Abstract
Chlamydia pecorum is a mucosal infection, which causes debilitating disease of the urinary tract, reproductive tract and ocular sites of koalas (Phascolarctos cinereus). While antibiotics are available for treatment, they are detrimental to the koalas’ gastrointestinal tract microflora leaving the implementation of a vaccine as an ideal option for the long-term management of koala populations. We have previously reported on the successes of an anti-chlamydial recombinant major outer membrane protein (rMOMP) vaccine however, recombinant protein based vaccines are not ideal candidates for scale up from the research level to small-medium production level for wider usage. Peptide based vaccines are a promising area for vaccine development, because peptides are stable, cost effective and easily produced. In this current study, we assessed, for the first time, the immune responses to a synthetic peptide based anti-chlamydial vaccine in koalas. Five healthy male koalas were vaccinated with two synthetic peptides derived from C. pecorum MOMP and another five healthy male koalas were vaccinated with full length recombinant C. pecorum MOMP (genotype G). Systemic (IgG) and mucosal (IgA) antibodies were quantified and pre-vaccination levels compared to post-vaccination levels (12 and 26 weeks). MOMP-peptide vaccinated koalas produced Chlamydia-specific IgG and IgA antibodies, which were able to recognise not only the genotype used in the vaccination, but also MOMPs from several other koala C. pecorum genotypes. In addition, IgA antibodies induced at the ocular site not only recognised recombinant MOMP protein but also, whole native chlamydial elementary bodies. Interestingly, some MOMP-peptide vaccinated koalas showed a stronger and more sustained vaccine-induced mucosal IgA antibody response than observed in MOMP-protein vaccinated koalas. These results demonstrate that a synthetic MOMP peptide based vaccine is capable of inducing a Chlamydia-specific antibody response in koalas and is a promising candidate for future vaccine development.
Introduction
Chlamydia (C) continues to be one of the major factors threatening the long-term survival of the koala (Phascolarctos cinereus). C. pecorum is primarily considered to be a sexually transmitted infection however, congenital transmission has also been shown [1]. C. pecorum is a mucosal infection, which causes debilitating disease at the urinary tract, reproductive tract and ocular sites of koalas [2]. When left untreated, C. pecorum infection can lead to cystitis, infertility and blindness [2–5]. The current treatment for Chlamydia in koalas involves the use of antibiotics, which can be detrimental to the koalas’ gastrointestinal tract microflora, which is essential for the digestion of their diet of eucalyptus leaves [6–8]. Although antibiotics can be a useful treatment, they offer no long-term protection from subsequent infections and have limited effect on severe cases of chlamydiosis [9]. Furthermore, as Chlamydia can be asymptomatic, showing no overt signs of disease in up to 50% of infected koalas, many infected koalas go untreated [1].
Our group has been working towards the successful development of an anti-chlamydial vaccine with considerable progress [10–20]. Until now, the vaccine has been composed of recombinant proteins derived from the full length chlamydial major outer membrane protein (MOMP) [21]. Previous vaccine trials have shown that, a) the vaccine is safe to use, in both healthy and infected koalas [11], b) there is a level of cross protection against other koala-C. pecorum genotypes [12], c) both humoral and cellular immune responses are stimulated [14, 15], d) the vaccine-induced immune responses are long lasting [14], and e) vaccination has both prophylactic as well as therapeutic effects [19]. Although the current recombinant based vaccine has shown considerable promise, its production is at a research level and scale up for wider use will be challenging. One alternative would be the development of a synthetic, peptide based vaccine. Peptide vaccines are becoming increasingly popular, primarily because peptide based antigens are, a) easier to produce, b) cost effective, c) customised, d) typically water-soluble, e) stable, and f) able to be freeze dried for long-term storage [22]. Furthermore, it has also been suggested that dendritic cells (DC) can process synthetic peptides more efficiently than full length proteins [23]. This would provide an ideal advantage as antigen presenting cells (APCs) are responsible for initiating adaptive T-cell responses, which are thought to be needed for cellular and/or humoral immunity to Chlamydia [22, 24]. However, peptide based antigens are often recognised as being poor immunogens, lacking essential immunostimulatory properties required for effective immune stimulation [22, 25–28]. The addition of an appropriate delivery system or adjuvant is therefore essential. Whilst there are a number of adjuvants suitable for use in animals the choice of adjuvant for use in humans is limited due to their adverse side effects and toxicity [29, 30]. For this reason, careful consideration should be taken when selecting an appropriate adjuvant. Peptide vaccines have also been shown to induce tolerance and autoimmunity, post vaccination [30–34]. This is of particular importance when developing anti-cancer peptide-based vaccines as tumor-associated antigens can also be expressed in normal cells where self-recognition can lead to tolerance or an induced immune response could lead to autoimmunity [30].
