Short abstract
Objectives
This study was performed to analyze the risk factors for early mortality (EM) in elderly patients undergoing treatment for multiple myeloma (MM) in real-world clinical practice.
Methods
Retrospective data from 108 elderly patients who were newly diagnosed with MM from January 2007 to July 2015 were analyzed in a single hematology center. EM was defined as death of any cause within 12 months after diagnosis. A multivariate regression model was used to evaluate EM.
Results
EM occurred in 16 (14.8%) elderly patients with newly diagnosed MM. The most common cause of death was infection (10/16, 62.5%). In the multivariate analysis, only an age of ≥75 years, International Staging System (ISS) stage III disease, and high lactate dehydrogenase concentration were significantly and independently associated with EM.
Conclusion
Our results suggest that infection is the leading cause of EM in elderly patients with MM. An age of ≥75 years, ISS stage III disease, and a high lactate dehydrogenase concentration are significant predictors of EM. We should further target this higher-risk patient population to define personalized therapy with which to improve outcomes.
Keywords: Elderly, multiple myeloma, early mortality, bortezomib, International Staging System, lactate dehydrogenase
Introduction
Multiple myeloma (MM) is a hematological malignancy of plasma cells and predominantly affects elderly patients. With the rapid evolution of treatment for MM in recent decades, the survival of affected patients has significantly improved.1 However, the improvements in elderly patients have been small compared with those in younger individuals.2 An estimate of the 5-year relative survival rate of patients with MM from 1989–1992 to 2001–2005 in the Netherlands did not show significantly improved survival among older patients, whereas more substantial increases were seen in patients aged ≤65 years (5-year relative survival of 34%–56%).3
A few studies have evaluated the impact of early mortality (EM) on the clinical outcomes in patients with MM.4–7 EM remains problematic, especially in elderly patients. Elderly patients with MM are more susceptible to adverse effects of treatment and are often unable to tolerate full drug doses because of their impaired performance status or comorbidities.8 This population is often excluded from clinical trials and has not been well studied despite the fact that most MM diagnoses and related mortality occur in persons aged ≥65 years.8 In the United Kingdom Medical Research Council trials, Augustson et al.9 reported that 10% of patients with MM died within 2 months after diagnosis and that 60% of the these deaths occurred in patients aged >65 years. In another analysis using the Greek Myeloma Study Group database, 22 of 155 octogenarians (14%) died within 2 months after therapy initiation.10 If patients can overcome the risk of EM, they will reap benefits from novel antimyeloma therapies to achieve longer remission and survival.
Determinants of EM are very important and will help to identify those patients with MM who are at risk of early death and optimize treatment accordingly. Previous studies have identified some parameters as independent predictive factors for EM, including age,4,6,9 International Staging System (ISS) stage III disease,4 performance status,4,5,9 lower serum albumin concentrations,5 and renal failure.7
Most data about EM are from clinical trials.11 However, limited information about EM among elderly patients with MM in the real-world setting is available. In addition, most studies published to date were from Western countries; data from China are scarce. Therefore, we performed a retrospective cohort study of patients with MM from a medical center in China. The aim of this study was to investigate the incidence of EM in older patients with newly diagnosed symptomatic MM. Various patient characteristics and laboratory parameters at diagnosis were analyzed to determine their impact on EM.
Patients and methods
This single-center retrospective study included elderly patients (≥65 years of age) who had been newly diagnosed with symptomatic MM from 1 January 2007 to 31 July 2015 in a typical regional university hospital. All patients received antimyeloma treatment at our hospital. The last follow-up date was 31 July 2016. Patients with amyloidosis at the time of diagnosis were excluded. Patients who refused or abandoned antimyeloma treatment were also excluded.
We collected all data by reviewing the patients’ medical charts and electronic records. The collected data comprised clinical characteristics and laboratory parameters at diagnosis and information concerning induction treatment. The Charlson comorbidity index (CCI) was calculated according to the criteria reported by Charlson et al.12,13 Determination of each patient’s clinical stage was based on the Durie–Salmon staging system and the ISS.14,15 EM was defined as death within the first 12 months after diagnosis.
