Short abstract
We present a case of a 69-year-old woman with rheumatoid arthritis. The patient’s condition was managed with steroid therapy for more than 12 years. She had a coexisting infected chronic ulceration in the left leg, which was treated with negative pressure wound therapy for 52 days. Use of this therapy within the wound reduced exudate and the bacterial count, which dramatically accelerated the process of wound healing.
Keywords: Negative pressure wound therapy, chronic leg wound, rheumatoid arthritis, bacterial count, infection, steroid
Introduction
The introduction of negative pressure wound therapy (NPWT) in the early 1990s resulted in a change in the concept of care and treatment of wounds of various aetiologies in the hospital and outpatient care settings. NPWT is increasingly being used in primary and home health care because of its non-invasiveness, high efficacy, shortening the time of wound healing, and improving wound healing, thereby greatly reducing the need for hospital treatment.1–3 NPWT requires specialized equipment with manual control of negative pressure. Negative pressure is maintained in the wound bed with ready-made sterile sets (sponge, proper dressing, adapter, polyurethane foil). Negative pressure causes wound shrinkage and reduction of the bacterial count by evacuating the effusion into a disposable canister placed on the device.4,5 Although the potential of NPWT is promising and clinically used worldwide, economic benefits remain controversial.6 We report here a unique case of a patient with rheumatoid arthritis (RA) who had a progressive leg wound that healed in a short time. In this case, there were many aggravating factors, such as the patient’s older age, steroid therapy, immunological disease, a large area of skin damage around the wound, and infection of the wound.
Case report
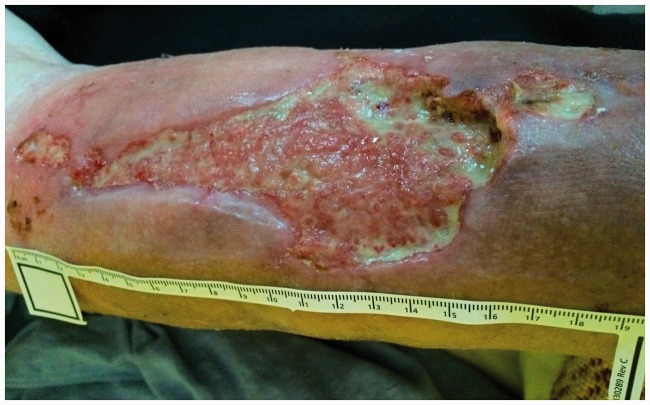
A woman aged 69 years had a history of RA for 12 years. She was treated with steroids (Metypred), was partially self-efficacious (score of 40 in the Barthel scale), and had a progressive wound with necrosis of the latero-posterior surface in the left lower leg for 8 weeks. The wound was caused by a contusion of this area with resulting tissue infection and necrosis. Initially, the wound was cleaned and dressed under hospital conditions. During the first examination that was performed at the patient's home, a wound of a mixed character was observed (arteriovenous). The wound was stage II/III according to the European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel guidelines. The wound also had a yellowish surface, was a size of 2–6 × 20 cm (approximately 180 cm2), was fibrous, and it had pronounced effusion with features of infection (tenderness, undermining, damage to the skin around the wound) (Figure 1). The patient’s lower limbs were covered with parchment-like skin with discolouration and local ecchymosis. Persistent oedema was found around the ankles. The serum albumin level was 3.62 g/dL, haematocrit was 39.0%, the red blood cell count was 4.2 × 10−6/mm3, haemoglobin was 14.3 g/L, and the C-reactive protein level was 79 mg/L. Superficial mechanical cleansing with surgical instruments was performed, and swabs from several areas of the wound were taken. The results of wound swabs showed cultures of Klebsiella oxytoca (++++growth) and Proteus mirabilis (++++).
Figure 1.
