Abstract
Pro-inflammatory cytokines cause pancreatic beta cell failure during the development of type 2 diabetes. This beta cell failure associates with mitochondrial dysfunction, but the precise effects of cytokines on mitochondrial respiration remain unclear. To test the hypothesis that pro-inflammatory cytokines impair glucose-stimulated insulin secretion (GSIS) by inhibiting oxidative ATP synthesis, we probed insulin release and real-time mitochondrial respiration in rat INS-1E insulinoma cells that were exposed to a combination of 2 ng/mL interleukin-1-beta and 50 ng/mL interferon-gamma. We show that 24-h exposure to these cytokines dampens both glucose- and pyruvate-stimulated insulin secretion (P < 0.0001 and P < 0.05, respectively), but does not affect KCl-induced insulin release. Mirroring secretory defects, glucose- and pyruvate-stimulated mitochondrial respiration are lowered after cytokine exposure (P < 0.01). Further analysis confirms that cytokine-induced mitochondrial respiratory defects occur irrespective of whether fuel oxidation is coupled to, or uncoupled from, ATP synthesis. These observations demonstrate that pro-inflammatory cytokines attenuate GSIS by restricting mitochondrial pyruvate oxidation capacity. Interleukin-1-beta and interferon-gamma also increase mitochondrial superoxide levels (P < 0.05), which may reinforce the inhibition of pyruvate oxidation, and cause a modest (20%) but significant (P < 0.01) loss of INS-1E cells. Cytokine-induced INS-1E cell failure is insensitive to palmitoleate and linoleate, which is at odds with the cytoprotection offered by unsaturated fatty acids against harm caused by nutrient excess. Our data disclose a mitochondrial mechanism for cytokine-impaired GSIS in INS-1E cells, and suggest that inflammatory and nutrient-related beta cell failure emerge, at least partly, through distinct paths.
Introduction
Impaired glucose-stimulated insulin secretion (GSIS) by pancreatic beta cells (β-cells) contributes to the hyperglycemic state that characterizes type 2 diabetes [1]. While certain conditions are strongly associated with this impairment, for example the excessive nutrient levels that circulate in obesity [2], the pathological mechanism of β-cell dysfunction in type 2 diabetes is incompletely understood. Several symptoms and complications of type 2 diabetes involve activation of the immune system and a consequent state of chronic low-grade inflammation [3,4]. Inflammation is associated with impaired β-cell function [4,5] and decreased β-cell mass [6,7]. The cause of β-cell inflammation in type 2 diabetes is debated, but likely relates to obesity as it is widely accepted that elevated nutrient levels stimulate the expression of interleukin-1β (IL-1β) in human pancreatic islets, via activation of the NLRP3 inflammasome [8–10] or the NF-κB pathway [11], which causes a pro-inflammatory state [12]. In turn, IL-1β upregulates numerous other cytokines and chemokines [13,14] and indeed reinforces its own expression causing a vicious cycle [15]. Chronic upregulation of pro-inflammatory cytokines is regarded a hallmark for impaired insulin secretion [5] and increased β-cell apoptosis [5] in the pathophysiology of type 2 diabetes.
The precise mechanisms by which cytokines alter β-cell function and mass have not been established conclusively. Cytokine-induced β-cell failure is likely mediated by nitric oxide (NO) that results from activation of inducible nitric oxide synthase [16], but NO-independent inflammatory mechanisms have also been suggested [17–19]. Cytokine-provoked NO may inhibit glycolysis [20–24] and/or the mitochondrial TCA cycle [25,26], but functional bioenergetic consequences of such inhibition have not been demonstrated to date. It is worth notice in this respect that NO may in fact benefit the bioenergetics of inflamed cells as it stimulates mitochondrial biogenesis under certain circumstances [27]. Moreover, it remains unclear if and how inflammatory GSIS defects relate mechanistically to β-cell failure caused by nutrient excess [28].
Mitochondria are essential for GSIS as glucose-fueled oxidative ATP synthesis causes a rise in the cytosolic ATP/ADP ratio, which triggers the electrophysiological events that are responsible for the eventual exocytosis of insulin-containing granules [29]. Moreover, β-cells fully depend on mitochondria to meet the high energy cost of insulin synthesis (cf. [30]) and exocytosis [31,32] because they are unable to make ATP via anaerobic glycolysis [29]. As mitochondrial dysfunction has indeed been associated with β-cell failure [33] and because mitochondrial targets of cytokine-induced NO have been reported [25,26], we set out to explore possible effects of pro-inflammatory cytokines on real-time mitochondrial activity of INS-1E insulinoma cells. Here we use IL-1β and interferon-gamma (IFN-γ) to model the inflammatory milieu in diabetes and show that these cytokines attenuate GSIS and dampen both glucose-sensitivity and coupling efficiency of oxidative phosphorylation. Concomitantly, these cytokines increase mitochondrial superoxide production and provoke a modest (20%) cell loss. Detailed real-time respiratory analysis reveals that IL-1β and IFN-γ impair ATP synthesis and, consequently, GSIS by restricting the mitochondrial capacity for oxidizing pyruvate.
Materials and methods
Cell culture
Rat INS-1E insulinoma cells (RRID: CVCL_0351), a well-established pancreatic beta-cell model [34] were maintained in RPMI-1640 medium containing 11 mM glucose, 5% (v/v) fetal bovine serum (FBS), 10 mM Hepes, 1 mM sodium pyruvate, 50 U/mL penicillin, 50 μg/mL streptomycin, 500 μM β-mercaptoethanol and 2 mM GlutaMAX [34]. Cells were seeded at 6 x 104 cells/well and, at 70–80% confluence, exposed to a mixture of 2 ng/mL IL-1β and 50 ng/mL IFN-γ, in the absence or presence of non-esterified fatty acids (NEFAs), for 24 hr in RPMI medium containing 11 mM glucose. Notably, the applied cytokine concentrations are used throughout the literature [11,17,35–37], as they reflect pathological levels observed during the development of diabetes. Palmitoleate and linoleate were conjugated to bovine serum albumin (BSA) at molar ratios of 2:1 and 4:1, respectively, yielding free fatty acid levels of 20 nM as estimated assuming binding parameters reported by Huber et al. (2006) [38]. FBS was omitted from the growth medium for NEFA experiments.
Insulin secretion
INS-1E cells seeded and exposed to cytokines ± NEFAs on 96-well culture plates were washed and starved for 1 h at 37 oC under air in low-glucose Krebs-Ringer Hepes (KRH) medium comprising 135 mM NaCl, 3.6 mM KCl, 10 mM Hepes (pH 7.4), 0.5 mM MgCl2, 1.5 mM CaCl2, 0.5 mM NaH2PO4, 2 mM GlutaMAX, 2.5 mM glucose and 0.2% (w/v) BSA. Assay buffer was then replaced with fresh KRH medium and cells were incubated for 30 min on a shaking plate incubator (Labnet International, Oakham, UK) at 100 rpm. Next, supernatants were collected on ice and replaced with KRH containing either 20 mM glucose, 5 mM sodium pyruvate or 30 mM KCl. After another 30-min incubation, supernatants were collected on ice. Supernatants were centrifuged at 1,000g to pellet any detached cells and assayed for insulin by enzyme-linked immunosorbent assay (#10-1247-01, Mercodia, Sweden) or homogenous time-resolved fluorescence (#62IN1PEG, Cisbio Bioassays, France). Secreted insulin was normalized to cell density (see below).
