 81.7 ± 8.0 | 80.1 ± 9.3 | 0.536 | 0.163 | 0.339 |
| Stage 3 + 4 sleep time/TST (%) | 1.3 ± 2.7 | 1.7 ± 2.5 | 2.6 ± 6.0 | 0.409 | 0.192 | 0.231 |
| AI (events/h) | 26.9 ± 13.8 | 25.4 ± 14.6 | 21.0 ± 12.3 | 0.576 | 0.052 | 0.119 |
| HI (events/h) | 10.1 ± 9.1 | 9.4 ± 8.6 | 11.4 ± 10.6 | 0.643 | 0.561 | 0.268 |
| AHI (total events/h) | 37.0 ± 10.3 | 34.7 ± 13.3 | 31.4 ± 7.7 | 0.328 | 0.009* | 0.169 |
| REM-AHI (events/h) | 38.8 ± 15.9 | 38.4 ± 21.0 | 42.5 ± 15.1 | 0.907 | 0.300 | 0.294 |
| NREM-AHI (events/h) | 36.9 ± 10.8 | 34.4 ± 14.1 | 28.6 ± 10.3 | 0.321 | 0.001* | 0.030* |
| REM-related OSA (%) | 0 (0.0) | 14 (16.9) | 13 (37.1) | 0.005 | <0.0001* | 0.030* |
| Mean respiratory event duration (s) | 27.0 ± 7.0 | 25.7 ± 6.3 | 25.8 ± 6.4 | 0.268 | 0.411 | 0.937 |
| Respiratory event duration (%) | 28.4 ± 12.1 | 25.0 ± 9.9 | 22.4 ± 8.3 | 0.093 | 0.014* | 0.168 |
| Minimum SpO2 (%) | 75.4 ± 11.3 | 76.5 ± 10.1 | 79.6 ± 8.1 | 0.611 | 0.069 | 0.100 |
| ODI (events/h) | 33.6 ± 11.1 | 31.3 ± 13.5 | 28.4 ± 9.7 | 0.355 | 0.036* | 0.255 |
| REM-ODI (events/h) | 37.4 ± 16.1 | 36.6 ± 21.6 | 41.3 ± 15.4 | 0.841 | 0.288 | 0.260 |
| NREM-ODI (events/h) | 33.1 ± 11.7 | 29.8 ± 14.5 | 25.5 ± 11.3 | 0.193 | 0.123 | 0.005* |
| CT90 (%) | 3.5 (1.3–8.7) | 2.8 (1.1–5.6) | 2.0 (0.5–4.0) | 0.584 | 0.024* | 0.133 |
| RERA index (events/h) | 39.0 ± 9.1 | 38.3 ± 15.1 | 36.1 ± 13.2 | 0.790 | 0.257 | 0.453 |
| LMI (events/h) | 0 (0–5.0) | 0 (0–7.8) | 0 (0.0–19.8) | 0.867 | 0.525 | 0.371 |
| Mean nadir SpO2 | 90.1 ± 3.5 | 90.6 ± 3.2 | 91.5 ± 2.6 | 0.404 | 0.048* | 0.132 |
Continuous variables are expressed as mean ± standard deviation or median (25th–75th percentile), and categorical variables are expressed as number (proportion).
OSA, obstructive sleep apnea; ESS, Epworth sleepiness scale; BMI, body mass index; NC, neck circumference; REM, rapid eye movement; NREM, non-rapid eye movement; TST, total sleep time; AI, apnea index; HI, hypopnea index; AHI, apnea–hypopnea index; ODI, oxygen desaturation index; CT90, cumulative percentage of time spent at saturation below 90%; RERA, respiratory event-related arousal; LMI, limb movement index; SpO2, peripheral capillary oxygen saturation. *p < 0.05
Compared with patients in the dropout group, those in the good adherence group had a significantly higher AHI (37.0 ± 10.3 vs. 31.4 ± 7.7, p = 0.009), NREM AHI (36.9 ± 10.8 vs. 28.6 ± 10.3, p = 0.001), ratio of total apnea and hypopnea event duration to total sleep time (28.4% ± 12.1% vs. 22.4% ± 8.3%, p = 0.014), ODI (33.6 ± 11.1 vs. 28.4 ± 9.7, p = 0.036), and ratio of sleep time with a peripheral capillary oxygen saturation (SpO2) of <90% to total sleep time [6.1% (7.5%) vs. 2.0% (3.5%), p = 0.024]. A higher percentage of patients had REM-related OSA in the dropout group than in the good adherence group (0.0% vs. 17.1%, p < 0.0001). The patients in the dropout group also had a higher minimum SpO2 than those in the good adherence group (90.1% ± 3.5% vs. 91.5% ± 2.6%, p = 0.048).