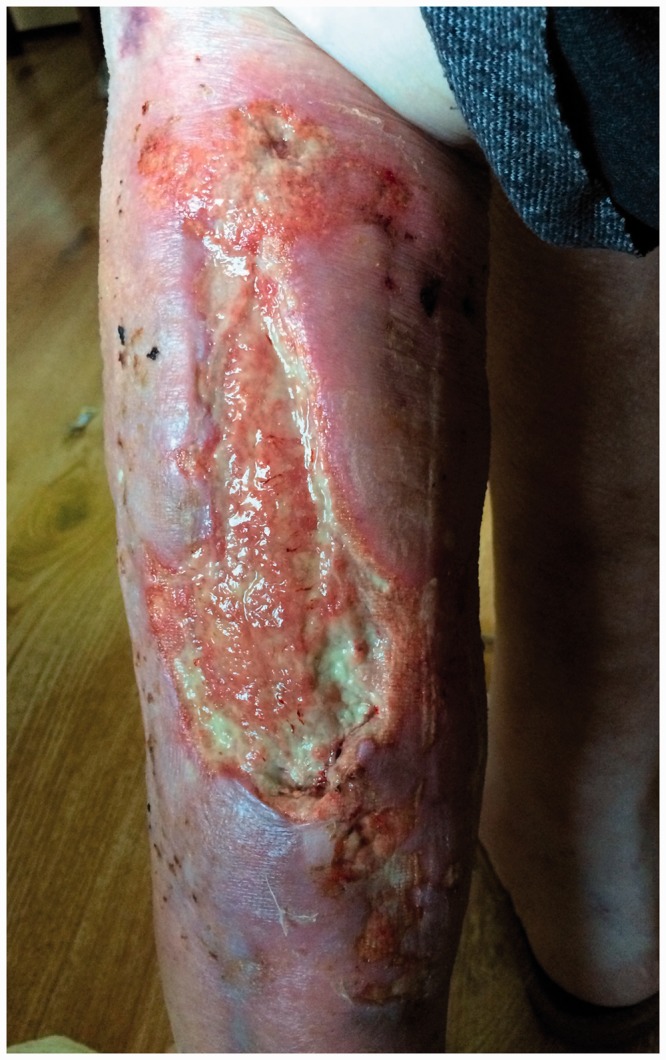
Photograph of the wound on the day of the first examination (03/09/2016).
Targeted therapy was implemented according to the antibiotics indicated in an antibiogram (amoxycycline 2 × 1 g, ciprofloksacin 2 × 0.5 g, orally). After 10 days of antibiotic therapy, NPWT was performed using an ActiV.A.C. Therapy Unit (KCI, San Antonio, TX, USA). NPWT was started from constant pressure (continuous mode) of 120 mmHg for 14 days (Figure 2) followed by 100 mmHg for 21 days. This pressure was reduced to 70 mmHg on the 36th day of therapy by changing the pressure mode from continuous to intermittent. Negative pressure dressings were dressed by a person in charge of the therapy at intervals of 3 to 5 days. At the beginning of the therapy, 100 to 120 mL of exudate was observed per day. In the last week of therapy, the exudate did not exceed 20 mL per day. To reduce the risk of polyurethane sponge ingrowth in the wound, Atrauman Ag (Paul Hartmann, Pabianice, Lodzkie, Poland) mesh dressings and Aquacel Ag Hydrofiber Dressings (ConvaTec, Princeton, NJ, USA) were used. These dressings were also intended to reduce the bacterial count within the wound. No damage to granulation with the mesh dressing was observed.
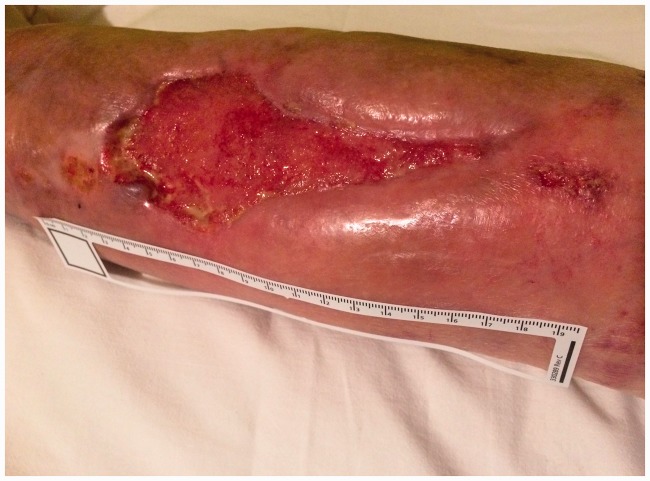
Figure 2.
Photograph of the wound on the day of NPTW implementation (24/09/2016).