Mitochondrial respiration and superoxide
Mitochondrial respiration was measured in intact attached INS-1E cells as described in detail before [39]. Briefly, INS-1E cells seeded and treated with cytokines ± NEFAs on XF24 cell culture plates (Seahorse Bioscience, Agilent Technologies) were washed into KRH assay buffer and incubated for 50 min at 37 oC under air. XF24 plates were then transferred to a Seahorse XF24 extracellular flux analyzer (controlled at 37°C) for a 10-min calibration and 4 measurement cycles to record basal cellular respiration. Glucose or sodium pyruvate were added at 20 and 5 mM, respectively, to stimulate respiration. Subsequently, 2 μg/mL oligomycin or 3 μM BAM 15 (Tocris Bioscience, Bristol, UK) and a mixture of 2 μM rotenone plus 2 μM antimycin A were added sequentially to, respectively, inhibit the ATP synthase or uncouple oxidative phosphorylation, and determine non-mitochondrial respiration. Superoxide was determined by monitoring MitoSOX and DHE (hydroethidine) oxidation in time as described before [40].
Cell density
Density of attached cells was determined in 96-well plates by 4',6-diamidino-2'-phenylindole dihydrochloride (DAPI) fluorescence [40]. Cells seeded and treated with cytokines ± NEFAs were washed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde (ThermoFisher Scientific, #28908). After fixation, cells were washed again in PBS and then loaded with 0.5 μg/mL DAPI. To limit background detection after loading with DAPI, cells were washed 4 times with PBS. DAPI fluorescence (λex/em = 350/460 nm) was detected using a PHERAstar FS plate reader (BMG LABTECH) in fluorescence intensity, bottom-reading and well-scanning mode.
Statistical analysis
Significance of mean differences was tested by unpaired Student’s t-tests or two-way ANOVA with Sidak multiple comparison analysis using GraphPad Prism Version 6.0 for Mac OS X (GraphPad software, San Diego, CA, USA). Data are presented as means ± SEM.
Results and discussion
Cytokines concomitantly impair GSIS and lower mitochondrial ATP synthesis
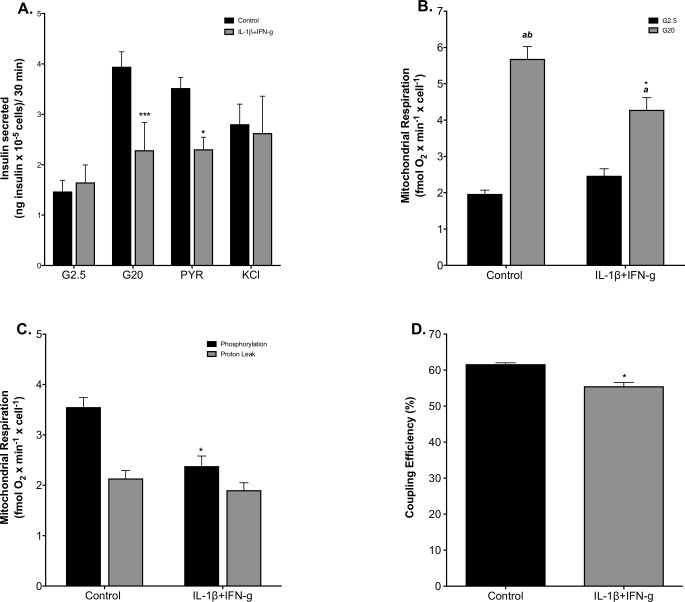
INS-1E insulinoma cells retain many characteristics of primary β-cells, including glucose-sensing ability, which is why they are a widely used β-cell model [34]. In this study, INS-1E cells increased their insulin secretion rate 2.7-fold in response to 20 mM glucose, a stimulation that lowered to 1.4-fold when cells had been exposed to a mixture of IL-1β (2 ng/mL) and IFN-γ (50 ng/mL) for 24 h (Fig 1A). Cytokine attenuation of GSIS is owing to decreased insulin release at a stimulatory glucose level since basal insulin release was unaffected (Fig 1A), which is consistent with the deleterious effects of pro-inflammatory cytokines (including IL-1β, IFN-ɣ and TNF-α) on insulin secretion reported by others [21,41,42]. Notably, cytokine-induced GSIS impairment thus differs from nutrient-provoked secretory defects, which are predominantly owing to stimulated basal insulin release [43]. Pyruvate stimulates insulin secretion to the same extent as glucose, and does so in a cytokine-sensitive manner, whereas KCl-induced insulin secretion is unaffected by cytokines (Fig 1A). Pyruvate stimulation of beta cell activity is not exclusive to insulinoma cells, as insulin secretion by isolated mouse islets is increased to the same extent in response to methyl-pyruvate as to glucose [44]. These data thus demonstrate that secretory defects emerge downstream from glycolysis and upstream from β-cell electrophysiology. Therefore, we determined how cytokines affected glucose-fuelled oxidative phosphorylation. Mirroring the GSIS phenotype, mitochondrial respiration in cytokine-exposed cells was stimulated significantly less by glucose (1.7-fold) than respiration in control cells—2.8-fold stimulation–(Fig 1B). This significant loss of glucose sensitivity is owing to decreased respiration at a high glucose level (Fig 1B) and is consistent with GSIS data. Like basal insulin release, basal mitochondrial respiration was unaffected by cytokines (Fig 1B). To establish the proportion of glucose-stimulated respiration that is used to make ATP, we inhibited respiration with oligomycin. Fig 1C shows that the oligomycin-sensitive oxygen uptake rate was 3.6 fmol oxygen/min/cell on average and the oligomycin-insensitive rate was 2.4 fmol oxygen/min/cell. Notably, IL-1β and IFN-γ lowered oligomycin-sensitive respiration, i.e., oxygen consumption coupled to ATP synthesis, whereas they left oligomycin-resistant respiration, i.e, oxygen consumption linked to mitochondrial proton leak, unaffected (Fig 1C). Consequently, cytokine exposure lowers coupling efficiency of oxidative phosphorylation from 62 ± 0.4% to 56 ± 1.0% (Fig 1D). This coupling efficiency phenotype is statistically significant (P < 0.05) and shows that cytokine exposure decreases the proportion of mitochondrial respiration used to make ATP [45]. Cytokine-induced impairment of GSIS thus coincides with dysfunctional oxidative phosphorylation, which suggests that deficient mitochondrial ATP synthesis is responsible for the insulin secretory defect.
Fig 1. Cytokine-induced GSIS impairment and oxidative phosphorylation defects.