Developing a successful peptide based vaccine requires the identification of key epitopes responsible for producing strong humoral and cell mediated immunity against C. pecorum. Our progression towards a peptide based vaccine, has identified the location of B and T cell epitopes within the full length MOMP from a diverse range of C. pecorum genotypes, with a focus on the immunostimulatory epitopes located within both the variable and conserved regions of MOMP [13, 35]. Further analysis was then performed to identify which epitopes were responsible for inducing antibodies with neutralising capabilities, essential for an effective vaccine. This led to the identification of a unique set of epitopes, all contained within the conserved region of MOMP, that induced antibodies capable of neutralising whole C. pecorum elementary bodies (EBs) [15, 16]. As C. pecorum vaccinated koalas have previously been shown to cross-recognise other C. pecorum genotypes [12] and given this unique set of neutralising epitopes that reside within the conserved region of MOMP, it is equally expected that a peptide vaccine, based on these epitopes, would also show cross-protective abilities against other C. pecorum genotypes. In this study, we present for the first time, the humoral responses in koalas vaccinated with a synthetic peptide antigen, derived from the conserved region of whole C. pecorum MOMP, combined with a Tri-Adjuvant [36]. We have shown that our peptide antigen is capable of inducing a systemic IgG antibody response as well as a mucosal IgA antibody response at the ocular site.
Materials and methods
Animals
Ten healthy captive adult male koalas were used for this study. All koalas had been bred and housed at, Lone Pine Koala Sanctuary (LPKS), Fig Tree Pocket, Brisbane, Queensland, Australia. A full veterinary health check was performed on all koalas before being given approval to participate in the vaccine trial. Enclosures and koala husbandry followed the Code of Practice for wildlife care (Queensland). Koala enclosures consisted of a sand or concrete floor with either wooden, brick, colourbond or glass walls with a tin or sail roof. Koalas remained housed in their usual enclosure with other koalas, which all met or exceeded zoo standards for koala exhibit size. They were supplied with fresh eucalyptus leaves daily, with the water in the leaf holding pots and water dishes topped up two to three times a day. The floors were cleaned once a day, and the koala climbing poles were cleaned once a week with disinfectant. All koalas were individually checked daily by a senior koala keeper and cared for by their regular koala keepers. All procedures relating to this study were approved by the University of the Sunshine Coast (USC) Animal Ethics Committee (Animal Ethics permit number AN/S/15/42) and by the Queensland Government (Scientific Purposes Permit number WISP16718315).
Chlamydia pecorum MOMP purification
The protein antigen vaccine consisted of recombinant MOMP G. ELISA’s were performed using recombinant MOMP genotypes, A, F and G. Chlamydia pecorum MOMP proteins, genotype A, F and G, was purified as previously described by Kollipara et al. (2012) [11].
Peptide synthesis and alignment
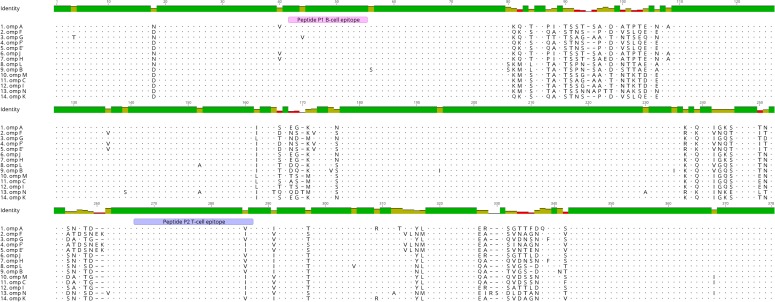
The peptide antigen vaccine consisted of two synthetic peptides located within the conserved regions of MOMP. P1, a 14 amino acid sequence, H-EGMSGDPCDPCATW-OH and P2, a 21 amino acid sequence H-INYHEWQVGAALSYRLNMLIP-OH (Fig 1). For use in the ELISA assays, both P1 and P2 were constructed as Biotin molecules linked to a serine glycine (SGSG) spacer. Peptides were reconstituted using endotoxin-free water. All peptides were produced by Mimotopes (Melbourne, Australia).