During NPWT therapy, the patient’s condition was stable and there were no signs of systemic infection, with a body temperature of 36.7°C to 36.9°C. The patient's locomotor capacity was increased. No local complications were observed and the wound healing process was satisfactory. However, there were concerns that the polyurethane film would damage and delaminate the delicate epidermis (Figures 3 and 4). During the therapy, a control swab from the wound was collected twice, and it showed Enterococcus fecalis bacteria with a growth level of ++. No general treatment was implemented because of the low bacterial count and satisfactory healing and epidermal processes. This form of treatment was discontinued on day 52 of treatment because of a small area of wounds and scarce effusion. During this period, the wound decreased in size to 4 × 6 cm (24 cm2) (Figure 5). Treatment with specialized dressings was then performed, with hydrofibres, hydrogels, and hydrocolloids as secondary dressings. We observed that after discontinuation of NPWT, the healing process greatly slowed, despite the fact that no infection was found in the wound. The wound treatment process was completed in May 2017.
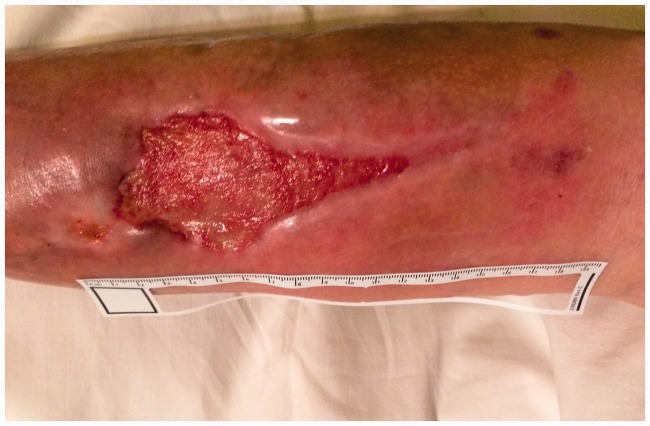
Figure 3.
Photograph of the wound on the 8th day of therapy (14/10/2016).
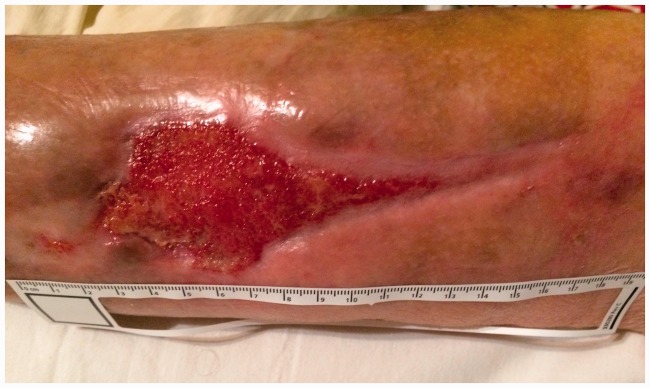
Figure 4.
Photograph of the wound on the 20th day of therapy (02/11/2016).
Figure 5.
Photograph of the wound on the 52nd day of therapy (15/11/2016).
The Bioethics Committee at the University of Rzeszów approved the study, which was carried out in compliance with the Declaration of Helsinki (No. 6/09/2016). The patient, whose identity has been protected, provided permission to publish the features of her case.
Discussion
Complementary care of a patient with a wound should be based on rational selection of methods and measures that are clinically justified based on the tissue debridement, infection or inflammation, moisture balance, and edge system and recommendations of scientific societies. NPWT has become a widely accepted method of supporting treatment of open wounds of different aetiologies. The mechanism of action of negative pressure within the wound reduces exudate, decreases the bacterial count, and increases blood flow in the wound, which accelerates migration of fibroblasts.7–10
NPWT in home care conditions in Poland is rarely used because of the high cost, difficult accessibility, and low level of knowledge about the method among nurses. Nevertheless, there have been an increasing number of reports on individual cases of using this method in non-hospital care in the literature. Ulcerations of the lower limbs are a common problem in patients with RA. Approximately 10% of patients experience chronic skin lesions in the lower limbs.11 The most common causes of these lesions are venous insufficiency, arterial failure, and immunological disorders.12 Our case of an ulcerated wound in the lower leg was ineffectively treated for 8 weeks with special dressings (hydrofibres) and antiseptics without the expected therapeutic effect. The patient was not eligible for plastic management because of an autoimmunological condition and was administered medication. Therefore, this led to the decision to use NPWT in the wound.