All analyses were performed using SPSS statistical software (version 19.0; IBM Corp., Armonk, NY, USA). A P-value of <0.05 was considered significant. Continuous variables are presented as median with range, and categorical data are presented as absolute with percentage. Categorical variables among different groups were compared with the chi-square test or Fisher’s exact test. Binary logistic regression analysis was performed to determine the risk factors for predicting EM. Parameters that were significant in the univariate analysis were entered into the multivariate analysis. Results are expressed as odds ratios and 95% confidence intervals.
This retrospective study was approved by the Institutional Review Board of Wuxi People’s Hospital in accordance with the Helsinki Declaration. Patient informed consent was unnecessary because of the retrospective nature of the study.
Results
Patient characteristics
In total, 108 elderly patients newly diagnosed with symptomatic MM were included in this study. The patients’ characteristics are listed in Table 1. The median age of the patients in this study was 69.5 years (range, 65–82 years), and 56.5% were male. The clinical stages according to the ISS were I, II, and III in 4.6%, 50.9%, and 44.5% of patients, respectively. The monoclonal protein isotypes were as follows: immunoglobulin (Ig) G, 38.9%; IgA, 38.0%; light chain, 16.7%; and others, 6.4%. A total of 72.2% of patients had a concurrent comorbidity. The median CCI was 4 for the entire study population (range, 3–9). A total of 43 patients (39.8%) received a cytotoxic agent as the first-line treatment, such as melphalan and prednisolone (MP); vincristine, adriamycin, and dexamethasone (VAD); pegylated liposomal doxorubicin, vincristine, and dexamethasone (DVD); or other combinations. Nine patients (8.3%) received a regimen containing thalidomide after the initial diagnosis, such as melphalan, prednisolone, and thalidomide (MPT) or thalidomide and dexamethasone (TD). In total, 56 patients (51.9%) were treated with a bortezomib-containing regimen such as bortezomib, thalidomide, and prednisolone (VTD); bortezomib, pegylated liposomal doxorubicin, and dexamethasone (PAD); or bortezomib and dexamethasone.
Table 1.
Patients’ characteristics
| Characteristics | Total | EM | Non-EM | P value |
|---|---|---|---|---|
| Number of patients | 108 | 16 (14.8) | 92 (85.2) | |
| Age of ≥75 years | 19 (17.6) | 8 (50.0) | 11 (12.0) | 0.001 |
| Male | 61 (56.5) | 9 (56.3) | 52 (56.5) | 0.984 |
| Myeloma type | 0.637 | |||
| IgG | 42 (38.9) | 4 (25.0) | 38 (41.3) | |
| IgA | 41 (38.0) | 8 (50.0) | 33 (35.9) | |
| Light chain | 18 (16.7) | 3 (18.8) | 15 (16.3) | |
| Others | 7 (6.4) | 1 (6.2) | 6 (6.5) | |
| DSS | 0.69 | |||
| II | 12 (11.1) | 1 (6.2) | 11 (12.0) | |
| III | 96 (88.9) | 15 (93.8) | 81 (88.0) | |
| ISS | 0.026 | |||
| I | 5 (4.6) | 0 (0.0) | 5 (5.4) | |
| II | 55 (50.9) | 4 (25.) | 51 (55.4) | |
| III | 48 (44.5) | 12 (75.0) | 36 (39.2) | |
| WHO performance status ≥3 | 52 (48.1) | 7 (43.8) | 45 (48.9) | 0.703 |
| Charlson comorbidity index ≥5 | 46 (42.6) | 11 (68.8) | 35 (38.0) | 0.029 |
| Hemoglobin <10 g/L | 85 (78.7) | 15 (93.8) | 70 (76.1) | 0.184 |
| Albumin <3.5 g/L | 103 (95.4) | 16 (100.0) | 87 (94.6) | 1.000 |
| Lactate dehydrogenase >normal | 28 (25.7) | 11 (68.8) | 17 (18.5) | 0.000 |
| Creatinine >177 µmol/L | 40 (37.0) | 10 (62.5) | 30 (32.6) | 0.028 |
| Calcium >2.75 mmol/L | 14 (13.0) | 1 (6.2) | 13 (14.1) | 0.688 |
| Bortezomib-based regimens | 56 (51.9) | 12 (75.0) | 44 (47.8) | 0.059 |
Data are presented as n or n (%).
