Short abstract
Objective
Dementia with Lewy bodies (DLB) is a common type of neurodegenerative dementia. Molecular neuroimaging using dopamine transporter (DaT), Pittsburgh compound B (PIB), and fluorodeoxyglucose (FDG) positron emission tomography (PET) has advantages in detecting dopaminergic neuron loss, abnormal amyloid β-protein deposition, and glucose metabolism changes in patients with neurodegenerative disorders. However, the multi-modality molecular imaging features of patients with DLB have rarely been reported.
Methods
Five patients with a probable diagnosis of DLB were enrolled. PET/magnetic resonance imaging was performed with three tracers: 11C-β-CFT, 11C-PIB, and 18F-FDG. Clinical and imaging characteristics were analyzed.
Results
All patients with DLB showed reduced uptake in the bilateral putamen on DaT PET, increased uptake throughout the cerebral cortex on PIB PET, and intact metabolism of the posterior cingulate gyrus on FDG PET.
Conclusion
Multimodal molecular imaging is helpful for early diagnosis of DLB. Studies with larger sample sizes are needed to confirm the molecular imaging differences between DLB and Alzheimer’s disease and Parkinson’s disease dementia.
Keywords: Molecular imaging, dementia with Lewy bodies, multi-modality, diagnosis, Alzheimer’s disease, Parkinson’s disease
Background
Dementia with Lewy bodies (DLB) is the second most common type of neurodegenerative dementia after Alzheimer’s disease (AD) and accounts for about 5% of all cases of dementia in older populations.1,2 A recent study showed that the prevalence of DLB was 1.05% in 5542 Chinese participants aged >60 years.3 DLB is often underdiagnosed because its symptoms overlap with those of AD and Parkinson’s disease (PD), and the definitive diagnosis of DLB is still dependent upon pathological evidence. A clinical diagnosis of probable and possible DLB can be established according to the consensus diagnostic criteria for DLB.4 However, the accuracy of a clinical diagnosis of DLB is poor, ranging from 34% to 65%, compared with a postmortem diagnosis.5 DLB is still a frequently misdiagnosed condition.
Molecular neuroimaging allows for the detection of abnormal molecular deposits on positron emission tomography (PET) in the early stage of neurodegenerative disease. Dopamine transporter (DaT) PET can reveal dopaminergic neuron loss in the nigrostriatal system in patients with PD and DLB. McKeith et al.6 reported that the specificity of DaT PET imaging for differentiation between DLB and AD was >90%. Pittsburgh compound B (PIB) PET can show amyloid beta (Aβ) deposits in the brain tissue of patients with DLB and AD.7 DLB is also associated with decreased metabolism in the cortex and occipital visual association area on fluorodeoxyglucose (FDG) PET.8 However, only one or two of these techniques (DaT PET, FDG PET, and PIB PET) have been performed separately to observe the differences between patients with DLB and AD or PD dementia (PDD). The molecular imaging features of patients with DLB using three tracers have rarely been reported. Therefore, we used DaT PET, PIB PET, and FDG PET to explore the multi-modality molecular imaging characteristics of patients with probable DLB.
Material and methods
Patients
Patients suspected to have DLB in the Department of Geriatric Neurology of the General Hospital of PLA from February 2014 to June 2016 were included in this study. All patients underwent routine blood testing, thyroid function testing, folic acid and vitamin B12 measurement, and biochemical testing. The diagnosis of probable DLB was based on the 3rd Consortium Criteria.4 The diagnosis was determined by a consensus opinion of two neurologists. Patients with known neurologic diseases such as epilepsy, brain tumors, or substance abuse that may impact cognitive function were excluded. The patients’ clinical information was obtained by personal interview, and a physical examination was performed by a staff member. Written informed consent was obtained from all patients. We had no access to information that could identify individual patients during or after the data collection. The study design was approved by the Ethics Committee of PLA General Hospital and performed in accordance with the Declaration of Helsinki.
Data collection
A medical history was obtained from each patient, and a physical examination was performed by one neurologist. The severity of parkinsonism was assessed using the Unified Parkinson’s Disease Rating Scale. Cognitive function was assessed using the Mini-Mental State Examination and Montreal Cognitive Assessment. Anxiety and depression were assessed using the Hamilton Anxiety Scale and Hamilton Depression Scale, respectively. Behavioral and psychiatric symptoms were assessed using the Neuropsychiatric Inventory.