Cells were grown in fully supplemented RPMI (black bars) or exposed for 24 h to a combination of 2 ng/mL IL-1β plus 50 ng/mL IFN-γ (grey bars). The rate of insulin secretion was normalized to cell number (A) and was measured at 2.5 mM glucose (G2.5), 20 mM glucose (G20), 5 mM sodium pyruvate (PYR) or 30 mM KCl. Absolute mitochondrial respiration normalized to cell number was measured ± 20 mM glucose (B)—grey and black bars, respectively. Glucose-stimulated respiration used to make ATP or associated with proton leak was determined as oligomycin-sensitive or oligomycin-resistant mitochondrial oxygen uptake, respectively (C)—black and grey bars, respectively. Coupling efficiency of oxidative phosphorylation was calculated as the percentage of glucose-stimulated oxygen uptake used to make ATP (D). Data are means ± SEM from 3–7 individual experiments (S1 Data) with each condition repeated 3–5 times. Statistical significance of mean differences was tested by 2-way ANOVA: asterisks indicate statistically significant differences from equivalent RPMI controls (*P < 0.05 and ***P < 0.001). a, ab differs from the low-glucose condition (P < 0.05 and P < 0.001, respectively).
Cytokines restrict mitochondrial pyruvate oxidation capacity
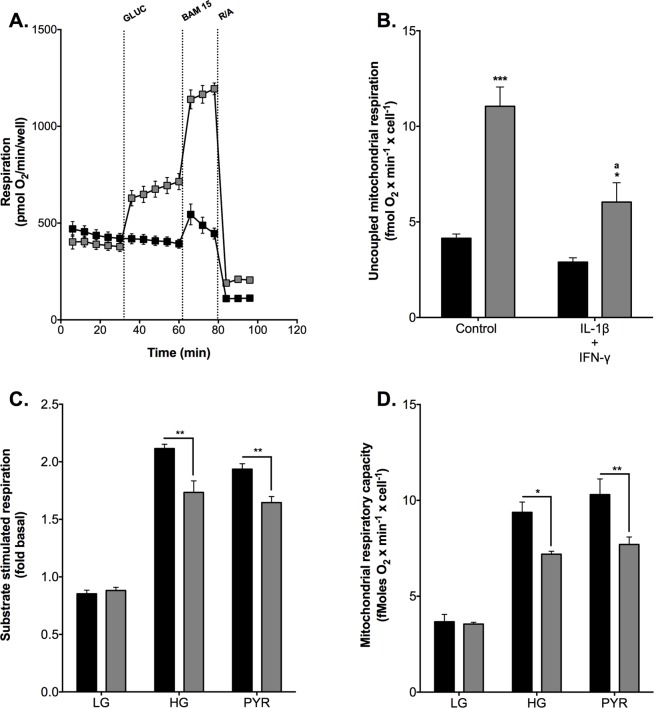
To better understand how cytokines inhibit mitochondrial ATP synthesis, we probed their effect on oxidative phosphorylation in more detail. Interestingly, glucose stimulation of respiration appeared most pronounced in the presence of BAM 15 (Fig 2A), a protonophore that uncouples oxidative phosphorylation. Uncoupled mitochondrial oxygen uptake is not controlled by ATP synthesis or ATP turnover, and thus generally reflects the capacity of cells for oxidizing fuel. The fuel oxidation capacity of INS-1E cells thus appears limited by the concentration of glucose (Fig 2B), which sits well with the glucose sensitivity of oxidative phosphorylation of pancreatic β-cells [46,47]. Cytokine exposure inhibits both coupled and uncoupled mitochondrial respiration (Fig 2B), which shows the respiratory inhibition emerges from a negative effect on glucose oxidation. Identical data were obtained when respiration was stimulated with pyruvate instead of glucose (Fig 2D). Pyruvate increases coupled (Fig 2C) and uncoupled (Fig 2D) mitochondrial oxygen consumption to the same extent as glucose, and the cytokine sensitivity of stimulated respiration is independent of fuel type Fig 2C and 2D. These respiratory data show that cytokines impair INS-1E cell function through inhibition of pyruvate oxidation, which is consistent with our GSIS data (Fig 1). The data rule out that IL-1β and IFN-γ exerted inflammatory effects upstream from mitochondria under the exposure conditions we applied.
Fig 2. Cytokine-induced restriction of mitochondrial pyruvate oxidation.
(A) mean respiratory traces illustrating the effect of glucose on coupled and uncoupled oxygen uptake. Cells were incubated in glucose-free KRH and then subjected to 20 mM glucose after 5 basal respiratory measurements (grey symbols)–the glucose level was not changed in control cells (black symbols). Subsequently, respiration was stimulated with 3 μM BAM 15 and then inhibited with a mix of 2 μM rotenone and 2 μM antimycin A (R/A). (B) cells were grown in fully supplemented RPMI (control) or exposed for 24 h to 2 ng/mL IL-1β plus 50 ng/mL IFN-γ. Mitochondrial respiration was uncoupled with 3 μM BAM 15 in the presence or absence of 20 mM glucose (grey and black symbols, respectively). Panels (C) and (D) cells were grown in full RPMI (black bars) or were exposed to 2 ng/mL IL-1β plus 50 ng/mL IFN-γ (grey bars). Respiration was stimulated with 20 mM glucose (HG) or 5 mM sodium pyruvate (PYR) in the absence (C) or presence (D) of 3 μM BAM 15, and substrate responses were normalized to basal oxygen uptake measured without added substrate–control cells were not stimulated by fuel (LG). Data are means ± SEM from 3 individual experiments (S1 Data) with each condition repeated 3–4 times. Mean differences were tested for statistical significance by 2-way ANOVA. (B) *,***differs from the no-glucose condition (P < 0.05 and P < 0.001, respectively); adiffers from high-glucose control condition (P < 0.01). Panels (C) and (D) *,**cytokine and control conditions differ (P < 0.05 and P < 0.01, respectively).
