Abstract
Objective
To investigate the vasodilative and endothelial-protective effects and the underlying mechanisms of total flavonoids from Astragalus (TFA).
Methods
The vasodilative activities of TFA were measured with a myograph ex vivo using rat superior mesenteric arterial rings. The primary human umbilical vein endothelial cell (HUVEC) viabilities were assayed using the cell counting kit-8 after hypoxia or normoxia treatment with or without TFA. Akt, P-Akt, eNOS, P-eNOS, Erk, P-Erk, Bcl-2 and Bax expression were analyzed using western blotting.
Results
TFA showed concentration-dependent vasodilative effects on rat superior mesenteric arterial rings, but had no effects on normal or potassium chloride precontracted arterial rings. TFA did not affect HUVEC viabilities in normoxia, but dramatically promoted cell proliferation in the concentration range of 1 to 30 µg/mL under hypoxia. Moreover, TFA significantly increased the ratios of P-Akt/Akt and P-eNOS/eNOS in vascular endothelial cells under hypoxic conditions, but did not change the P-Erk/Erk or Bcl-2/Bax ratios.
Conclusions
TFA might exhibit vasorelaxant and endothelial-protective effects via the Akt/eNOS signaling pathway.
Keywords: Total flavonoids from Astragalus, vasodilation, endothelial protection, superior mesenteric artery, Akt/eNOS signaling pathway, hypoxia
Introduction
Coronary heart disease (CHD) is the leading cause of morbidity and mortality in patients worldwide.1 Endothelial dysfunction responds to low myocardial oxygen (O2) levels, fatty deposits, calcium deposits, and abnormal inflammatory cells, and plaque build-up inside the coronary arteries decreases blood flow and eventually leads to unstable angina, myocardial infarction and heart failure.2 Therapeutic strategies such as angioplasty and bypass surgery are often limited to palliative interventions.3 Recent research indicates that the endothelium plays a crucial role in vascular reactivity by releasing vasoactive factors, such as nitric oxide (NO).4,5 Therefore, the optimal treatment strategy may be to restore the endothelium function and increase dilation.
In traditional Chinese medicine, Astragalus membranaceus supplements Qi and is prescribed for general debility and chronic illnesses.6,7 The herb is widely used to treat CHD in China.8 Total flavonoids from Astragalus (TFA) are some of the main active components of the herb and have been associated with several biological activities, such as antitumor,9 anti-inflammatory10 and antioxidant effects.11 However, there are few reports on the vasodilatory action and endothelial effects of Astragalus flavonoids.
The aims of this study were to identify the vasodilative and endothelial-protective effects of TFA with a series of ex vivo and in vitro studies. We hoped to clarify the potential mechanisms of TFA on endothelial dysfunction and contribute to the further development of drugs for CHD treatment.
Materials and methods
Materials and cell line
TFA extract was obtained from Shaanxi Sciphar Natural Products Co., Ltd. (Xi’an, China); its purity was 81.2% according to UV detection. The TFA extract used for cell experiments was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA); the final concentration of DMSO did not exceed 0.1% (v/v). Antibodies for Akt, P-Akt, Erk, P-Erk, Bcl-2 and Bax were purchased from Cell Signaling Technology® (Danvers, MA, USA). Antibodies for eNOS and P-eNOS were purchased from Abcam (Cambridge, MA, USA). Antibody for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was obtained from Kangchen (Shanghai, China).
Primary human umbilical vein endothelial cells (HUVECs) were a gift from Dr Yi Wang (Zhejiang University, Zhejiang, China).
Animals and tissue preparation
All experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the Animal Care and Use Committee of Zhejiang Chinese Medical University.
Superior mesenteric arterial rings were prepared using a previously reported method with modifications.12 Male Sprague–Dawley rats (220 g ± 20 g, Silaike Co., Shanghai, China) were anesthetized by isoflurane inhalation. The main branches of the superior mesenteric arteries were excised promptly and placed in precooled oxygenated (95% O2, 5% carbon dioxide (CO2)) physiological saline solution (PSS). The arteries were freed from adhering tissues, such as fat and veins, under a dissecting microscope and cut into cylindrical rings (2 mm in length) for tension measurements.