Imaging protocols
PET/magnetic resonance (MR) imaging was performed using an integrated, simultaneous, hybrid PET/MR device (Biograph mMR; Siemens, Erlangen, Germany) operating at 3T, with high-performance gradient systems (45 mT/m) and a slew rate of 200 T/m/s. For PET, the system offers an axial field of view of 256 mm, a sensitivity of 13.2 cps/kBq, and a transaxial resolution of 4.4 mm. PET was performed 100 to 150 minutes after the original tracer administration with 5 minutes per bed position, 3 iterations, 21 subsets, a 4.2-mm slice thickness, and a 172 × 172 matrix using the point-spread function–based reconstruction algorithm of high-definition PET. All patients underwent PET/MR scans by three tracers (11C-β-CFT, 11C-PIB, and 18F-FDG). The scans using each of the tracers were performed separately on 3 different days to avoid the effects of drug residues on the results. The dose of the three tracers was 3.70 MBq/kg. PET images of the head were collected for 10 minutes in each bed position. Attenuation correction was performed based on ultra-short echo time sequences. PET/MR images were reconstructed using the following parameters: ordered subset expectation maximization algorithm, 3 iterations, 21 subsets, Gaussian filtering, full width at half maximum (4.0 mm), and scattering correction. MR scans were performed using a head/neck coil for the entire brain. MR images were simultaneously acquired for each bed position with the applied sequences omitted. 11C-PIB PET imaging was performed as follows: PET image acquisition began 40 minutes after the intravenous injection of 11C-PIB and continued for 20 minutes with three-dimensional list-mode reconstruction. Before 18F-FDG PET imaging, the patients were fasted for 4 to 6 hours to control the fasting glucose concentration at <11 mmol/L. The PET scan was begun in prostration 50 minutes following the intravenous injection of 18F-FDG and continued for 10 minutes with the three-dimensional mode. The 11C-β-CFT PET scan was performed 40 minutes after the intravenous injection of 11C-β-CFT and continued for 10 minutes.
Imaging analysis
The uptake of 11C-β-CFT in the putamina and caudate nucleus was evaluated to determine the function of DaT on the presynaptic membrane of dopaminergic neurons in the striatum. The uptake of 11C-PIB in the frontal, parietal, occipital, and temporal lobes; basal ganglia; and cerebellum was used to detect Aβ deposition. The uptake of 18F-FDG was evaluated to determine the metabolic condition of different brain areas.
We used visual evaluation of the uptake of 11C-β-CFT, 11C-PIB, and 18F-FDG to determine the patients’ molecular imaging characteristics. The uptake status of the three tracers was classified as increased, decreased, or normal by comparison with the normal model established by the Department of Nuclear Medicine of Chinese PLA General Hospital. The PET/MR images were evaluated by two nuclear medicine specialists (X-BX and F-LP) who were blinded to the patients’ clinical data.
Results
Baseline information and clinical features
Five patients were studied (3 male, 2 female; age range, 62–81 years; average age, 70 years). The duration of the disease course was 3 to 5 years upon diagnosis. Of the five patients, two initially had parkinsonian symptoms, which were followed by cognitive impairment; the other three patients began with cognitive impairment and then developed parkinsonian symptoms. The time interval between the onset of parkinsonian symptoms and cognitive impairment ranged from 7 to 30 months. Three of the five patients had fluctuating cognitive impairment. Four of the five patients had apparent visual hallucinations during the disease course. Two patients had delusions, which were accompanied by mental and behavioral abnormalities in one patient. One patient had orthostatic hypotension and another had urticaria. All five patients exhibited mild to severe rigidity and movement slowness, and three patients also had static tremors (Table 1).
Table 1.