Compared with patients in the dropout group, those in the poor adherence group had significantly higher sleep efficiency (89.6% ± 8.7% vs. 85.6% ± 10.6%, p = 0.036) and NREM AHI (34.4 ± 14.1 vs. 28.6 ± 10.3, p = 0.030). A significantly higher percentage of patients had REM-related OSA in the dropout group than in the poor adherence group (16.9% vs. 37.1%, p < 0.0030).
Age was significantly different between the poor adherence and good adherence groups (59.2 ± 10.4 vs. 52.4 ± 13.6 years, respectively; p = 0.005) (Table 2) and between the poor adherence and dropout groups (52.4 ± 13.6 vs. 59.1 ± 11.1, years, respectively; p = 0.013).
The multivariate logistic regression analysis of the good and poor adherence groups revealed no parameters associated with adherence to CPAP use. However, REM-related OSA was likely to be associated with adherence to CPAP use (OR, 16.585; 95% CI, 0.870–316.1) (Table 3). Multivariate logistic regression analysis of the good adherence and dropout groups revealed that REM-related OSA was an important factor associated with adherence to CPAP use (OR, 64.024; 95% CI, 3.127–999.999) (Table 4). Multivariate logistic regression analysis of the poor adherence and dropout groups revealed that REM-related OSA was an important factor associated with adherence to CPAP use (OR, 3.246; 95% CI, 1.267–8.318) (Table 5).
Table 3.
Results of multivariate logistic regression analysis between good and poor adherence groups to predict adherence to use of continuous positive airway pressure
| OR | 95% CI | p value | |
|---|---|---|---|
| REM-related OSA | 16.585 | 0.87–316.1 | 0.062 |
| Sleep latency | 1.042 | 1.004–1.082 | 0.031* |
Dependent variable: 0 = good adherence, 1 = poor adherence.
OR, odds ratio; CI, confidence interval; REM, rapid eye movement; OSA, obstructive sleep apnea. *p < 0.05
Table 4.
Results of multivariate logistic regression analysis between good adherence and dropout groups to predict adherence to use of continuous positive airway pressure
| OR | 95% CI | p value | |
|---|---|---|---|
| REM-related OSA | 64.024 | 3.127–∞ | 0.001* |
| Lowest arterial oxygen saturation | 1.073 | 0.999–1.152 | 0.05 |
Dependent variable: 0 = good adherence, 1 = dropout.
OR, odds ratio; CI, confidence interval; REM, rapid eye movement; OSA, obstructive sleep apnea. *p < 0.05
Table 5.
Results of multivariate logistic regression analysis between poor adherence and dropout groups to predict adherence to use of continuous positive airway pressure
| OR | 95% CI | p value | |
|---|---|---|---|
| REM-related OSA | 3.246 | 1.267–8.318 | 0.014* |
| Age | 1.043 | 1.009–1.079 | 0.013* |
Dependent variable: 0 = poor adherence, 1 = dropout.
OR, odds ratio; CI, confidence interval; REM, rapid eye movement; OSA, obstructive sleep apnea. *p < 0.05
Discussion
We sought to identify factors associated with adherence to CPAP use and found that REM-related OSA was an important factor that was independently associated with dropout from CPAP therapy. In fact, among the 27 patients with REM-related OSA, none had good adherence to CPAP use.
A previous study showed that the prevalence of REM-related OSA ranges from 10% to 36%.29 This variability is due to the use of inconsistent definitions for REM-related OSA. As described above, two definitions were used to determine the presence of REM-related OSA:20,21 (i) an overall apnea–hypopnea index (AHI) of ≥5 and a ratio of the AHI during REM sleep to the AHI during non-REM (NREM) sleep of >2 and (ii) an overall AHI of ≥5, a ratio of the AHI during REM sleep to the AHI during NREM sleep of >2, and an AHI during NREM sleep of <15. Of the patients in the present study, 27 patients met the criteria for definition (i) and 5 patients met the criteria for definition (ii). We used definition (i) to have a larger sample size; therefore, the prevalence of REM-related OSA was 16.8% in this study. This is consistent with the results of a previous report29 and one study reporting that REM-related OSA occurs more commonly in women and patients with mild to moderate, rather than severe, OSA.21
We found one other report describing the effects of REM-related OSA on adherence to CPAP use.20 Conwell et al.20 reported that adherence to CPAP use is similar in patients with REM-related and non-stage-specific OSA. Our study differs from that of Conwell et al.20 with respect to the period between CPAP therapy initiation and assessment. In our study, the CPAP usage data were analyzed 6 months after CPAP treatment initiation, whereas Conwell et al.20 analyzed data from the first 30 days following the initiation of therapy. In addition, the two studies used different definitions for REM-related OSA. We used definition (i), described above, while Conwell et al.20 used definition (ii). Furthermore, we confirmed that the five patients who fulfilled definition (ii) in our study sample did not have good adherence to CPAP treatment, indicating that our results would not change if we had used definition (ii). Finally, Conwell et al.20 did not provide a definition for adherence in their report.