We would like to emphasize that caution is required for using NPWT in infected wounds because of the limited possibility of wound environment control. Despite evacuation of secretion from the wound, there is a risk of infection progressing.6 During the entire duration of NPWT, our patient and other occupants of her home were educated on basic kinesitherapy and preventive measures to minimize infection in the wound during wound decontamination and disinfection or damage to the epidermis while removing the kit. Similar results to those in our patient were obtained by Moues et al.13 in NPWT of a group of 54 patients. These authors showed that better wound healing was associated with elimination of exudate and increased granulation, which resulted in a shorter wound healing time. The healing process was not disturbed by bacteria colonizing the wound. Complications were only observed in two patients. These complications were development of sepsis and tissue necrosis, which resulted in discontinuation of NPWT in these patients. A retrospective analysis by Driver et al.14 showed that, in patients with diabetic foot ulcers, the cost of total wound closure for 1 cm2 was 1227 USD for NPWT and 1695 USD for specialist dressings. This finding indicated greater cost-effectiveness in the NPWT-treated group. In Poland, treatment with NPWT is still expensive, and the estimated cost of 1 day of therapy is 12 to 15 USD.
Findings in our case of NPWT in a patient with a chronic lower leg wound with coexisting RA indicate that this form of therapy is safe and that the risk of complications is negligible. Any complications can be successfully managed by trained medical staff in the non-hospital setting.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was financed by the University of Rzeszów and performed within the project “Centre for Innovative Research in Medical and Natural Sciences.” This project was co-financed within the Regional Operational Programme for the Podkarpackie Province for 2007–2013 (contract number: UDA-RPPK.01.03.00-18-004/12-00). This study was also financed from a subsidy designated for statutory research at the University of Rzeszów, and was granted by the Ministry of Health for 2016/2017.
References
- 1.Argenta LC, Morykwas MJ, Marks MW, et al. Vacuumassisted closure: state of clinic art. Plast Reconstr Surg 2006; 117: 127–142. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki T, Minehara A, a Matsuura T, et al. Negative-pressure wound therapy over surgically closed wounds in open fractures. J Orthop Surg 2014; 22: 30–34. [DOI] [PubMed] [Google Scholar]
- 3.Miller C. The History of Negative Pressure Wound Therapy (NPWT): From ‘‘Lip Service’’ to the Modern Vacuum System. J Am Coll Clin Wound Spec 2014; 4: 61–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997; 38: 563–577. [PubMed] [Google Scholar]
- 5.Wolvos T. The use of negative pressure wound therapy with an automated, volumetric fluid administration: an advancement in wound care. Wounds 2013; 25: 75–83. [PubMed] [Google Scholar]
- 6.Apleqvist J, Willy C, Fagerdahl AM, et al. EWMA document: negative pressure wound therapy. J Wound Care 2017; 26: 1–154. [DOI] [PubMed] [Google Scholar]
- 7.Phillips PL, Yang Q, Schultz GS. The effect of negative pressure wound therapy with periodic instillation using antimicrobial solutions on Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J 2013; 10: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kairinos N, Solomons M, Hudson DA. Negative-pressure wound therapy I: the paradox of negative-pressure wound therapy. Plast Reconstr Surg 2009; 123: 589–598. [DOI] [PubMed] [Google Scholar]
- 9.Chen D, Zhao Y, Li Z, et al. Circulating fibrocyte mobilization in negative pressure wound therapy. J Cell Mol Med 2017; 21: 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li PY, Yang D, Liu D, et al. Reducing surgical site infection with negative-pressure wound therapy after open abdominal surgery: a prospective randomized controlled study. Scand J Surg 2017; 106: 189–195. [DOI] [PubMed] [Google Scholar]
- 11.Hafner J, Schneider E, Burg G, et al. Management of leg ulcers in patients with rheumatoid arthritis or systemic sclerosis: the importance of concomitant arterial and venous disease. J Vasc Surg 2001; 32: 322–329. [DOI] [PubMed] [Google Scholar]
- 12.Shanmugam VK, Schilling A, Germinario A, et al. Prevalence of immune disease in patients with wounds presenting to a tertiary wound healing centre. Int Wound J 2012; 9: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moues CM, van den Bemd GJ, Heule F, et al. Comparing conventional gauze therapy to vacuum-assisted closure wound therapy: a prospective randomised trial. J Plast Reconstr Aesthet Surg 2007; 60: 672–681. [DOI] [PubMed] [Google Scholar]
- 14.Driver VR, Blume PA. Evaluation of wound care and health-care use costs in patients with diabetic foot ulcers treated with negative pressure wound therapy versus advanced moist wound therapy. J Am Podiatr Med Assoc 2014; 104: 147–153. [DOI] [PubMed] [Google Scholar]