Fig 1. Vaccine peptide antigens are located within the conserved regions of the chlamydial MOMP.
Sequence alignment showing the location of the vaccine peptide antigens at 45–55 (P1) and 265–285 (P2) are both within the conserved regions of the major outer membrane protein across the 14 koala Chlamydia pecorum ompA genotypes (A, F, G, F’, E’, J, H, L, B, M, C, I, N and K).
Koala C. pecorum ompA genotypes were compared for similarity within the conserved regions, where the two synthetic peptides (P1 and P2) are located. In total, 14 C. pecorum ompA genotypes (A, F, G, F’, E’, J, H, L, B, M, C, I, N and K), were retrieved from GenBank for sequence comparison (Fig 1). All C. pecorum ompA sequences can be found through GenBank using accession numbers, AGV52054 (A), AGV52057 (F), AGV52059 (G), AGV52058 (F’), AGV52056 (E’), AGV52062 (J), AGV52060 (H), AMO26059 (L), AGV52055 (B), AMO26056 (M), AMO26054 (C), AGV52061 (I), AMO26053 (N) and AGV52063 (K). Sequence alignment was performed using Geneious 9.1 software.
Immunisation schedule and sample collection
Koalas were randomly assigned to two groups of five animals. One group (n = 5) received a single subcutaneous injection containing recombinant MOMP G (50μg) with Tri-Adjuvant (MOMP-protein vaccinated group), and the other group (n = 5) received a single subcutaneous injection containing synthetic peptides P1 and P2 (50μg or each peptide) (MOMP-peptide vaccinated group) combined with Tri-Adjuvant and made up to a total volume of 500μL with sterile endotoxin-free PBS. The Tri-Adjuvant contained a 1:2:1 ratio of Poly I;C (250μg), Host Defence Peptide–Innate Defence Regulator IDR-1002 (500μg), and Polyphosphazene EP3 (250μg) all produced and supplied by VIDO-Intervac (University of Saskatchewan, Saskatoon, SK, CA). Each vaccine was prepared in a 2mL sterile endotoxin free amber glass vials, stored on ice and administered within 2 hours of preparation.
All koalas were sampled pre-vaccination and again at 12 and 26 weeks post-vaccination. Samples were collected from the koalas whilst they were being restrained by an experienced staff member, as per standard Lone Pine Koala Sanctuary procedure. Briefly, koalas were held facing outwards from the handler and seated on a table. Forelimb and hindlimb were held together, right with right and left with left in the handlers’ respective right and left hands. Antiseptic was applied to the forelimb and whole blood (3mL) was collected from either the left or right cephalic vein. Whole blood was then placed into EDTA collection tubes (Interpath Services) and stored at 4°C until centrifugation where the plasma was removed and then stored at -20°C. Swabs were collected from the ocular site (Aluminium rayon swabs; Copan), placed into 1% protease inhibitor cocktail and stored at -20°C until processed.
Koala specific Chlamydia pecorum IgG ELISA
The IgG ELISA assay was performed to determine the systemic antibody response utilising recombinant MOMP proteins A, F and G, from plasma samples, collected pre-vaccination and again at 12 and 26 weeks post-vaccination. Initially, 96 well plates (Greiner Bio-One medium binding) where coated with 50μL of carbonate-bicarbonate coating buffer containing, 2μg/well of recombinant MOMP G, then incubated at 4°C overnight. After incubation, wells were emptied then coated with 100μL per well of blocking buffer consisting of 5% skim milk in PBS containing 0.01% Tween-20 then incubated for 2 hours at 37°C. After incubation, wells were emptied then 1:3 serially diluted plasma, with dilutions starting at 1:50, was added in duplicate then incubated for 1 hour at 37°C. After incubation, wells were washed 3 times with PBS containing 0.05% Tween-20 then coated with 50μL/well of sheep anti-koala IgG diluted 1:8000 in PBS containing 0.01% Tween-20 then incubated for 1 hour at 37°C. After incubation, wells were washed 3 times with PBS containing 0.05% Tween-20 then coated with 50μL/well of HRP-conjugated donkey anti-sheep IgG diluted 1:20000 (Abcam) in PBS containing 0.01% Tween-20 then incubated for 1 hour at 37°C. After incubation, wells were washed 3 times with PBS then 50μL/well of TMB substrate (Sigma-Aldrich) was added and incubated at room temperature for 30 mins before stopping the reaction with 50μL/well of 1M sulphuric acid. The end point titre (EPT) was calculated as per [20].