Tension measurements
The tension of the superior mesenteric arterial rings was measured using a previously described method with modifications.12–14 The superior mesenteric arterial rings were immersed in individual temperature-controlled (37℃) bath chambers containing 5 mL PSS in a multiwire myograph system (model 620, Danish Myo Tech, Aarhus, Denmark) for isometric tension recording. During the 60 min equilibration period, the PSS was constantly aerated with gas (95% O2, 5% CO2) at 37℃ and refreshed per 20 min. The arterial rings were then sequentially exposed to 60 mM KCl PSS (KPSS, 5 mL, 3 min) and PSS washing (5 mL, 5 min) twice for vascular reactivity recovery. The 60 mM KPSS solution was prepared by replacing sodium chloride with KCl in PSS to reach a final potassium concentration of 60 mM. Then the arterial rings were precontracted by adding 5 µL 1 mM phenylephrine to the chamber. After the contraction was stabilized, 5 µL 10 mM acetylcholine was applied to relax the arterial rings for 2 min. Only arterial rings with 80% relaxation rate were used in further experiments.
The vascular reactivity effect of TFA was studied by treating normal arterial rings, KCl (60 mM) precontracted arterial rings, phenylephrine (0.02 mM) precontracted arterial rings and phenylephrine (0.02 mM) precontracted arterial rings in the presence of N(ω)-nitro-L-arginine methyl ester (L-NAME) (0.1 mM, pretreated 20 min) with a series concentration (0.1, 1, 10, 20, 40, 100, 200, 300, 400 and 500 mg/L).
Cell culture
HUVECs were cultured in gelatin-coated flasks in Dulbecco’s modified Eagle’s medium (Gibco BRL, Life Technologies Inc., Gaithersburg, MD, USA) supplemented with 20% fetal bovine serum (Gibco BRL, Life Technologies Inc., Gaithersburg, MD, USA), 50 U/mL heparin (Shanghai No. 1 Biochemical Pharmaceutical Co, Shanghai, China), 100 U/mL penicillin (Zhongguo Pharmaceutical (Shijiazhuang) Co, Shijiazhuang, China), and 100 mg/ml streptomycin (Northern China Pharmaceutical Co, Shijiazhuang, China) (pen/strep). The HUVECs were used at fewer than eight passages in this study.
Cell viability analysis
Cell viability was assessed using the cell counting kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan). HUVECs were seeded in collagen-coated 96-well plates at 2 × 104 cells per well. Three repeated wells were set in each group. The cells in the hypoxia and serum deprivation environment were treated with TFA at various concentrations (1, 2.5, 5, 10, 15, 20, 30 and 40 µg/mL) or DMSO (hypoxia control) and placed in a controlled-environment chamber (Thermo Fisher Scientific Inc., Rockford, IL, USA) aerated with 1% O2/5% CO2/94% nitrogen at 37℃ for 24 h. Cells in the normal environment were treated with TFA at the same concentrations as hypoxia or DMSO (normal control) under standard culture conditions at 37℃ for 24 h. At the end of the experiment, CCK-8 (10 µL) was added to each well and incubation was performed at 37℃ for 2 h. The optical density of each well was measured at 450 nm with a universal microplate spectrophotometer (BioTek, Winooski, VT, USA).
Western blot
Each well, with 3 × 105 cells/well, was treated with 15 µg/mL TFA or DMSO for 24 h under hypoxic and serum deprivation or normal conditions. Cells were washed twice with cold phosphate-buffered saline and lysed in 2.5 × SDS gel loading buffer (30 mM Tris-HCl (pH 6.8), 1% SDS, 2.5% mercaptoethanol, 12.5% glycerol and 0.05% bromophenol blue) followed by boiling for 30 min. After centrifuging at 12000 × g for 5 min at 4℃, equal amounts of cell lysate supernatants were separated by 8% or 12% SDS polyacrylamide gels and the proteins were electrotransferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were physically cut and incubated with the indicated primary antibodies: Akt (1:1000), P-Akt (1:1000), eNOS (1:500), P-eNOS (1:500), Erk (1:1000), P-Erk (1:1000), Bcl-2 (1:1000), Bax (1:1000) and GAPDH (1:3000). Proteins were visualized using horseradish peroxidase-conjugated secondary antibody (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and chemiluminescence ECL western-blotting system (Millipore, Billerica, MA, USA).