Cytokines increase mitochondrial superoxide and cause cell loss
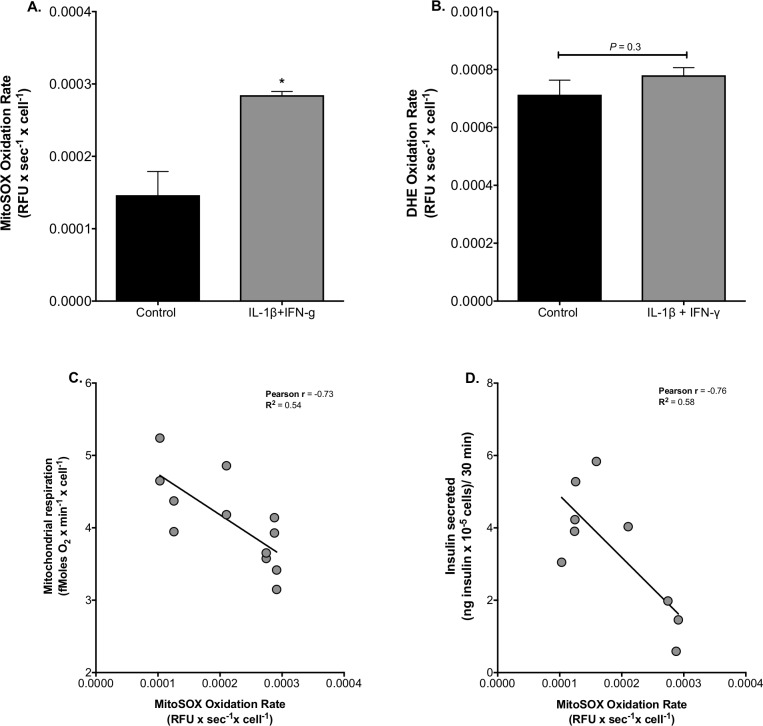
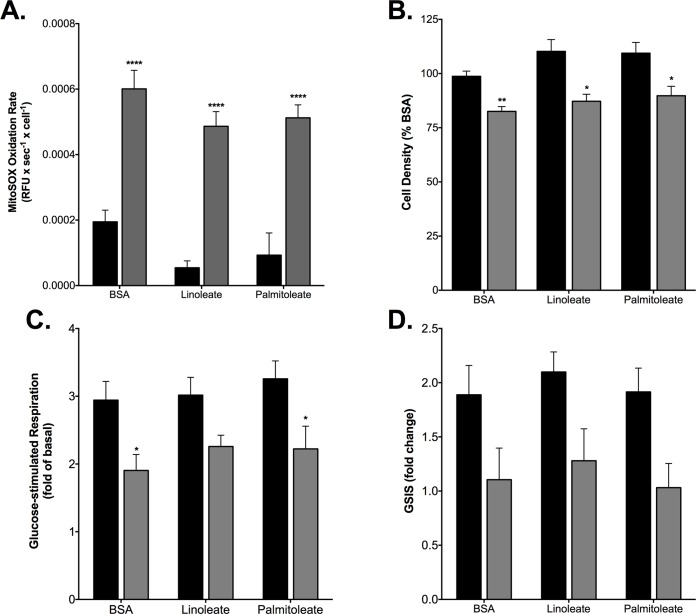
Pro-inflammatory cytokines are known to provoke production of reactive oxygen species (ROS) in β-cells [48] and, consistently, IL-1-β plus IFN-γ exposure increases the rate at which INS-1E cells oxidize MitoSOX ~ 2-fold (Fig 3A). MitoSOX is a mitochondria-targeted hydroethidine(DHE)-based probe that is widely used to detect superoxide, although it should be noted that the probe can also be oxidized by hydrogen peroxide (in the presence of peroxidases) and intracellular oxidases [49]. It is conceivable that cytokine-induced mitochondrial ROS are owing to the restricted pyruvate oxidation capacity. Oxidation of non-targeted DHE is not affected by cytokine exposure (Fig 3B). Glucose-stimulated mitochondrial respiration (Fig 3C) and glucose-stimulated insulin secretion (Fig 3D) both correlate inversely with MitoSOX oxidation rate, suggesting the possibility that cytokine-induced superoxide reinforces the inhibition of pyruvate oxidation that accounts for GSIS impairment. The cytokine effect on MitoSOX oxidation (Fig 3A) is reminiscent of that induced by NEFAs: 24-h palmitate exposure, against a high-glucose background, increases mitochondrial superoxide, an effect that correlates strongly with the loss of INS-1E cells and, notably, is largely prevented by palmitoleate, the monounsaturated counterpart of palmitate [40,50]. In contrast, unsaturated NEFAs do not attenuate cytokine-stimulated superoxide, as neither linoleate nor palmitoleate significantly lowers the cytokine-provoked MitoSOX oxidation rate seen in BSA-treated control cells (Fig 4A). Exposure to IL-1-β plus IFN-γ causes a 20% decrease of cell density (Fig 4B), which is likely owing to ROS-induced apoptosis [51,52], however we cannot exclude other mechanisms of cytokine-induced beta-cell death which have also been suggested [53,54]. This 20% decrease is small compared to that caused by palmitate [40,50] and, importantly, is not prevented by linoleate or palmitoleate (Fig 4B). Together, these data suggest that cytokine-induced generation of ROS and associated loss of cell density are mechanistically unrelated to palmitate-induced changes in ROS production. Cytokine inhibition of mitochondrial respiration (Fig 4C) and insulin secretion in response to 20 mM glucose (Fig 4D) are also not influenced by linoleate or palmitoleate.
Fig 3. Cytokine-induced mitochondrial superoxide.
(A) cells were grown in full RPMI (black bars) or were exposed for 24 h to 2 ng/mL IL-1β plus 50 ng/mL IFN-γ (grey bars). MitoSOX (A) and DHE (B) oxidation rates were measured without added glucose as described before [40] and the presented data are means of 3 independent experiments with each condition repeated 4–5 times. Mean differences were tested for statistical significance by unpaired Student's t-tests: *differs from the control condition (P < 0.05). Glucose-stimulated mitochondrial respiration (C) and glucose-stimulated insulin secretion (D) correlate inversely with the MitoSOX oxidation rate as confirmed by Pearson correlation analysis (P < 0.01 and P < 0.05, respectively). Data are stand alone repeats and were collected from 3 independent experiments (S1 Data).
Fig 4. Unsaturated NEFAs do not protect against cytokine-induced β-cell failure.
Cells were exposed for 24 h to 2 ng/mL IL-1β plus 50 ng/mL IFN-γ (grey bars) in serum-deprived RPMI containing BSA-conjugated linoleate or palmitoletae, or BSA alone. Control cells were incubated in the same media lacking the cytokines (black bars). NEFA effects were determined on MitoSOX oxidation rate (A)—normalized to cell number), cell density (B)—normalized to BSA control not exposed to cytokines, glucose-stimulated coupled mitochondrial respiration (C)—normalized to coupled respiration without added glucose and GSIS (D). Data are means ± SEM of 4 independent experiments (S1 Data) with each condition repeated 5 times. Mean differences were tested for statistical significance by 2-way ANOVA: *,**,****differs from the control condition (P < 0.05, P < 0.01 and P < 0.0001, respectively).
Discussion
Pro-inflammatory cytokines have well-established detrimental effects on the function and viability of pancreatic β-cells [4,35,52,55]. Our detailed real-time respiratory analysis reported here reveals functional consequences of cytokine exposure for β-cell oxidative phosphorylation, and highlights INS-1E cells as powerful model of β-cell bioenergetics. Previously, we predicted a dual role of mitochondrial uncoupling protein-2 in GSIS regulation and oxidative stress protection [56], which has proven correct in pancreatic islets [57,58]. Moreover, toxic nutrient effects on the bioenergetic behaviour of INS-1E cells align closely with those observed in islets [43]. Our current findings offer novel insight in the effects of cytokines on real-time mitochondrial function in β-cells and conclusively link a restricted pyruvate oxidation capacity to cytokine-impaired GSIS. Moreover, our results dissociate inflammatory defects from palmitate-induced defects, and provide clues as to how unsaturated NEFAs may protect against nutrient-induced β-cell damage.