EM: early mortality; Ig: immunoglobulin; DSS: Durie–Salmon staging system; ISS: International Staging System; WHO: World Health Organization.
Characteristics of EM
EM occurred in 16 of 108 (14.8%) elderly patients undergoing treatment for newly diagnosed MM. Of all 108 patients, 1 (0.9%) died within 3 months of diagnosis, and 10 (9.0%) died within 6 months. Patients with EM were older and had more comorbidities, higher lactate dehydrogenase (LDH) concentrations, and more severely impaired renal function than those without EM (Table 1). The causes of death are shown in Figure 1. Overall, the most common cause of death among patients with EM was infection (10/16, 62.5%). Pneumonia was the cause of death in 80% (8/10) of those who died early of infection.
Figure 1.
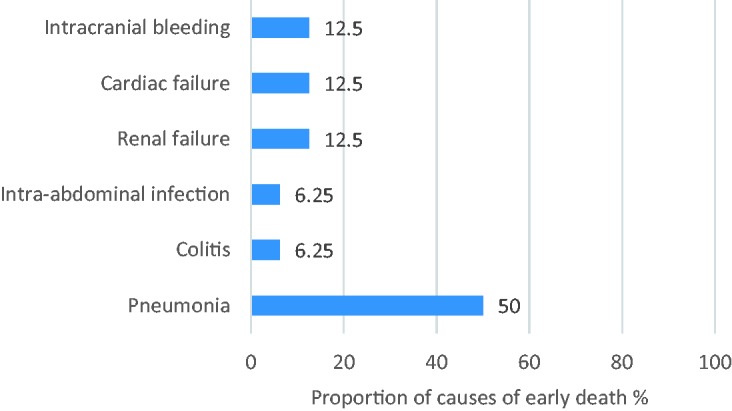
Causes of early death (n = 16)
Risk factors for EM
We further investigated the patients’ clinical variables to identify risk factors for EM using univariate and multivariate analyses (Table 2). In the univariate analysis, we found that age, ISS stage, CCI, LDH concentration, and serum creatinine concentration were significantly associated with EM. In contrast, the patient’s performance status, type of monoclonal protein, serum calcium concentration, and hemoglobin level were not significantly associated with EM. In the multivariate analysis, only an age of ≥75 years (P = 0.005), ISS stage III (P = 0.041), and high LDH concentration (P = 0.001) were independent predictors of EM. Initial bortezomib therapy was not associated with EM in the logistic regression analysis.
Table 2.
Univariate and multivariate analysis of factors associated with early mortality
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age of ≥75 years | 7.364 (2.297–23.605) | 0.001 | 8.583 (1.932–38.128) | 0.005 |
| IgA | 1.788 (0.614–5.205) | 0.287 | ||
| DS III | 2.037 (0.245–16.97) | 0.511 | ||
| ISS III | 4.667 (1.396–15.596) | 0.012 | 5.506 (1.077–28.157) | 0.041 |
| WHO performance status ≥3 | 0.812 (0.279–2.366) | 0.703 | ||
| Charlson comorbidity index ≥5 | 3.583 (1.148–11.179) | 0.028 | 0.987 (0.19–5.112) | 0.987 |
| Hemoglobin <10 g/L | 4.714 (0.589–37.741) | 0.144 | ||
| Lactate dehydrogenase >normal | 9.706 (2.98–31.617) | 0.000 | 10.234 (2.596–40.341) | 0.001 |
| Creatinine >177 µmol/L | 3.444 (1.144–10.368) | 0.028 | 1.692 (0.327–8.751) | 0.531 |
| Calcium >2.75 mmol/L | 0.405 (0.049–3.333) | 0.401 | ||
| Bortezomib-based regimen from diagnosis to RRMM, range |
3.273 (0.983–10.901) | 0.053 | ||
ISS: International Staging System; DSS: Durie–Salmon staging system; WHO: World Health Organization; OR: odds ratio; CI: confidence interval.