Statistical analysis
All data were expressed as mean ± SD. One-way analysis of variance was performed using Minitab 14 (Minitab, Inc., State College, PA, USA). Values with P < 0.05 were considered statistically significant.
Results
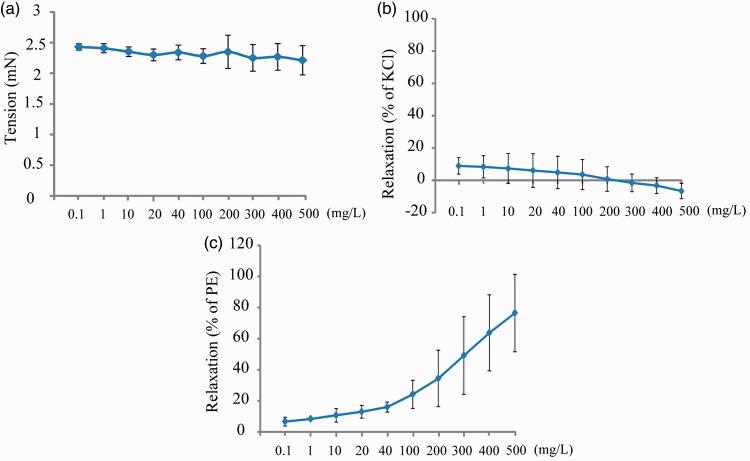
To evaluate the vascular effects of TFA, the superior mesenteric arterial rings of rats were pretreated with KCl (60 mM) or phenylephrine (0.02 mM), and various concentrations of TFA were added to the bath chamber when the plateau was achieved. The results showed that TFA had no vasodilative effect on normal or KCl precontracted arterial rings (Figure 1(a) and 1(b)), but showed a concentration-dependent relaxation of phenylephrine contracted arterial rings (Figure 1(c)).
Figure 1.
Vasodilation effects of total flavonoids from Astragalus on normal superior mesenteric arterial rings (a), precontracted with (b) KCl (60 mM) or (c) phenylephrine (PE, 0.02 mM). Data shown are mean ± SD (n = 3).
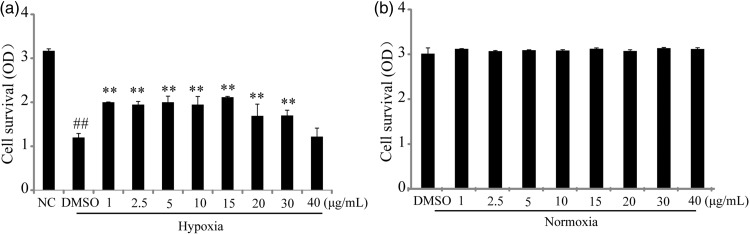
To access the vascular endothelium protective effect, series ranges of 1–40 µg/mL of TFA were applied to HUVECs under hypoxia or normoxic conditions. The results indicated that TFA significantly stimulated cell proliferation at the range of 1 to 30 µg/mL under hypoxic conditions and 15 µg/mL TFA demonstrated the optimal effect (Figure 2(a)). We chose 15 µg/mL TFA for treatment in the western blot assay. Under normoxic conditions, there were no changes among these groups (Figure 2(b)).
Figure 2.
Proliferation effects of total flavonoids from Astragalus (TFA) on human umbilical vein endothelial cells under hypoxia and serum deprivation or normoxia. (a) Cells were incubated in various concentrations of TFA under hypoxia and serum deprivation for 24 h. (b) Cells were incubated in various concentrations of TFA under normoxia for 24 h. Cell growth was determined by the cell counting kit-8 assay. Data shown are mean ± SD (n = 3). ##P < 0.01 compared with normoxia control (NC); **P < 0.01 compared with hypoxia control (HC).