Mechanism of cytokine-induced GSIS impairment
Mitochondrial ATP synthesis is crucial for nutrient-secretion coupling in β-cells as bioenergetic fuel sensitivity is responsible for the glucose-induced rise in the cytosolic ATP/ADP ratio that triggers insulin secretion [47]. Our functional respiratory data reveal that IL-1β plus IFN-γ impair GSIS by restricting mitochondrial capacity for oxidising pyruvate (Fig 2), which is consistent with a well-established aconites inhibition by cytokine-induced NO [25,26]. Reported glucokinase inhibition [20–24] unlikely accounts for the inflammatory GSIS phenotype we report here (Fig 1A) as glucose- and pyruvate-stimulated electron transfer capacity are equally sensitive to IL-1β plus IFN-γ. Restricted pyruvate oxidation capacity dampens glucose sensitivity (Fig 2B) and coupling efficiency of oxidative phosphorylation (Fig 2C), and thus likely prevents an increase in the ATP/ADP ratio that initiates the electrophysiological events that are necessary for Ca2+ influx and eventual exocytosis of insulin-containing granules. Indeed, this pathological order of events (Fig 5) agrees with the lack of cytokine effect on KCl-induced insulin secretion (Fig 1A). The mechanism by which IL-1β plus IFN-γ inhibit pyruvate oxidation cannot be concluded directly from our data, but NO-involvement is likely [16,59]. Indeed, aconitase inhibition by NO [25,26] is expected to lower pyruvate oxidation as is NO inhibition of cytochrome c oxidase [60]. Moreover, cytokine-induced superoxide (Fig 3), which could arise from several sites [61] following increased reduction of the pyruvate oxidation apparatus, may react with NO to form peroxynitrite [62] that could exacerbate inflammatory respiratory dysfunction and consequent GSIS impairment (Fig 5). Our mitochondrial superoxide data (Fig 3) are consistent with a well-established ROS involvement in cytokine-induced beta cell failure [21,63–65]. Worth notice, it has been suggested that superoxide may in fact ameliorate cytokine-provoked β-cell dysfunction by converting NO to peroxynitrite [66].
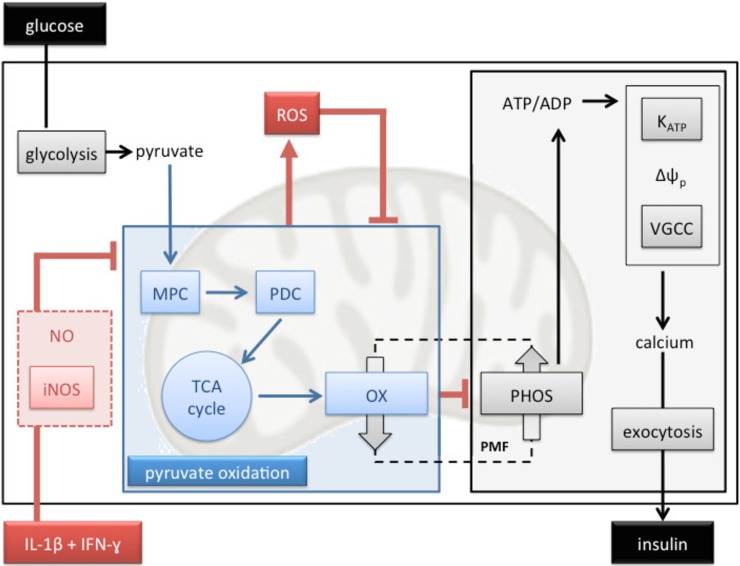
Fig 5. Mechanistic model of cytokine-induced GSIS impairment.
Pancreatic β-cells respond to a rise in the blood glucose level by increasing their oxidative metabolism. Pyruvate that results from increased glycolysis is imported to mitochondria by the mitochondrial pyruvate carrier (MPC) and then oxidized by the pyruvate dehydrogenase complex (PDC) to acetyl-CoA, which fuels the tricarboxylic acid (TCA) cycle. Reducing power generated by these processes is oxidized by the mitochondrial electron transfer chain (OX) that conserves liberated energy in a protonmotive force (PMF), which drives ADP phosphorylation (PHOS). The consequent rise in the cytosolic ATP/ADP ratio inhibits ATP-sensitive potassium channels (KATP), decreases the plasma membrane potential (Δψp), opens voltage-gated calcium channels (VGCC), causes Ca2+ influx, and triggers exocytosis of insulin-containing granules. Exposure to IL-1β plus IFN-γ inhibits mitochondrial pyruvate oxidation (blue box) likely via activation of inducible nitric oxide synthase (iNOS) and consequent NO formation. Inhibition of pyruvate oxidation dampens bioenergetic glucose sensitivity and thus attenuates GSIS. Cytokine-induced reactive oxygen species (ROS) are a plausible consequence of inhibited pyruvate oxidation and may reinforce this inhibition.
Palmitate and cytokine phenotypes are mechanistically distinct
Circulating NEFAs and cytokines are considered important molecular mediators that link obesity to type 2 diabetes [12] through mechanisms that are possibly related. Specifically, nutrient excess that characterizes obesity has been shown to stimulate the production of pro-inflammatory cytokines in human islets by NEFA-binding to β-cell toll-like receptors [67] and consequent upregulation of NF-κB signaling [12]. Nutrient surplus further causes β-cell inflammation by activating the NLRP3 inflammasome [12]. Although IL-1β plus IFN-γ indeed cause dysfunction and loss of INS-1E cells that appear similar to palmitate-induced defects [40,43,50,68], our data reveal that the underlying mechanisms are different. Both cytokines and palmitate cause oxidative phosphorylation defects, but attenuation of GSIS coincides with deficient ATP synthesis in the case of inflammatory stress (Fig 1), whereas it precedes mitochondrial dysfunction in the case of nutrient stress [43]. Furthermore, restricted oxidative capacity appears the sole cause of cytokine-impaired GSIS (Fig 5), whereas the deleterious palmitate GSIS phenotype is largely owing to stimulation of basal insulin secretion, most likely secondary to electrophysiological defects [43]. Cytokine effects on electrophysiology and insulin exocytosis are unlikely in our experiments as KCl-induced insulin secretion from INS-1E cells was not affected by IL-1ß and IFN-γ (Fig 1A). In agreement with [69], we also reveal that palmitate and cytokines stimulate mitochondrial superoxide production via distinct mechanisms, since palmitate-induced oxidative stress and consequent loss of INS-1E cells are prevented by palmitoleate [40,50], whilst equivalent cytokine phenotypes are unaffected by palmitoleate and linoleate (Fig 4).
Protection against nutrient-induced β-cell failure
It is generally unclear how unsaturated NEFAs protect against damage caused by their saturated counterparts [70]. As discussed above, the cytokine defects we report here are not prevented by unsaturated NEFAs, which disagrees with observations published by others [35]. This disagreement may arise from experimental differences, which would suggest that protection by unsaturated NEFAs against inflammatory cell stress is not particularly robust. Because the design of our palmitate and cytokine experiments is comparable, the differential protection by unsaturated NEFAs against distinct oxidative stress mechanisms offers clues as to how palmitoleate prevents palmitate-induced cell loss. Specifically, our findings reveal that palmitoleate does not prevent ROS from triggering apoptosis, and, therefore, that protection must arise during ROS production. Both palmitate [40,50] and cytokines Figs 3A and 4A stimulate mitochondrial superoxide, but by distinct mechanisms given the differential palmitoleate effect on the rate of MitoSOX oxidation. Our cytokine exposures likely stimulate superoxide generation from one or more of the mitochondrial sites that are involved in (restricted) pyruvate oxidation (Fig 5), which include the pyruvate dehydrogenase complex and respiratory complexes I, II and III [61]. Palmitate exposure is expected to increase ROS production from the same sites against a background of high glucose [71], but, in addition, may cause superoxide production from the electron-transferring flavoprotein [61], which reduces the mitochondrial electron transfer chain during fatty acid β-oxidation. We speculate that unsaturated NEFAs may lower ROS production from this site.