Discussion
Some studies have shown that the use of novel agents in first-line chemotherapy significantly improved survival of elderly patients.16 However, a few patients do not benefit from new treatments for MM because of early death. EM is an uncommon event in most recently reported phase 3 trials for newly diagnosed MM.11 Patients enrolled in clinical trials are highly selected and often not representative of elderly patients in real-life settings. Thus, little is known about EM in elderly patients in the real-world setting.
In this study, we found that 14.8% of elderly patients with MM died within 1 year after diagnosis. Few studies have mentioned the incidence of EM in elderly patients with MM. A Mayo Clinic study by Kumar et al.17 revealed a 12-month overall mortality rate of 10% among patients treated from 2006 to 2010. They found that an age of >70 years, serum albumin concentration of <3.5 mg/dL, and serum beta-2 microglobulin concentration of >6.5 mg/dL were independent predictors of early mortality.17 The EM rate was 10.8% in another Japanese single-center study performed from 2004 to 2014.18 Among 18,885 patients with MM diagnosed from 1993 to 2010 in the National Cancer Institute Surveillance Epidemiology and End Result Registry, 31.7% died within 12 months.6 This discrepancy in the EM rate is mostly dependent upon the population included, therapeutic agents used, and local health care systems in place among studies from different countries.
In the current study, 19 (17.6%) of the 108 patients were aged ≥75 years, and a high proportion of patients had a performance status of ≥3 (48.1%) and CCI of ≥5 (42.6%). A total of 56 patients (51.9%) received a regimen containing bortezomib as the initial treatment. Our results revealed an elevated risk of EM among patients aged ≥75 years, those with ISS stage III disease, and those with a high LDH concentration. We also observed that the most common causes of death among patients with EM were infection, cardiac failure, and renal failure. The high infection rate observed in this study suggests that prophylactic antibiotic therapy should be considered for patients with MM.
A recent analysis demonstrated that early mortality tends to be lower with the widespread use of novel MM agents in upfront regimens.17 In contrast, Chen et al.19 reported that bortezomib therapy did not appear to reduce the incidence of early mortality in patients with MM. In the present study, 75% of patients with EM received induction therapy with a bortezomib-based regimen, but bortezomib therapy was not an independent predictor of EM. Due to health insurance limitations in China, diagnostic delays and lack of immediate adequate medical care may largely explain the high EM rate in our cohort.20,21
Age is an important factor in determining the optimal treatments for patients with MM. Aging-related gradual and progressive reductions in renal, gastric, and cardiac function; hepatic mass; blood flow; and bone marrow status result in high rates of treatment-related adverse events, contributing to the reduced tolerability of MM treatments observed in elderly patients.22 Therefore, a bortezomib-based regimen should be cautiously used as an induction therapy for elderly patients newly diagnosed with MM with ISS stage III disease and/or comorbidities. Personalized bortezomib therapy such as less frequent dosing, lower dosing, or changing the administration route from intravenous to subcutaneous is urgently needed to reduce toxicity in these patients.23 Furthermore, improvements in supportive care can help to avoid and overcome early complications.
This study had some limitations. First, this was a retrospective study in a single center. It may have had potential bias, and the sample size was relatively small. Second, data regarding cytogenetic risk factors were not available for most of the patients. In addition, the drugs chosen as frontline therapy were determined by the physician, the patient, or the patient’s family. Due to health insurance limitations in China, the patients who received the bortezomib-based regimens and treatment schedules were relatively heterogeneous. This made it difficult to analyze the association between induction treatment and early mortality of patients with MM without confounding factors.
In conclusion, our study showed that infection was the major cause of death in elderly patients with MM. An age of ≥75 years, ISS stage III disease, and a high LDH concentration were significant predictors of EM. Further exploration to identify prognostic factors for EM in real-life unselected elderly patients with MM is warranted. We should target this higher-risk patient population who are left out of trials to define personalized therapy that will improve outcomes.
Acknowledgments
The authors thank all the doctors and nursing staff of the Department of Hematology in Wuxi People’s Hospital for their dedicated patient care.