Koala specific Chlamydia pecorum IgA ELISA
The IgA ELISA assay was performed to determine the mucosal antibody response utilising recombinant MOMP protein, and heat inactivated semi-purified C. pecorum G EBs (purified as per Carey et al. (2010)) [10] on ocular swab samples, stored in 1% PIC, collected at pre-vaccination and again at 12 and 26 weeks post-vaccination. Initially, 96 well plates (Greiner Bio-One medium binding) where coated with 50μL of carbonate-bicarbonate coating buffer containing either, 2μg/well of recombinant MOMP G or 50000 IFU/well of heat inactivated semi-purified C. pecorum G EBs, then incubated at 4˚C overnight. After incubation, wells were emptied then coated with 100μL per well of blocking buffer consisting of 5% skim milk in PBS containing 0.01% Tween-20 then incubated for 2 hours at 37˚C. After incubation, wells were emptied then 50μL/well of swab sample solution, defrosted at room temperature then vortexed for 3 minutes, was added in duplicate then incubated for 1 hour at 37°C. After incubation, wells were washed 3 times with PBS containing 0.05% Tween-20 then coated with 50μL/well of rabbit anti-koala IgA diluted 1:3000 in PBS containing 0.01% Tween-20 then incubated for 1 hour at 37°C. After incubation, wells were washed 3 times with PBS containing 0.05% Tween-20 then coated with 50μL/well of HRP-conjugated goat anti-rabbit IgG (ab6721; Abcam) diluted 1:20000 in PBS containing 0.01% Tween-20 then incubated for 1 hour at 37°C. After incubation, wells were washed 3 times with PBS then 50μL/well of TMB substrate (Sigma-Aldrich) was added and incubated at room temperature for 30 mins before stopping the reaction with 50μL/well of 1M sulphuric acid. The optical density (OD) was measured at 450nm and the absorbance value was calculated as the mean of duplicate samples minus the mean of the no sample control wells.
Koala specific synthetic peptide IgG and IgA ELISA
Peptide ELISA’s were performed to determine the antibody response to P1 and P2, as described by Bommana et al. (2017) [37] with the following modifications. Streptavidin plates were initially coated with either, 1.5μg/well of P1 or 1.5μg/well of P2. For the IgG ELISA, 100μL/well of 1:3 serially diluted plasma, starting with a 1:50 dilution, was added in duplicate, following incubation, 100μL/well of sheep anti-koala IgG, diluted 1:8000, was added followed by a final incubation of 100μL/well of HRP-conjugated donkey anti-sheep IgG diluted 1:20000 (Abcam). For the IgA ELISA, 50μL/well of swab sample solution, defrosted at room temperature then vortexed for 3 minutes, was added in duplicate, following incubation, 100μL/well rabbit anti-koala IgA, diluted 1:3000, was added followed by a final incubation of 100μL/well HRP-conjugated goat anti-rabbit IgG (ab6721; Abcam) diluted 1:20000. The optical density (OD) was measured at 450nm and the absorbance value was calculated as the mean of duplicate samples minus the mean of the no sample control wells.
Statistical analysis
All statistical analysis was performed using GraphPad Prism version 7 (GraphPad Software, LaJolla, CA, USA). To evaluate the difference between time-points of each group, a one-way ANOVA Tukey’s multiple comparison test was performed with P-values set at *p<0.05, **p<0.01, ***p<0.005.