Patient baseline data and clinical features
| Features | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Baseline information | |||||
| Age, years | 70 | 62 | 61 | 81 | 78 |
| Sex | Female | Female | Male | Male | Male |
| Disease course, years | 3 | 4 | 3 | 5 | 5 |
| Education, years | 11 | 5 | 8 | 5 | 5 |
| Clinical features | |||||
| Onset with parkinsonian symptoms | Yes | No | No | No | Yes |
| Onset with cognitive impairment | No | Yes | Yes | Yes | No |
| Time interval between parkinsonian symptoms and cognitive impairment, months | 12 | 23 | 7 | 30 | 12 |
| Fluctuating cognitive impairment | Yes | Yes | No | Yes | No |
| Visual hallucinations | Yes | Yes | Yes | No | Yes |
| Delusions | No | Yes | No | Yes | No |
| Mental and behavioral abnormalities | No | Yes | No | No | No |
| Orthostatic hypotension | No | No | No | Yes | No |
| Constipation | Mild | Severe | Moderate | Mild | Moderate |
| Urinary and fecal incontinence | No | Yes | No | No | No |
| Spasticity | Mild | Severe | Moderate | Mild | Moderate |
| Bradykinesia | Mild | Severe | Moderate | Mild | Moderate |
| Static tremors | No | Mild | No | Moderate | Mild |
| Evaluation with rating scales | |||||
| MMSE score | 25 | 5 | 22 | 19 | 17 |
| MoCA score | 18 | 3 | 14 | 11 | 13 |
| HAMA score | 12 | 11 | 9 | 17 | 15 |
| HAMD score | 17 | 12 | 11 | 24 | 21 |
| UPDRS-III | 53 | 62 | 43 | 37 | 32 |
MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; HAMA, Hamilton Anxiety Scale; HAMD, Hamilton Depression Scale; UPDRS, Unified Parkinson’s Disease Rating Scale.
PET/MR imaging findings
DaT PET imaging
Patient No. 2 showed decreased uptake in the putamina and caudate nuclei bilaterally. However, Patient No. 4 showed normal uptake in the putamina and caudate nuclei bilaterally. The other three patients had decreased uptake of DaT in the putamina and normal uptake in the caudate nuclei (Table 2).
Table 2.
Positron emission tomography/magnetic resonance imaging findings
| Tracer | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| DaT | |||||
| Putamina | ↓ | ↓ | ↓ | Norm | ↓ |
| Caudate nucleus | Norm | ↓ | Norm | Norm | Norm |
| PIB | |||||
| Frontal lobe | ++ | + | ++ | + | – |
| Parietal lobe | ++ | ++ | ++ | ++ | – |
| Occipital lobe | + | + | + | ++ | – |
| Temporal lobe | + | ++ | + | ++ | – |
| Basal ganglia | – | – | – | – | – |
| Cerebellum | – | – | – | – | – |
| FDG | |||||
| Frontal lobe | ↓ | ↓ | Norm | ↓ | Norm |
| Parietal lobe | ↓ | ↓ | ↓ | ↓ | ↓ |
| Occipital lobe | Norm | ↓ | ↓ | Norm | ↓ |
| Temporal lobe | ↓ | ↓ | ↓ | ↓ | Norm |
| Posterior cingulate cortex | Norm | Norm | Norm | ↓ | Norm |
| Basal ganglia | ↓ | ↓ | ↓ | Norm | Norm |
| Cerebellum | Norm | Norm | Norm | Norm | Norm |
+ indicates increased uptake of PIB; ++ indicates an apparent increase in PIB uptake; – indicates no apparent uptake of PIB; ↓ indicates decreased uptake of FDG and DaT; Norm indicates normal uptake of FDG and DaT. DaT, dopamine transporter; PIB, Pittsburgh compound B; FDG, fluorodeoxyglucose.
PIB PET imaging
All patients except Patient No. 5 had a diffuse pattern of increased uptake of PIB in the frontal, parietal, temporal, and occipital lobes. No apparent uptake of radioactivity was observed in the basal ganglia or cerebellum in any patient (Table 2).
FDG PET imaging
All five patients had decreased metabolism in the parietal lobe. Three patients showed decreased metabolism in the frontal lobe. Three patients had decreased metabolism in the occipital lobe. Four patients had decreased metabolism in the temporal lobe. Three patients showed decreased metabolism in the basal ganglia. Four patients had normal metabolism in the posterior cingulate cortex. All five patients had normal metabolism in the cerebellum (Table 2).