DMSO: dimethyl sulfoxide; OD: optical density.
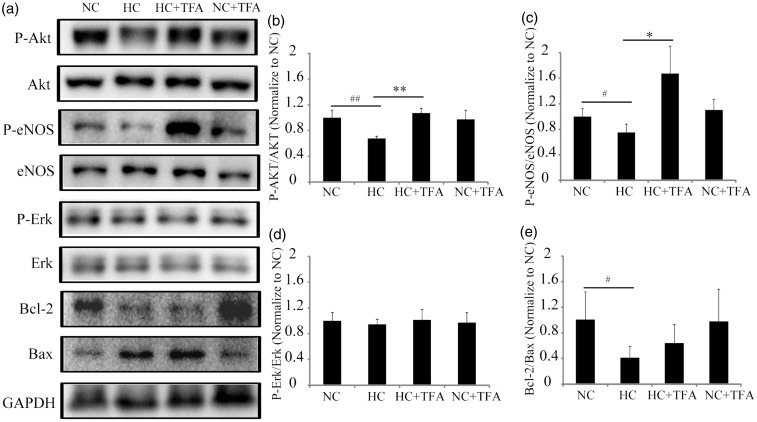
To determine whether the Akt/eNOS, Bcl-2/Bax or Erk pathways influenced the TFA effects, western blot assays were used to detect the expression of indicated proteins. The ratios of P-Akt/Akt and P-eNOS/eNOS significantly decreased when HUVECs were under hypoxia, but dramatically increased in the TFA-treated hypoxic HUVECs group (Figure 3(a), 3(b) and 3(c)). No changes were detected in the P-Erk/Erk ratio when HUVECs were under hypoxia with or without TFA (Figure 3(a) and 3(d)). The Bcl-2/Bax ratio was significantly downregulated in HUVECs after hypoxia for 24 h, whereas no obvious changes were discovered in the TFA treatment group compared with the hypoxia group (Figure 3(a) and 3(e)). TFA changed none of the above ratios in the normoxia control group (Figure 3(a), 3(b), 3(c), 3(d) and 3(e)). These findings suggested that the Akt/eNOS pathway participated in the protection of vascular endothelial cells by TFA under hypoxic conditions.
Figure 3.
Total flavonoids from Astragalus (TFA) affected the expression of cell survival and vasoactive proteins in human umbilical vein endothelial cells under hypoxia and serum deprivation or normoxia. The four groups were as follows: normoxia control group (NC), hypoxia control group (HC), normoxia treated with TFA group (NC + TFA), hypoxia treated with TFA group (HC + TFA). (a) Representative western blots showing cell survival and vasoactive proteins. Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control; (b-e) Quantification of P-Akt/Akt, P-eNOS/eNOS, P-Erk/Erk and Bcl-2/Bax ratios by western blot. Data are expressed as mean ± SD (n = 3). #P < 0.05 compared with NC; ##P < 0.01 compared with NC; *P < 0.05 compared with HC; **P < 0.01 compared with HC.
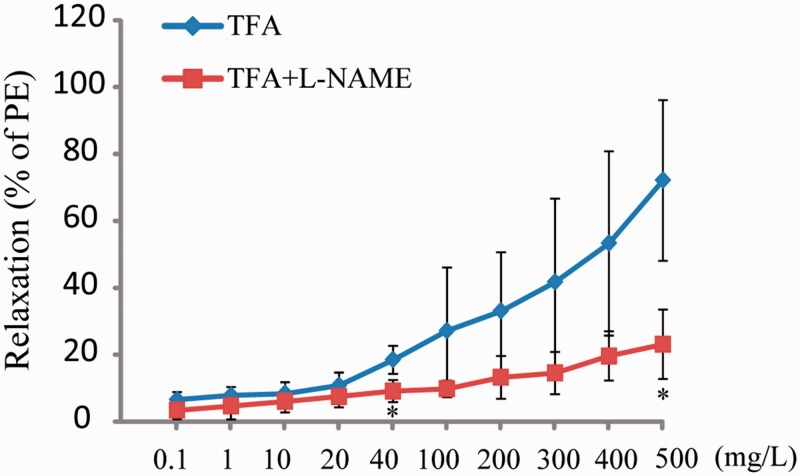
The eNOS inhibitor L-NAME (0.1 mM) was used to identify eNOS associated with the vasodilative effects of TFA. The results showed that the vasodilative activities of TFA were significantly reduced in the presence of L-NAME (Figure 4).