Conclusion
Our results identify pyruvate oxidation defects as an early step in cytokine-induced GSIS impairment in INS-1E cells. Given the previous consistency between bioenergetic effects in INS-1E cells and pancreatic islets (cf. papers cited above), the reported data suggest the mitochondrial electron transfer chain as a possible target for therapeutic prevention of pro-inflammatory loss of β-cell function and mass. As palmitate- and cytokine-induced defects arise via distinct mechanisms, multiple pharmacological interventions may be necessary to manage β-cell failure in obesity. Although the mechanism by which unsaturated NEFAs protect against obesity-related β-cell loss and dysfunction remains to be elucidated, our data suggest that their use against inflammatory stress is limited.
Supporting information
Excel spreadsheet containing all relevant individual data values that were used to generate mean averages and standard errors for all data figures presented throughout this manuscript.
(XLSX)
Acknowledgments
We thank Professor Noel Morgan (Institute of Biomedical and Clinical Science, University of Exeter, Exeter, UK) for donating INS-1E insulinoma cells. Current address for Jonathan Barlow is School of Sport, Exercise and Rehabilitation Sciences, University of Birmingham, UK and should be used for any correspondence.
Data Availability
All of the data used to generate the figures within this manuscript are either contained within the figures themselves or are available in the excel supporting document file.
Funding Statement
This work was supported by the Medical Research Council (New Investigator Research Grant G1100165 to CA), and the European Commission (Marie Skłodowska-Curie Research Fellowship 656775 to TS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kahn BB. Type 2 Diabetes: When Insulin Secretion Fails to Compensate for Insulin Resistance. Cell. Elsevier; 1998;92: 593–596. doi: 10.1016/S0092-8674(00)81125-3 [DOI] [PubMed] [Google Scholar]
- 2.Briaud I, Harmon JS, Kelpe CL, Segu VBG, Poitout V. Lipotoxicity of the Pancreatic β-Cell Is Associated With Glucose-Dependent Esterification of Fatty Acids Into Neutral Lipids. Diabetes. American Diabetes Association; 2001;50: 315–321. doi: 10.2337/diabetes.50.2.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotamisligil GOKS. Inflammation and metabolic disorders. Nature. Nature Publishing Group; 2006;444: 860–867. doi: 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- 4.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. Journal of Clinical Investigation. 2002;110: 851–860. doi: 10.1172/JCI15318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donath MY, Böni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet Inflammation Impairs the Pancreatic β-Cell in Type 2 Diabetes. Physiology. American Physiological Society; 2009;24: 325–331. doi: 10.1152/physiol.00032.2009 [DOI] [PubMed] [Google Scholar]
- 6.Holohan C, Szegezdi E, Ritter T, O'Brien T, Samali A. Cytokine-induced beta-cell apoptosis is NO-dependent, mitochondria-mediated and inhibited by BCL-XL. J Cell Mol Med. Blackwell Publishing Ltd; 2008;12: 591–606. doi: 10.1111/j.1582-4934.2007.00191.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen CS, Christensen DP, Lundh M, Dahllöf MS, Haase TN, Velasquez JM, et al. Skeletal Muscle to Pancreatic β-Cell Cross-talk: The Effect of Humoral Mediators Liberated by Muscle Contraction and Acute Exercise on β-Cell Apoptosis. J Clin Endocrinol Metab. 2015;100: E1289–98. doi: 10.1210/jc.2014-4506 [DOI] [PubMed] [Google Scholar]
- 8.De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends in Immunology. 2011;32: 373–379. doi: 10.1016/j.it.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant RW, Dixit VD. Mechanisms of disease: inflammasome activation and the development of type 2 diabetes. Frontiers in Immunology. Frontiers Media SA; 2013;4: 50 doi: 10.3389/fimmu.2013.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donath MY. Targeting inflammation in the treatment of type 2 diabetes. Diabetes Obes Metab. Blackwell Publishing Ltd; 2013;15 Suppl 3: 193–196. doi: 10.1111/dom.12172 [DOI] [PubMed] [Google Scholar]
- 11.Burke SJ, Stadler K, Lu D, Gleason E, Han A, Donohoe DR, et al. IL-1β reciprocally regulates chemokine and insulin secretion in pancreatic β-cells via NF-κB. American Journal of Physiology—Endocrinology and Metabolism. American Physiological Society; 2015;309: E715–26. doi: 10.1152/ajpendo.00153.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donath MY, Dalmas É, Sauter NS, Böni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metabolism. 2013;17: 860–872. doi: 10.1016/j.cmet.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 13.Jaksch C, Thams P. A critical role for CK2 in cytokine-induced activation of NFκB in pancreatic β cell death. Endocrine. Springer US; 2014;47: 117–128. doi: 10.1007/s12020-013-0133-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27: 519–550. doi: 10.1146/annurev.immunol.021908.132612 [DOI] [PubMed] [Google Scholar]
- 15.Böni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, et al. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. Journal of Clinical Endocrinology & Metabolism. 2008;93: 4065–4074. doi: 10.1210/jc.2008-0396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbett JA, Sweetland MA, Wang JL, J R Lancaster J, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proceedings of the National Academy of Sciences. National Acad Sciences; 1993;90: 1731–1735. doi: 10.1073/pnas.90.5.1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eizirik DL, Sandler S, Welsh N, CETKOVICCVRLJE M, NIEMAN A, GELLER DA, et al. Cytokines Suppress Human Islet Function Irrespective of Their Effects on Nitric-Oxide Generation. Journal of Clinical Investigation. American Society for Clinical Investigation; 1994;93: 1968–1974. doi: 10.1172/JCI117188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu DB, Pavlovic D, Chen MC, Flodstrom M, Sandler S, Eizirik DL. Cytokines induce apoptosis in beta-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS(-/-)). Diabetes. American Diabetes Association; 2000;49: 1116–1122. doi: 10.2337/diabetes.49.7.1116 [DOI] [PubMed] [Google Scholar]
- 19.Andersson AK, Flodstrom M, Sandler S. Cytokine-induced inhibition of insulin release from mouse pancreatic beta-cells deficient in inducible nitric oxide synthase. Biochemical and Biophysical Research Communications. 2001;281: 396–403. doi: 10.1006/bbrc.2001.4361 [DOI] [PubMed] [Google Scholar]
- 20.Meredith M, Rabaglia ME, Corbett JA, Metz SA. Dual functional effects of interleukin-1beta on purine nucleotides and insulin secretion in rat islets and INS-1 cells. Diabetes. 1996;45: 1783–1791. [DOI] [PubMed] [Google Scholar]