Case descriptions
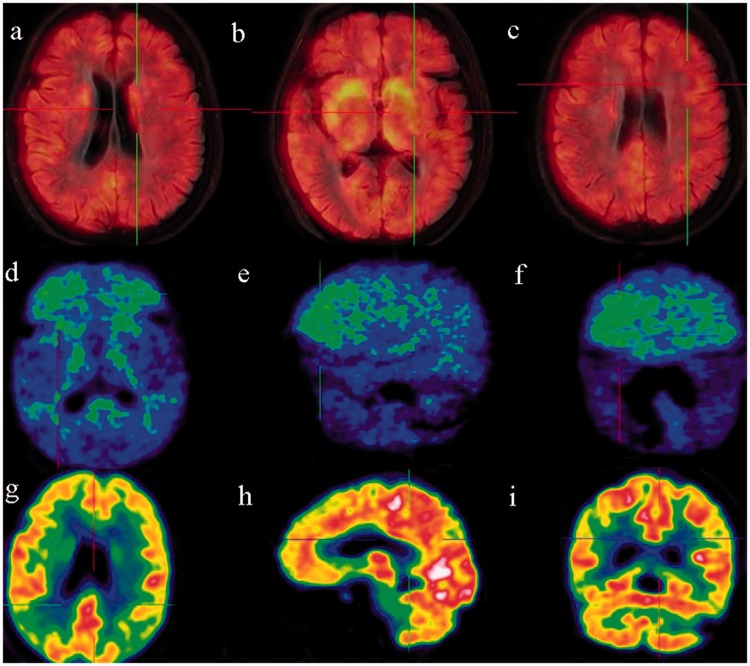
Patient No. 1 was a 70-year-old woman. She had developed heaviness and reduced mobility of the lower limbs in April 2013, but she had no abnormalities of the upper limbs or tremors. Weakness and slowness accompanied by memory loss developed in April 2014. The patient reported hallucinations (“strangers moving about in the house”) and anxiety. She was intermittently medicated with benserazide (125 mg three times daily), pramipexole (0.125 mg three times daily), and amantadine (0.1 g twice daily) but showed little improvement. The patient was transported to our clinic on 21 January 2015 with severe visual hallucinations (“puddles in the quilt with frogs jumping here and there”). A physical examination revealed decreased facial expressions, a low pitch when talking, and slightly retarded responses. Her eyeballs moved in all directions with midline protrusion of the tongue, but no difficulty in swallowing. Her muscle volume was normal in all four limbs, and her muscular strength was grade 5. Muscle tension was increased in the upper limbs, especially in the right arm. The limb joints had moderate mobility. The finger-to-nose test was normal; however, alternating movements were clumsy (finger-to-finger test and clenching of the fists, especially on the right side). No apparent static or intention tremor was observed. Pathologic signs were negative. The patient had no family history of AD or essential tremor. DaT PET imaging indicated decreased uptake in the putamina bilaterally (Figure 1(a)–(c)). PIB PET imaging indicated obviously increased uptake in the frontal and parietal lobes and mildly increased uptake in the temporal and occipital lobes (Figure 1(d)–(f)). FDG PET imaging indicated decreased metabolism in the frontal, parietal, and temporal lobes and basal ganglia. FDG metabolism was normal in the occipital lobe, posterior cingulate cortex, and cerebellum (Figure 1(g)–(i)).
Figure 1.
A 70-year-old woman with a 3-year disease course. (a)–(c) DaT PET imaging revealed reduced uptake in the bilateral caudate nuclei and putamina. (d)–(f) PIB PET imaging revealed clear uptake in the frontal lobe and basal ganglia and a slight increase in the parietal, temporal, and occipital lobes. (g)–(i) FDG PET imaging revealed decreased metabolism in the frontal lobe and the junction between the parietal, occipital, and temporal lobes. FDG metabolism was normal in the occipital lobe, posterior cingulate cortex, basal ganglia, and cerebellum. DaT, dopamine transporter; PET, positron emission tomography; PIB, Pittsburgh compound B; FDG, fluorodeoxyglucose.
Patient No. 4 was an 81-year-old man. He complained of memory loss for 5 years and developed weakness and movement slowness 30 months later. He presented with mild spasticity and bradykinesia and moderate static tremor. He had normal uptake of DaT in the putamina and caudate nuclei bilaterally with obviously increased uptake of PIB in the parietal, occipital, and temporal lobes. Based on these clinical and radiological characteristics, we diagnosed this patient with AD rather than DLB.
Patient No. 5 was a 78-year-old man. He had experienced weakness and movement slowness for 5 years and reported memory loss and hallucinations about 12 months after the onset of parkinsonism. Despite the clinical diagnosis of DLB, no obvious Aβ deposition was revealed by PIB PET. Moreover, the patient’s cognitive condition was stable, and he achieved relief from hallucinations for the next 4 years. Based on these clinical and imaging findings, we changed his diagnosis to PDD.