Authors’ contributions
Hongfeng Guo provided the conception and design of the study and wrote the manuscript. Jun Xia and Lingling Wang designed the study and collected and analyzed the data. Xin Zhou, Jing Wang, and Huan Wang were involved in the data analysis and interpretation. All authors approved the final version of the manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Landgren O, Iskander K. Modern multiple myeloma therapy: deep, sustained treatment response and good clinical outcomes. J Intern Med 2017; 281: 365–382. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Gondos A, Pulte D. Recent major improvements in long-term survival of younger patients with multiple myeloma and the impact of novel therapies. Blood 2008; 111: 2521–2526. [DOI] [PubMed] [Google Scholar]
- 3.Schaapveld M, Visser O, Siesling S, et al. Improved survival among younger but not among older patients with Multiple Myeloma in the Netherlands, a population-based study since 1989. Eur J Cancer 2010; 46: 160–169. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S. Risk of early death in multiple myeloma. Clin Adv Hematol Oncol 2012; 10: 172–174. [PubMed] [Google Scholar]
- 5.Holmström MO, Gimsing P, Abildgaard N, et al. Causes of early death in multiple myeloma patients who are ineligible for high-dose therapy with hematopoietic stem cell support: A study based on the nationwide Danish Myeloma Database. Am J Hematol 2015; 90:E73–74. [DOI] [PubMed] [Google Scholar]
- 6.Costa LJ, Gonsalves WI, Kumar SK. Early mortality in multiple myeloma. Leukemia 2015; 29: 1616–1618. [DOI] [PubMed] [Google Scholar]
- 7.Jung SH, Cho MS, Kim HK, et al. Risk factors associated with early mortality in patients with multiple myeloma who were treated upfront with a novel agents containing regimen. BMC Cancer 2016: 8; 16: 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood 2011; 118: 4519–4529. [DOI] [PubMed] [Google Scholar]
- 9.Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: Analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002—Medical Research Council Adult Leukaemia Working Party. J Clin Oncol 2005; 23: 9219–9226. [DOI] [PubMed] [Google Scholar]
- 10.Dimopoulos MA, Kastritis E, Delimpasi S, et al. Multiple myeloma in octogenarians: Clinical features and outcome in the novel agent era. Eur J Haematol 2012; 89: 10–15. [DOI] [PubMed] [Google Scholar]
- 11.Gonsalves WI, Godby K, Kumar SK, et al. Limiting early mortality: Do’s and don’ts in the management of patients with newly diagnosed multiple myeloma. Am J Hematol 2016; 91: 101–108. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 13.Charlson M, Szatrowski TP, Peterson J, et al. Validaton of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 14.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 1975; 36: 842–854. [DOI] [PubMed] [Google Scholar]
- 15.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23: 3412–3420. [DOI] [PubMed] [Google Scholar]
- 16.Mateos MV, Ocio EM, Paiva B, et al. Treatment for patients with newly diagnosed multiple myeloma in 2015. Blood Rev 2015; 29: 387–403. [DOI] [PubMed] [Google Scholar]
- 17.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 2014; 28: 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsue K, Matsue Y, Fujisawa M, et al. Clinical features and treatment outcome of very elderly patients over 80 years old with multiple myeloma: comparison with patients in different age groups in the era of novel agents. Leuk Lymphoma 2016; 57: 110–115. [DOI] [PubMed] [Google Scholar]
- 19.Chen YK, Han SM, Yang Y, et al. Early mortality in multiple myeloma: experiences from a single institution. Hematology 2016; 21: 392–398. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Wong H, Liu K. Outcome-based health equity across different social health insurance schemes for the elderly in China. BMC Health Serv Res 2016; 16: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friese CR, Abel GA, Magazu LS, et al. Diagnostic delay and complications for older adults with multiple myeloma. Leuk Lymphoma 2009; 50: 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bringhen S, Mateos MV, Zweegman S, et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica 2013; 98: 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerrato C, Mina R, Palumbo A. Optimal management of elderly patients with myeloma. Expert Rev Anticancer Ther 2014; 14: 217–228. [DOI] [PubMed] [Google Scholar]