Figure 4.
Vasodilation effects of total flavonoids from Astragalus (TFA) on superior mesenteric arterial rings precontracted with phenylephrine (0.02 mM) in the presence of eNOS inhibitor L-NAME (0.1 mM). Data shown are mean ± SD (n = 3). *P < 0.05 compared with TFA. L-NAME: N(ω)-nitro-L-arginine methyl ester: PE: phenylephrine.
Discussion
In this study, we described the key role of TFA in improvement of endothelial dysfunction. Our results provide evidence of a pathway in which TFA activates phosphorylation of Akt and then acts on phosphorylation of eNOS to promote NO production. Thus, our results indicate that the Akt/eNOS pathway is involved in TFA-mediated improvements in endothelial cell survival and augmentation of vasodilator effects.
In normal coronary circulation, multiple factors act together to regulate blood flow in response to myocardial O2 requirements. To adjust blood flow, endothelial cells and smooth muscle cells jointly control contractility.14 Research indicates that NO production contributes to the endothelial-mediated dilation of the aorta.15 Atherosclerosis or other risk factors cause endothelial dysfunctions, such as increased vascular contractility and decreased dilation, which are the result of increased production of oxygen-free radicals that consume NO, decrease tissue O2 levels and impair endothelial cells. In the current study, we found that TFA significantly stimulated cell proliferation under hypoxic conditions, but had no influence under normoxic conditions, indicating the endothelial-protective effect of TFA under hypoxia. The Erk pathway regulates endothelial cell proliferation and antiapoptosis by up-regulating Bcl-2 expression and down-regulating Bax,16,17 but we did not observe any differences between the hypoxia TFA treatment group and the hypoxia control group. The PI3K/Akt signaling pathway is activated during heart failure.18 It regulates the eNOS pathway to control the balance between cell survival and apoptosis.19,20 Our results further confirmed that the Akt/eNOS pathway participates in the endothelial-protective effects of TFA under hypoxic conditions.
TFA showed a dose-dependent relaxation of the phenylephrine contracted mesenteric artery but had no vasodilative effects on normal or KCl precontracted arteries. NO is an important regulator in vasodilator effects. eNOS plays a key role in inhibiting endothelial dysfunction and mediating NO synthesis. Our results further suggested that the vasodilative activities of TFA were significantly reduced in the presence of L-NAME. Our data suggest that TFA-mediated vasodilative effects occur via the eNOS pathway.
Taken together, our findings demonstrate that TFA can relax the phenylephrine contracted mesenteric artery and significantly stimulate cell proliferation under hypoxic conditions, but have no influence under normoxic conditions. Activation of the Akt/eNOS signaling pathway may be involved in the vasorelaxative and antiapoptotic effects of TFA.
Declaration of conflicting interest
The Authors declare that there is no conflict of interest.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81573641 for LZ, No. 81471889 for ZCZ), Zhejiang Provincial Natural Science Foundation (No. LY16H280003 for LZ, No. LY14H020003 for ZCZ), Fundamental Research Funds for the Central Universities (No. 2017FZA7011 for LZ), Zhejiang Provincial Program of medical sciences (No. 2013KYA142 for MFZ), Zhejiang Provincial Program for the Cultivation of High-level Innovation Health Talents (No. 2014-108 for MFZ) and Scientific Research Foundation of Zhejiang Chinese Medical University (No. 2015ZY25 for QL, No. 2015ZY26 for QYS).