Discussion
DLB was first reported by Okazaki et al.9 in 1961 as a neurodegenerative disease that shares some symptoms with PDD and AD. Most often occurring in the elderly, DLB has typical symptoms such as fluctuating cognitive impairment, repeated and persistent visual hallucinations, and parkinsonian symptoms. Fluctuating cognitive impairment is the core feature of DLB and occurs in 80% to 90% of affected patients.10 Fluctuating cognitive impairment is usually described by patients as “being sometimes conscious and sometimes not.” Attentiveness and alertness decline in a fluctuating manner, manifesting as an alternation between normal and abnormal states within several weeks or even within a given day. In the present study, 60% of patients had fluctuating cognitive impairment, which presented as marked fluctuation of cognitive function within a few days or weeks. Visual hallucination is the most common psychiatric symptom associated with DLB and occurs in >70% of affected patients. Visual hallucinations usually occur in the early stage of DLB and arise as the first symptom in about 20% of patients with DLB.11 Tiraboschi et al.5 reported that upon the initial presentation, patients with DLB displayed a higher frequency of visual hallucinations than patients with AD. The specificity of the presence or a recent history of visual hallucinations in patients with early-stage DLB is 99%. In the present study, 80% of patients reported that they had experienced typical visual hallucinations, and the patients’ descriptions of the hallucinations were clear and vivid and included specific details (“frogs jumping here and there in the quilt” and “strangers moving in the house”).
The major diagnostic challenge in patients with DLB is the variability and similarity of symptoms compared with AD, which leads to the misdiagnosis of DLB. The clinical diagnostic accuracy of DLB has been poor (34% to 65%) in studies using postmortem diagnosis as the gold standard.5 Dopaminergic neuron loss in the nigrostriatal system can result in an apparent reduction of uptake in the putamina on DaT PET imaging in patients with DLB patients. In contrast, DaT uptake is usually normal in the putamina and caudate nuclei of the normal striate body in patients with AD. Therefore, reduced DaT uptake on PET imaging was added to the latest revision criteria for the diagnosis of DLB.4 In the present study, DaT PET/MR imaging was performed in patients with probable DLB to visualize the dopaminergic neuron loss in the nigrostriatal system. The results showed that four patients had decreased uptake of DaT in the putamina bilaterally and that one of these four patients also had decreased uptake in the caudate nuclei. The remaining patient had normal DaT PET imaging. In a previous study, the sensitivity of DaT PET imaging for differentiating between DLB and AD was 78%, while the specificity was >90%.6 Autopsy data have confirmed that the specificity of DaT PET imaging for identifying DLB from AD is 100%.12 van der Zande et al.13 investigated patients with DLB with a normal initial DaT SPECT scan and abnormal subsequent scans during disease progression. Of 67 patients with DLB who underwent DaT SPECT scans, 7 (10.4%) had normal results. In five patients with a negative DaT result, a second DaT SPECT was performed after an average of 1.5 years, and all of these scans were abnormal. The authors suggested that normal DaT scans in patients with DLB could represent a relatively rare DLB subtype with a different severity or spread of alpha-synuclein pathology.13
DLB and PDD are very similar clinically and pathologically.14 The 1-year rule has been used to differentiate between DLB and PDD; i.e., DLB is diagnosed if cognitive impairment appears earlier than parkinsonian symptoms or within 1 year after parkinsonian symptoms, while PDD is diagnosed if the cognitive impairment appears > 1 year after parkinsonian symptoms.15 Notably, the 1-year threshold is somewhat arbitrary. Considering the complex and degenerative nature of DLB and PDD, the reliability of making a diagnosis based only on a time threshold is doubtful.
Although tracer uptake decreased asymmetrically with PDD, this should not be considered adequate for a differential diagnosis. In the present study, 80% of the patients had a diffuse pattern of radioactivity uptake in the frontal, parietal, temporal, and occipital lobes on PIB PET. Gomperts et al.7 used 11C-PIB PET imaging to demonstrate Aβ deposition, which revealed more severe Aβ deposition in patients with DLB than PDD. It is generally believed that most patients with DLB have increased PIB uptake, while PDD is usually not accompanied by obvious changes in PIB uptake. Thus, Aβ deposition in the brain is more severe in DLB than in PDD.16 Brooks17 applied PIB PET imaging to discriminate between PDD and DLB. Of 13 patients with DLB, 11 had a significant increase in cortical 11C-PIB uptake. In contrast, 10 of 12 patients with PDD had normal 11C-PIB uptake and only 2 patients had increased uptake. A systematic review showed a higher prevalence of PIB-positive studies among patients with DLB than PDD.18
In the present study, 80% of patients had normal metabolism in the posterior cingulate cortex. Another patient had decreased metabolism in the posterior cingulate cortex. DLB is associated with decreased metabolism on FDG PET in the cortex and occipital visual association area; however, decreased metabolism is not observed in the posterior cingulate cortex, and the metabolism may even be higher than that in the surrounding area. This radiologic phenomenon is called the “cingulate island sign” and is considered as a characteristic change in patients with DLB.8 The decreased metabolism in the posterior cingulate cortex in the prodromal stage of AD can be regarded as a differentiating feature from DLB.