Results
Systemic IgG antibody response of vaccinated koalas to full length recombinant MOMP protein
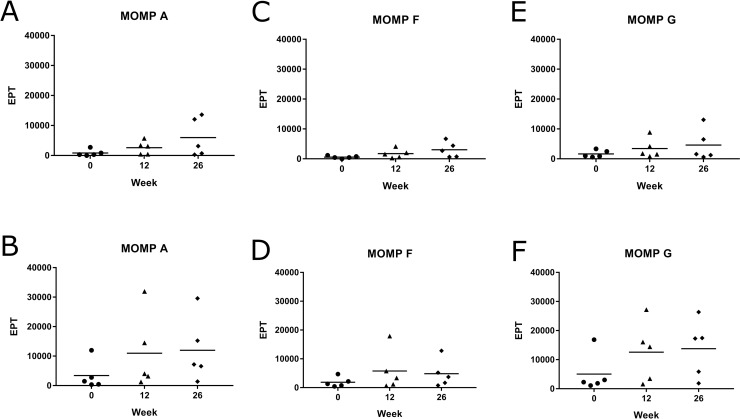
We initially assessed the ability of vaccine induced antibodies to recognise full length recombinant MOMP protein in an ELISA. The systemic IgG antibody response was measured against recombinant MOMP protein from three different C. pecorum genotypes (A, F and G) to assess their ability to cross-recognise varying MOMP genotypes (Fig 2). These genotypes were chosen as representatives of the previously identified genotypes circulating in wild koala populations, particularly in northern Australia. MOMP-peptide vaccinated koalas produced a good systemic IgG response, to all three recombinant MOMP genotypes (A, F and G) (Fig 2A, 2C and 2E), with the response increasing gradually to 26 weeks post-vaccination. As expected, MOMP-protein vaccinated koalas also produced a strong systemic IgG response, to all three recombinant MOMP genotypes (A, F and G) (Fig 2B, 2D and 2F), which was, overall, slightly stronger than that produced in the MOMP-peptide vaccinated group. Eventhough the vaccine was designed against the C. pecorum genotype G MOMP sequence, both groups produced antibodies that also recognised MOMP protein from C. pecorum genotypes A and F, in addition to G.
Fig 2. MOMP-peptide vaccinated koalas (Phascolarctos cinereus) produced a systemic IgG antibody response to rMOMP proteins.
Systemic IgG antibody response (from plasma) against recombinant protein from Chlamydia pecorum MOMP genotypes A, F and G measured pre-vaccination and at 12 and 26 weeks post-vaccination. Samples were analysed by ELISA and measurments are shown as end point titre (EPT). (A, C and E) MOMP-peptide vaccinated koalas (n = 5) response against recombinant MOMP genotypes A, F and G, respectively. (B, D and F) MOMP-protein vaccinated koalas (n = 5) response against recombinant MOMP genotypes A, F and G, respectively.
Systemic IgG antibody response of vaccinated koalas to the synthetic peptides P1 and P2
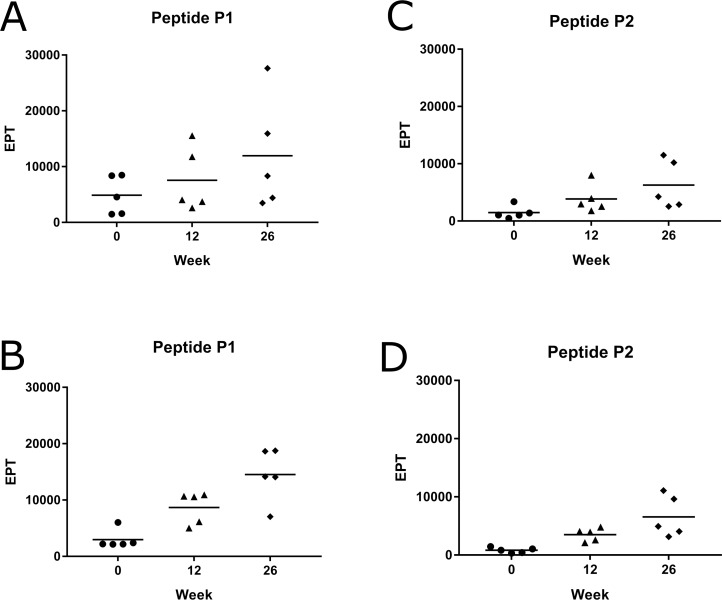
We then determined the level of antibodies produced post-vaccination that specifically recognised the peptides P1 and P2. All MOMP-peptide vaccinated koalas made an increasing vaccine-induced systemic IgG antibody response, from pre-vaccination to 26 weeks post-vaccination, when tested against each peptide (P1 and P2) (Fig 3A and 3C). The MOMP-protein vaccinated koalas also produced vaccine-induced systemic IgG antibodies, which recognised the peptides, P1 and P2 (Fig 3B and 3D) that increased to 26 weeks post-vaccination. Intersestingly, similar results were seen between the two vaccinated groups from pre-vaccination to 26 weeks post-vaccination.