Disease severity, as measured using the AHI, is associated with adherence to CPAP use.19,30 In our study, the AHI was significantly higher in the good adherence group than in the dropout group, which is consistent with previous reports. In addition, we found that the AHI during NREM sleep was significantly higher in the good adherence group than in the dropout group. The AHI during REM sleep was not significantly different between the two groups.
Upper airway collapse can occur during both REM and NREM sleep; however, upper airway muscle tone is severely suppressed by the withdrawal of excitatory noradrenergic and serotonergic inputs to upper airway motor neurons during REM sleep. This increases the tendency for upper airway collapse.31 As a result, patients with OSA, regardless of whether it is REM-related, have more respiratory events with greater severity during REM sleep.32 This may be why we observed no significant difference in the AHI during REM sleep between the good adherence and dropout groups. In addition, REM sleep comprises approximately 20% of total sleep in adults.22 This means that NREM sleep comprises approximately 80% of sleep time. Therefore, we hypothesized that disease severity during NREM sleep is significantly associated with adherence to CPAP use. Patients with REM-related OSA and thus a lower disease severity during a large proportion of sleep time have difficulty with adherence. In addition, it is possible that fluctuations in CPAP pressure caused by a disproportionate distribution of respiratory events during sleep may be the result of arousal from sleep, which may affect CPAP adherence. Similarly, patients with non-stage-specific OSA who have uniform disease severity during a large proportion of sleep are more likely to show good adherence. In our study, REM-related OSA was the only factor independently associated with dropout from CPAP therapy. Moreover, no patients in the good adherence group had REM-related OSA. These results support the above hypothesis.
Su et al.33 reported that general functional outcomes show greater improvements after CPAP therapy in patients with REM-related OSA than in patients with OSA. Therefore, it is important to treat patients with REM-related OSA using CPAP therapy.
However, some patients who drop out from CPAP therapy may also drop out from sleep therapy. It is important to predict adherence to therapy prior to the initiation of CPAP treatment because patterns of CPAP use are established early during the treatment period.15 We can use the ratio of the AHI during REM sleep to NREM sleep to predict which patients will drop out from treatment prior to the initiation of CPAP therapy. These patients are likely to require other interventions or treatment options in addition to CPAP therapy, including the use of auto bilevel pressure-relief positive airway pressure or other oral appliances.34,35 Using the above approach, we will be able to use patient-specific intensive interventions and decrease the rate of dropout from sleep therapy.
Our study has several limitations. First, selection bias was present because this study was conducted in a single facility. Second, a single type of CPAP machine was used by all patients in this study (REMstar M Series Auto CPAP) to eliminate differences arising from the use of different algorithms in CPAP machines; this also may have led to selection bias. Third, the effects of unknown confounding factors were not excluded because this was a retrospective study. Multicenter studies using other CPAP machines are required to confirm the external validity of our study results.