Conclusion
Patients with DLB show reduced uptake in the bilateral putamen on DaT PET, increased uptake throughout the cerebral cortex on PIB PET, and intact metabolism of the posterior cingulate gyrus on FDG PET. These characteristics can help to achieve a diagnosis of DLB in the early stage of the disease, even when symptoms are not obvious and classic. However, studies with larger numbers of patients are still needed to confirm the molecular imaging differences between DLB and AD or PDD.
Acknowledgments
The authors sincerely thank the staff for assisting with the data collection in the study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Capital Characteristic Clinical Project (No. Z151100004015206), the Chinese Central Healthcare Committee Foundation (No. W2013BJ14), the Clinical Supportive Grant of PLA General Hospital (No. 2015FC-TSYS-1016), and the Medical Big Data Research and Development Project of Chinese PLA General Hospital (No. 2016MBD-020).
References
- 1.Hogan DB, Fiest KM, Roberts JI, et al. The prevalence and incidence of dementia with Lewy bodies: a systematic review. Can J Neurol Sci 2016; 43: S83–95. [DOI] [PubMed] [Google Scholar]
- 2.McKeith I, Mintzer J, Aarsland D, et al. Dementia with Lewy bodies. Lancet Neurol 2004; 3: 19–28. [DOI] [PubMed] [Google Scholar]
- 3.Yue W, Wang XD, Shi Z, et al. The prevalence of dementia with Lewy bodies in a rural area of China. Parkinsonism Relat Disord 2016; 29: 72–77. [DOI] [PubMed] [Google Scholar]
- 4.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65: 1863–1872. [DOI] [PubMed] [Google Scholar]
- 5.Tiraboschi P, Salmon DP, Hansen LA, et al. What best differentiates Lewy body from Alzheimer's disease in early-stage dementia? Brain 2006; 129(Pt 3): 729–735. [DOI] [PubMed] [Google Scholar]
- 6.McKeith I, O'Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol 2007; 6: 305–313. [DOI] [PubMed] [Google Scholar]
- 7.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology 2008; 71: 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graff-Radford J, Murray ME, Lowe VJ, et al. Dementia with Lewy bodies: basis of cingulate island sign. Neurology 2014; 83: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okazaki H, Lipkin LE, Aronson SM. Diffuse intracytoplasmic ganglionic inclusions (Lewy type) associated with progressive dementia and quadriparesis in flexion. J Neuropathol Exp Neurol 1961; 20: 237–244. [DOI] [PubMed] [Google Scholar]
- 10.Walker MP, Ayre GA, Cummings JL, et al. The Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale. Two methods to assess fluctuating confusion in dementia. Br J Psychiatry 2000; 177: 252–256. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Wang XD, Wang Y, et al. Clinical and neuroimaging characteristics of Chinese dementia with Lewy bodies. PloS One 2017; 12: e0171802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker Z, Jaros E, Walker RW, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry 2007; 78: 1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Zande JJ, Booij J, Scheltens P, et al. [(123)]FP-CIT SPECT scans initially rated as normal became abnormal over time in patients with probable dementia with Lewy bodies. Eur J Nucl Med Mol Imaging 2016; 43: 1060–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo L, Itaya M, Takanashi M, et al. Relationship between Parkinson disease with dementia and dementia with Lewy bodies. Parkinsonism & related disorders 2005; 11: 305–309. [DOI] [PubMed] [Google Scholar]
- 15.Lippa CF, Duda JE, Grossman M, et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology 2007; 68: 812–819. [DOI] [PubMed] [Google Scholar]
- 16.Quigley H, Colloby SJ, O'Brien JT. PET imaging of brain amyloid in dementia: a review. Int J Geriatr Psychiatry. 2011; 26: 991–999. [DOI] [PubMed] [Google Scholar]
- 17.Brooks DJ. Imaging amyloid in Parkinson's disease dementia and dementia with Lewy bodies with positron emission tomography. Mov Disord 2009; 24 (Suppl 2): S742–S747. [DOI] [PubMed] [Google Scholar]
- 18.Petrou M, Dwamena BA, Foerster BR, et al. Amyloid deposition in Parkinson’s disease and cognitive impairment: a systematic review. Mov Disord 2015; 30: 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]