- 21.Vasu S, McClenaghan NH, McCluskey JT, Flatt PR. Mechanisms of toxicity by proinflammatory cytokines in a novel human pancreatic beta cell line, 1.1B4. 2014;1840: 136–145. doi: 10.1016/j.bbagen.2013.08.022 [DOI] [PubMed] [Google Scholar]
- 22.Beggs M, Beresford G, Clarke J, Mertz R, Espinal J, Hammonds P. Interleukin-1? inhibits glucokinase activity in clonal HIT-T15?-cells. FEBS Lett. 2001;267: 217–220. doi: 10.1016/0014-5793(90)80928-C [DOI] [PubMed] [Google Scholar]
- 23.Ma Z, Landt M, Bohrer A, Ramanadham S, Kipnis DM, Turk J. Interleukin-1 reduces the glycolytic utilization of glucose by pancreatic islets and reduces glucokinase mRNA content and protein synthesis by a nitric oxide-dependent mechanism. Journal of Biological Chemistry. American Society for Biochemistry and Molecular Biology; 1997;272: 17827–17835. doi: 10.1074/jbc.272.28.17827 [DOI] [PubMed] [Google Scholar]
- 24.Takeda T, Tsuura Y, Fujita J, Fujimoto S, Mukai E, Kajikawa M, et al. Heat Shock Restores Insulin Secretion after Injury by Nitric Oxide by Maintaining Glucokinase Activity in Rat Islets. Biochemical and Biophysical Research Communications. 2001;284: 20–25. doi: 10.1006/bbrc.2001.4933 [DOI] [PubMed] [Google Scholar]
- 25.Welsh N, Eizirik DL, Bendtzen K, Sandler S. Interleukin-1-Beta-Induced Nitric-Oxide Production in Isolated Rat Pancreatic-Islets Requires Gene-Transcription and May Lead to Inhibition of the Krebs Cycle Enzyme Aconitase. Endocrinology. 1991;129: 3167–3173. doi: 10.1210/endo-129-6-3167 [DOI] [PubMed] [Google Scholar]
- 26.Welsh N, Sandler S. Interleukin-1? induces nitric oxide production and inhibits the activity of aconitase without decreasing glucose oxidation rates in isolated mouse pancreatic islets. Biochemical and Biophysical Research Communications. 1992;182: 333–340. doi: 10.1016/S0006-291X(05)80149-4 [DOI] [PubMed] [Google Scholar]
- 27.D C, A P. Regulation of Mitochondrial Biogenesis and Its Intersection with Inflammatory Responses. Antioxid Redox Signal. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA; 2015;22: 965–976. doi: 10.1089/ars.2014.6200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontés G. Glucolipotoxicity of the pancreatic beta cell. Biochimica et Biophysica Acta (BBA)—Molecular and Cell Biology of Lipids. 2010;1801: 289–298. doi: 10.1016/j.bbalip.2009.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutter GA, Pullen TJ, Hodson DJ, Martinez Sanchez A. Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochemical Journal. 2015;466: 203–218. doi: 10.1042/BJ20141384 [DOI] [PubMed] [Google Scholar]
- 30.Nisr RB, Affourtit C. Palmitate-induced changes in energy demand cause reallocation of ATP supply in rat and human skeletal muscle cells. Biochimica et Biophysica Acta (BBA)—Bioenergetics. 2016;1857: 1403–1411. doi: 10.1016/j.bbabio.2016.04.286 [DOI] [PubMed] [Google Scholar]
- 31.Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46: 1029–1045. doi: 10.1007/s00125-003-1153-1 [DOI] [PubMed] [Google Scholar]
- 32.Barg S. Mechanisms of exocytosis in insulin-secreting B-cells and glucagon-secreting A-cells. Pharmacol Toxicol. 2003;92: 3–13. [DOI] [PubMed] [Google Scholar]
- 33.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. American Association for the Advancement of Science; 2005;307: 384–387. doi: 10.1126/science.1104343 [DOI] [PubMed] [Google Scholar]
- 34.Merglen A. Glucose Sensitivity and Metabolism-Secretion Coupling Studied during Two-Year Continuous Culture in INS-1E Insulinoma Cells. Endocrinology. 2003;145: 667–678. doi: 10.1210/en.2003-1099 [DOI] [PubMed] [Google Scholar]
- 35.Welters HJ, Tadayyon M, Scarpello JHB, Smith SA, Morgan NG. Mono-unsaturated fatty acids protect against β-cell apoptosis induced by saturated fatty acids, serum withdrawal or cytokine exposure. FEBS Lett. 2004;560: 103–108. doi: 10.1016/S0014-5793(04)00079-1 [DOI] [PubMed] [Google Scholar]
- 36.Collier JJ, Burke SJ, Eisenhauer ME, Lu D, Sapp RC, Frydman CJ, et al. Pancreatic β-cell death in response to pro-inflammatory cytokines is distinct from genuine apoptosis. PLoS One. Public Library of Science; 2011;6: e22485 doi: 10.1371/journal.pone.0022485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hostens K, Pavlovic D, Zambre Y, Ling Z, Van Schravendijk C, Eizirik DL, et al. Exposure of human islets to cytokines can result in disproportionately elevated proinsulin release. Journal of Clinical Investigation. American Society for Clinical Investigation; 1999;104: 67–72. doi: 10.1172/JCI6438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huber AH, Kampf JP, Kwan T, Zhu B, Kleinfeld AM. Fatty acid-specific fluorescent probes and their use in resolving mixtures of unbound free fatty acids in equilibrium with albumin. Biochemistry. 2006;45: 14263–14274. doi: 10.1021/bi060703e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Affourtit C, Brand MD. Chapter 23 Measuring Mitochondrial Bioenergetics in INS-1E Insulinoma Cells Mitochondrial Function, Part B: Mitochondrial Protein Kinases, Protein Phosphatases and Mitochondrial Diseases. Elsevier; 2009. pp. 405–424. doi: 10.1016/S0076-6879(09)05023-X [DOI] [PubMed] [Google Scholar]
- 40.Barlow J, Hirschberg-Jensen V, Affourtit C. Uncoupling protein-2 attenuates palmitoleate protection against the cytotoxic production of mitochondrial reactive oxygen species in INS-1E insulinoma cells. Redox Biology. Elsevier; 2015;4: 14–22. doi: 10.1016/j.redox.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim H-E, Choi S-E, Lee S-J, Lee J-H, Lee Y-J, Kang SS, et al. Tumour necrosis factor-alpha-induced glucose-stimulated insulin secretion inhibition in INS-1 cells is ascribed to a reduction of the glucose-stimulated Ca2+ influx. Journal of Endocrinology. BioScientifica; 2008;198: 549–560. doi: 10.1677/JOE-08-0131 [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Kim K-H. TNF‐α inhibits glucose‐induced insulin secretion in a pancreatic β‐cell line (INS‐1). FEBS Lett. 1995;377: 237–239. doi: 10.1016/0014-5793(95)01272-9 [DOI] [PubMed] [Google Scholar]
- 43.Barlow J, Hirschberg-Jensen V, Jastroch M, Affourtit C. Palmitate-induced impairment of glucose-stimulated insulin secretion precedes mitochondrial dysfunction in mouse pancreatic islets Portland Press Limited; 2016;473: 487–496. doi: 10.1042/BJ20151080 [DOI] [PubMed] [Google Scholar]
- 44.Kabra UD, Pfuhlmann K, Migliorini A, Keipert S, Lamp D, Korsgren O, et al. Direct Substrate Delivery into Mitochondrial-Fission Deficient Pancreatic Islets Rescues Insulin Secretion. Diabetes. 2017. doi: 10.2337/db16-1088 [DOI] [PubMed] [Google Scholar]
- 45.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochemical Journal. 3rd ed. 2011;435: 297–312. doi: 10.1042/BJ20110162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Affourtit C, Alberts B, Barlow J, Carré JE, Wynne AG. Control of pancreatic β-cell bioenergetics Biochem Soc Trans. Portland Press Limited; 2018;: BST20170505. doi: 10.1042/BST20170505 [DOI] [PubMed] [Google Scholar]