Fig 3. MOMP-peptide vaccinated koalas (Phascolarctos cinereus) produced a systemic IgG antibody response to the peptides P1 and P2.
Systemic IgG antibody response (from plasma) against two different synthetic peptides (P1 and P2) measured pre-vaccination and at 12 and 26 weeks post-vaccination. Samples were analysed by ELISA and measurments are shown as EPT. (A) MOMP-peptide vaccinated koalas (n = 5) response against P1. (B) MOMP-protein vaccinated koalas (n = 5) response against P1. (C) MOMP-peptide vaccinated koalas (n = 5) response against P2. (D) MOMP-protein vaccinated koalas (n = 5) response against P2.
Mucosal IgA antibody response in vaccinated koalas to full length recombinant MOMP protein, synthetic peptides P1 and P2 and whole Chlamydia pecorum elementary bodies
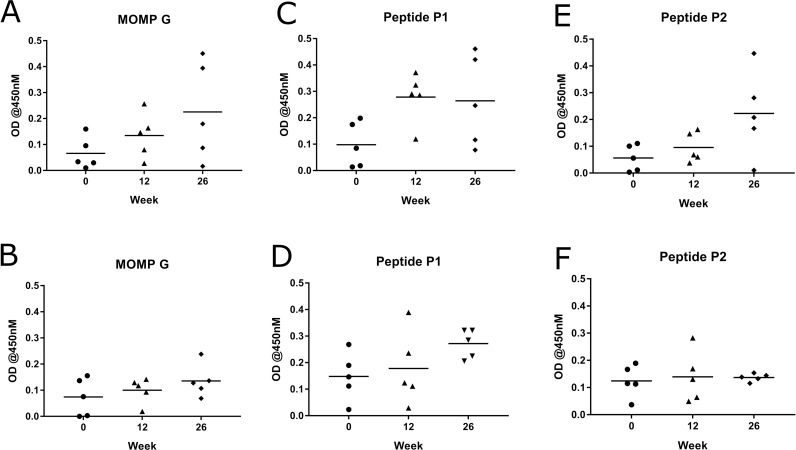
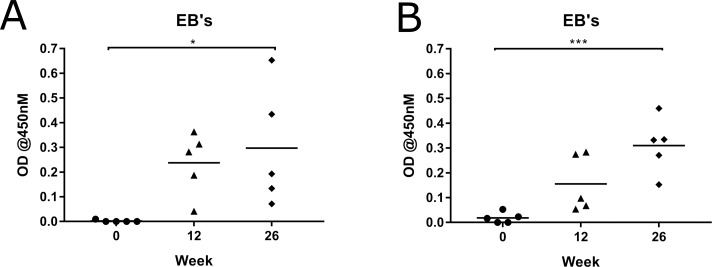
Because Chlamydia is a mucosal pathogen, causing disease at the ocular and urogenital sites, it is important for any vaccine to induce the correct immune response at these mucosal sites. We analysed the IgA antibody response to vaccination at the ocular site in this study. The mucosal IgA antibody response was measured against C. pecorum recombinant MOMP protein (G), the two synthetic peptides (P1 and P2) and whole C. pecorum EBs (G). The MOMP-peptide vaccinated koalas produced Chlamydia-specific mucosal IgA antibodies at the ocular site to all three forms of chlamydial antigen tested. They produced antibodies that recognised full length recombinant MOMP protein as well as peptides P1 and P2 (Fig 4A, 4C and 4D). Importantly, they produced a significant increase in IgA antibodies that recongised whole native chlamydial EBs (P = 0.0299) (Fig 5A). Perhaps not surprisingly, the MOMP-protein vaccinated koalas also produced IgA antibodies at the ocular site to C. pecorum recombinant MOMP protein (G), two synthetic peptides (P1 and P2) (Fig 4B, 4D and 4F) and a significant increase to whole native chlamydial EBs (P = 0.0009) (Fig 5B). Interestingly, although some koalas showed a stronger vaccine-induced mucosal IgA antibody response than others, at 12 and 26 weeks post-vaccination, some of the MOMP-peptide vaccinated koalas made a stronger and more sustained antibody response, compared to the MOMP-protein vaccinated group.
Fig 4. MOMP-peptide vaccinated koalas (Phascolarctos cinereus) produced a stronger mucosal IgA antibody response to rMOMP protein than MOMP-protein vaccinated koalas (Phascolarctos cinereus).