References
- 1.Hwang SJ, Ballantyne CM, Sharrett AR, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation 1997; 96: 4219–4225. [DOI] [PubMed] [Google Scholar]
- 2.Shrivastava AK, Singh HV, Raizada A, et al. C-reactive protein, inflammation and coronary heart disease. Egyptian Heart Journal 2015; 67: 89–97. [Google Scholar]
- 3.Heil M, Schaper W. Insights into pathways of arteriogenesis. Curr Pharm Biotechnol 2007; 8: 35–42. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Cheang WS, Yang H, et al. Nuciferine relaxes rat mesenteric arteries through endothelium-dependent and -independent mechanisms. Br J Pharmacol 2015; 172: 5609–5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanhoutte PM, Shimokawa H, Tang EH, et al. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009; 196: 193–222. [DOI] [PubMed] [Google Scholar]
- 6.Han L, Liu N, Yang L, et al. Astragalus membranaceus extract promotes angiogenesis by inducing VEGF, CD34 and eNOS expression in rats subjected to myocardial infarction. Int J Clin Exp Med 2016; 9: 5709–5718. [Google Scholar]
- 7.Wang D, Zhuang Y, Tian Y, et al. Study of the effects of total flavonoids of Astragalus on atherosclerosis formation and potential mechanisms. Oxid Med Cell Longev 2012; 2012: 282383–282383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan KP, Guo Y, Xing Z, et al. Dan-Shen-Yin protects the heart against inflammation and oxidative stress induced by acute ischemic myocardial injury in rats. Exp Ther Med 2012; 3: 314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D, Zhuang Y, Pan J, et al. Investigation of effects and mechanisms of total flavonoids of Astragalus and calycosin on human erythroleukemia cells. Oxid Med Cell Longev 2012; 2012: 209843–209843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Sun YN, Yan XT, et al. Flavonoids from Astragalus membranaceus and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch Pharm Res 2014; 37: 186–192. [DOI] [PubMed] [Google Scholar]
- 11.Guo Z, Xu HY, Xu L, et al. In Vivo and in vitro immunomodulatory and anti-inflammatory effects of total flavonoids of astragalus. Afr J Tradit Complement Altern Med 2016; 13: 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukao M, Hattori Y, Kanno M, et al. Thapsigargin- and cyclopiazonic acid-induced endothelium-dependent hyperpolarization in rat mesenteric artery. Br J Pharmacol 1995; 115: 987–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Zhang H, Liu H, et al. Mechanisms of simvastatin-induced vasodilatation of rat superior mesenteric arteries. Biomed Rep 2016; 5: 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peixoto-Neves D, Wang Q, Leal-Cardoso JH, et al. Eugenol dilates mesenteric arteries and reduces systemic BP by activating endothelial cell TRPV4 channels. Br J Pharmacol 2015; 172: 3484–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozaeva LP, Gorodetskaya EA, Ruuge EK, et al. Beneficial effect of coenzyme Q10 injection on nitric oxide -related dilation of the rat aorta. Eur J Pharmacol 2017; 794: 15–19. [DOI] [PubMed] [Google Scholar]
- 16.Song H, Wu F, Zhang Y, et al. Irisin promotes human umbilical vein endothelial cell proliferation through the ERK signaling pathway and partly suppresses high glucose-induced apoptosis. PloS one 2014; 9: e110273–e110273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma C, Liu Y, Wang Y, et al. Hypoxia activates 15-PGDH and its metabolite 15-KETE to promote pulmonary artery endothelial cells proliferation via ERK1/2 signalling. Br J Pharmacol 2014; 171: 3352–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Z, Zhou P, von Gise A, et al. Pi3kcb links Hippo-YAP and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ Res 2015; 116: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim BR, Seo SH, Park MS, et al. sMEK1 inhibits endothelial cell proliferation by attenuating VEGFR-2-dependent-Akt/eNOS/HIF-1alpha signaling pathways. Oncotarget 2015; 6: 31830–31843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun N, Wang H, Wang L. Vaspin alleviates dysfunction of endothelial progenitor cells induced by high glucose via PI3K/Akt/eNOS pathway. Int J Clin Exp Pathol 2015; 8: 482–489. [PMC free article] [PubMed] [Google Scholar]