- 47.Nicholls DG. The Pancreatic β-Cell: A Bioenergetic Perspective. Physiological reviews. American Physiological Society; 2016;96: 1385–1447. doi: 10.1152/physrev.00009.2016 [DOI] [PubMed] [Google Scholar]
- 48.Newsholme P, Morgan D, Rebelato E, Oliveira-Emilio HC, Procopio J, Curi R, et al. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia. 2009;52: 2489–2498. doi: 10.1007/s00125-009-1536-z [DOI] [PubMed] [Google Scholar]
- 49.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proceedings of the National Academy of Sciences. 2006;103: 15038–15043. doi: 10.1073/pnas.0601945103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barlow J, Affourtit C. Novel insights into pancreatic β-cell glucolipotoxicity from real-time functional analysis of mitochondrial energy metabolism in INS-1E insulinoma cells Portland Press Ltd; 2013;456: 417–426. doi: 10.1042/BJ20131002 [DOI] [PubMed] [Google Scholar]
- 51.Lin N, Chen H, Zhang H, Wan X, Su Q. Mitochondrial reactive oxygen species (ROS) inhibition ameliorates palmitate-induced INS-1 beta cell death. Endocrine. 2012;42: 107–117. doi: 10.1007/s12020-012-9633-z [DOI] [PubMed] [Google Scholar]
- 52.Morgan D, Oliveira-Emilio HR, Keane D, Hirata AE, Santos da Rocha M, Bordin S, et al. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia. 2006;50: 359–369. doi: 10.1007/s00125-006-0462-6 [DOI] [PubMed] [Google Scholar]
- 53.Collier JJ, Fueger PT, Hohmeier HE, Newgard CB. Pro- and antiapoptotic proteins regulate apoptosis but do not protect against cytokine-mediated cytotoxicity in rat islets and beta-cell lines. Diabetes. 2006;55: 1398–1406. [DOI] [PubMed] [Google Scholar]
- 54.Steer SA, Scarim AL, Chambers KT, Corbett JA. Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med. Public Library of Science; 2006;3: e17 doi: 10.1371/journal.pmed.0030017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morgan D, Rebelato E, Abdulkader F, Graciano MFR, Oliveira-Emilio HR, Hirata AE, et al. Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta-cells. Endocrinology. 2009;150: 2197–2201. doi: 10.1210/en.2008-1149 [DOI] [PubMed] [Google Scholar]
- 56.Affourtit C, Jastroch M, Brand MD. Uncoupling protein-2 attenuates glucose-stimulated insulin secretion in INS-1E insulinoma cells by lowering mitochondrial reactive oxygen species. Free Radical Biology and Medicine. 2011;50: 609–616. doi: 10.1016/j.freeradbiomed.2010.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic β-cell function. Diabetes Obes Metab. 2010;12: 141–148. doi: 10.1111/j.1463-1326.2010.01269.x [DOI] [PubMed] [Google Scholar]
- 58.Robson-Doucette CA, Sultan S, Allister EM, Wikstrom JD, Koshkin V, Bhattacharjee A, et al. -Cell Uncoupling Protein 2 Regulates Reactive Oxygen Species Production, Which Influences Both Insulin and Glucagon Secretion. Diabetes. 2011;60: 2710–2719. doi: 10.2337/db11-0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newsholme P 1, Cruzat VF ,2, Keane KN 1, Carlessi R 1, de Bittencourt PI Jr 3 Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochemical Journal. 2016;473: 4527–4550. doi: 10.1042/BCJ20160503C [DOI] [PubMed] [Google Scholar]
- 60.Brown GC. Nitric oxide and mitochondria. Front Biosci. 2007. [DOI] [PubMed] [Google Scholar]
- 61.Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radical Biology and Medicine. 2016. doi: 10.1016/j.freeradbiomed.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 62.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271: C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424 [DOI] [PubMed] [Google Scholar]
- 63.Hohmeier HE, Thigpen A, Tran VV, Davis R, Newgard CB. Stable expression of manganese superoxide dismutase (MnSOD) in insulinoma cells prevents IL-1beta- induced cytotoxicity and reduces nitric oxide production. Journal of Clinical Investigation. American Society for Clinical Investigation; 1998;101: 1811–1820. doi: 10.1172/JCI1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azevedo-Martins AK, Lortz S, Lenzen S, Curi R, Eizirik DL, Tiedge M. Improvement of the mitochondrial antioxidant defense status prevents cytokine-induced nuclear factor-kappa B activation in insulin-producing cells. Diabetes. American Diabetes Association; 2003;52: 93–101. doi: 10.2337/diabetes.52.1.93 [DOI] [PubMed] [Google Scholar]
- 65.Lortz S, Gurgul-Convey E, Naujok O, Lenzen S. Overexpression of the antioxidant enzyme catalase does not interfere with the glucose responsiveness of insulin-secreting INS-1E cells and rat islets. Diabetologia. Springer-Verlag; 2013;56: 774–782. doi: 10.1007/s00125-012-2823-7 [DOI] [PubMed] [Google Scholar]
- 66.Broniowska KA, Oleson BJ, McGraw J, Naatz A, Mathews CE, Corbett JA. How the Location of Superoxide Generation Influences the beta-Cell Response to Nitric Oxide. Journal of Biological Chemistry. American Society for Biochemistry and Molecular Biology; 2015;290: 7952–7960. doi: 10.1074/jbc.M114.627869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metabolism. 2012;15: 518–533. doi: 10.1016/j.cmet.2012.01.023 [DOI] [PubMed] [Google Scholar]
- 68.Hirschberg-Jensen V, Affourtit C. Mitochondrial uncoupling protein-2 is not involved in palmitate-induced impairment of glucose-stimulated insulin secretion in INS-1E insulinoma cells and is not needed for the amplification of insulin release. Biochemistry and Biophysics Reports. Elsevier; 2015;1: 8–15. doi: 10.1016/j.bbrep.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kharroubi I. Free Fatty Acids and Cytokines Induce Pancreatic -Cell Apoptosis by Different Mechanisms: Role of Nuclear Factor- B and Endoplasmic Reticulum Stress. Endocrinology. 2004;145: 5087–5096. doi: 10.1210/en.2004-0478 [DOI] [PubMed] [Google Scholar]
- 70.Morgan NG, Dhayal S, Diakogiannaki E, Welters HJ. The cytoprotective actions of long-chain mono-unsaturated fatty acids in pancreatic β-cells. Biochem Soc Trans. 2008;36: 905 doi: 10.1042/BST0360905 [DOI] [PubMed] [Google Scholar]
- 71.Navarro G, Xu W, Jacobson DA, Wicksteed B, Allard C, Zhang G, et al. Extranuclear Actions of the Androgen Receptor Enhance Glucose-Stimulated Insulin Secretion in the Male. Cell Metabolism. Elsevier; 2016;23: 837–851. doi: 10.1016/j.cmet.2016.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Excel spreadsheet containing all relevant individual data values that were used to generate mean averages and standard errors for all data figures presented throughout this manuscript.
(XLSX)
Data Availability Statement
All of the data used to generate the figures within this manuscript are either contained within the figures themselves or are available in the excel supporting document file.