In conclusion, REM-related OSA is a reliable risk factor for dropout from CPAP therapy. The ratio of the AHI during REM sleep to NREM sleep will enable us to identify patients requiring intervention treatment options other than CPAP therapy prior to the initiation of treatment.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing and Publication Support.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Olson LG, King MT, Hensley MJ, et al. A community study of snoring and sleep-disordered breathing prevalence. Am J Respir Crit Care Med 1995; 152: 711–716. [DOI] [PubMed] [Google Scholar]
- 2.Hoch CC, Reynolds CF, 3rd, Monk TH, et al. Comparison of sleep-disordered breathing among healthy elderly in the seventh, eighth, and ninth decades of life. Sleep 1990; 13: 502–511. [DOI] [PubMed] [Google Scholar]
- 3.Kripke DF, Ancoli-Israel S, Klauber MR, et al. Prevalence of sleep-disordered breathing in ages 40-64 years: a population-based survey. Sleep 1997; 20: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000; 283: 1829–1836. [DOI] [PubMed] [Google Scholar]
- 5.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001; 163: 19–25. [DOI] [PubMed] [Google Scholar]
- 6.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010; 182: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tregear S, Reston J, Schoelles K, et al. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med 2009; 5: 573–581. [PMC free article] [PubMed] [Google Scholar]
- 8.Monasterio C, Vidal S, Duran J, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea–hypopnea syndrome. Am J Resp Crit Care Med 2001; 164: 939–943. [DOI] [PubMed] [Google Scholar]
- 9.Krieger J, Grucker D, Sforza E, et al. Left ventricular ejection fraction in obstructive sleep apnea. Effects of long-term treatment with nasal continuous positive airway pressure. Chest 1991; 100: 917–921. [DOI] [PubMed] [Google Scholar]
- 10.Chin K, Shimizu K, Nakamura T, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation 1999; 100: 706–712. [DOI] [PubMed] [Google Scholar]
- 11.Durán-Cantolla J, Aizpuru F, Martínez-Null C, et al. Obstructive sleep apnea-hypopnea and systemic hypertension. Sleep Med Rev 2009; 13: 323–331. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez AI, Martínez P, Miró E, et al. CPAP and behavioral therapies in patients with obstructive sleep apnea: effects on daytime sleepiness, mood, and cognitive function. Sleep Med Rev 2009; 13: 223–233. [DOI] [PubMed] [Google Scholar]
- 13.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep 1997; 20: 278. [DOI] [PubMed] [Google Scholar]
- 14.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 2008; 5: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep 2007; 30: 320–324. [PubMed] [Google Scholar]
- 16.Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnea: implications for future interventions. Indian J Med Res 2010; 131: 245–258. [PMC free article] [PubMed] [Google Scholar]
- 17.McArdle N, Devereux G, Heidarnejad H, et al. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999; 159: 1108–1114. [DOI] [PubMed] [Google Scholar]
- 18.Sin DD, Mayers I, Man GC, et al. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest 2002; 121: 430–435. [DOI] [PubMed] [Google Scholar]
- 19.Meurice JC, Marc I, Series F, et al. Efficacy of auto-CPAP in the treatment of obstructive sleep apnea/hypopnea syndrome. Am J Respir Care Med 1996; 153: 794–798. [DOI] [PubMed] [Google Scholar]
- 20.Conwell W, Patel B, Doeing D, et al. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath 2012: 519–526. [DOI] [PubMed] [Google Scholar]
- 21.Haba-Rubio J, Janssens JP, Rochat T, et al. Rapid eye movement related disordered breathing: clinical and polysomnographic features. Chest. 2005; 128: 3350–3357. [DOI] [PubMed] [Google Scholar]
- 22.Carskadon MA, Rechtschaffen A. Monitoring and staging human sleep. In: Kryger MH, Roth TT, Dement WC, (eds). Principles and Practice of Sleep Medicine, 4th ed. Philadelphia, PA: Elsevier Science Health Science Division; 2005, pp. 1359-1377.
- 23.Yetkin O, Kunter E, Gunen H. CPAP compliance in patients with obstructive sleep apnea syndrome. Sleep breath 2008; 12: 365–367. [DOI] [PubMed] [Google Scholar]
- 24.Corlateanu A, Pylchenko S, Sircu V, et al. Predictors of daytime sleepiness in patients with obstructive sleep apnea. Pneumologia 2015; 64: 21–25. [PubMed] [Google Scholar]
- 25.Rechtschaffen A, Kales A, eds. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles, CA: BI/BR, 1968. [DOI] [PubMed]
- 26.Iber C, Ancoli-Israel S, Chesson A, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed Westchester, IL: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 27.O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 2000; 161: 1465–1472. [DOI] [PubMed] [Google Scholar]
- 28.Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993; 80: 27–38. [Google Scholar]
- 29.Koo BB, Patel SR, Strohl K, et al. Rapid eye movement related sleep-disordered breathing: influence of age and gender. Chest 2008; 134: 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gay P, Weaver T, Loube D, et al. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep 2006; 29: 381–401. [DOI] [PubMed] [Google Scholar]
- 31.Mokhlesi B, Punjabi NM. “REM-related” obstructive sleep apnea: an epiphenomenon or a clinically important entity? Sleep 2012; 35: 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koo BB, Dostal J, Ioachimescu O, et al. The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath 2008; 12: 259–264. [DOI] [PubMed] [Google Scholar]
- 33.Su CS, Liu KT, Panjapornpon K, et al. Functional outcomes in patients with REM-related obstructive sleep apnea treated with positive airway pressure therapy. J Clin Sleep Med 2012; 8: 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients–a pilot study. Sleep Breath 2012; 16: 773–779. [DOI] [PubMed] [Google Scholar]
- 35.Doff MH, Hoekema A, Wijkstra PJ, et al. Oral appliance versus continuous positive airway pressure in obstructive sleep apnea syndrome: a 2-year follow-up. Sleep 2013; 36: 1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]