Mucosal IgA antibody response to recombinant MOMP (G) and synthetic peptides (P1 and P2) in ocular swab samples collected pre-vaccination and 12 and 26 weeks post-vaccination. Samples were analysed by ELISA and are shown as optical density (OD) measured at 450nM. (A, C and E) MOMP-peptide vaccinated koalas (n = 5) response to recombinant MOMP G, peptide P1 and peptide P2, respectively. (B, D and F) MOMP-protein vaccinated koalas (n = 5) response to recombinant MOMP G, peptide P1 and peptide P2, respectively.
Fig 5. MOMP-peptide vaccinated koalas (Phascolarctos cinereus) produced a stronger mucosal IgA antibody response to whole Chlamydia pecorum elementary bodies than MOMP-protein vaccinated koalas (Phascolarctos cinereus).
Mucosal IgA antibody response to heat inactivated Chlamydia pecorum elementary bodies from genotype G in ocular swab samples collected pre-vaccination and 12 and 26 weeks post-vaccination. Samples were analysed by ELISA and are shown as OD measured at 450nM. (A) MOMP-peptide vaccinated koalas (n = 5) response with a P value 0.0009. (B) MOMP protein-vaccinated koalas (n = 5) response with a P value 0.0299.
Discussion
Wild koala populations continue to have significant levels of infection with C. pecorum and as a result are suffering debilitating disease, which is threatening their long-term survival [1, 38, 39]. In many populations, these levels of infection and disease are actually higher than previously reported [1] and current treatment options are showing little to no impact on the decline in the level of infection and disease, with hospital admission records remaining stable over time [39]. The widespread implementation of a vaccine in wild koalas could offer the protection needed to reverse this progression. To date, previous rMOMP protein vaccine trials conducted on both infected and diseased wild koala populations have been very successful [11, 13, 15, 16, 18, 19]. These trials have shown that a rMOMP protein vaccine stimulates the immune system and is responsible for an increase in neutralising antibodies [11, 15, 18]. Vaccinated koalas have shown a decrease in their chlamydial infectious load [18, 19] as well as ocular disease status [19], post-vaccination, and importantly, rMOMP vaccinated wild koalas have also shown a decrease in the progression to disease over a 12 month period [18–20]. However, in spite of this success, there are many challenges in producing and implementing a recombinant protein format vaccine on a wider scale. A synthetic peptide based vaccine could overcome some of these challenges, assuming that it induces a strong and relevant immune response [22]. The development of a peptide based anti-chlamydial vaccine that can elicit the same, or even stronger immune responses as the current C. pecorum MOMP vaccine, with the potential to be mass produced, would be an ideal candidate for future anti-chlamydial vaccine development. In this study, we have shown that a vaccine consisting of two relatively short peptides, derived from the full length MOMP, is capable of inducing an immune response in koalas up to 26 weeks post-vaccination. We have shown a mucosal IgA antibody response to full length rMOMP (G), in both the MOMP-peptide and MOMP-protein vaccinated koalas. Most importantly, for the first time, we found that the MOMP-peptide vaccinated koalas produced a mucosal IgA antibody response to whole chlamydial EBs with some MOMP-peptide vaccinated koalas showing a stronger response than the MOMP-protein vaccinated koalas. Furthermore, our study has also shown that the MOMP derived peptide vaccine is safe to use in koalas with no adverse effects reported in any of the koalas involved in this trial.
For the successful development of an anti-C. pecorum vaccine, it is important that the vaccine antigen is specific, induces a strong humoral and cell mediated response and can elicit long-lasting immunity. Additionally, it is advantageous that an anti-chlamydial vaccine antigen is capable of cross recognising other C. pecorum strains circulating in wild koala populations causing infection and disease [1, 38]. Previously, we have shown that using a full length rMOMP protein induced antibodies that could recognise other chlamydial genotypes (A, F and G) [12]. This current study also found that using just two peptides (14 aa and 21 aa in length) located at positions 42–55 (P1) and 265–285 (P2) in the MOMP, can also induce antibodies to epitopes of chlamydial genotypes A, F and G. Presumably, this is because these peptides were designed from regions of MOMP that are conserved across genotypes A, F and G. By carefully selecting peptides located in these conserved regions of MOMP, we further predict that these peptides should also be able to cross-recognise other C. pecorum genotypes, as these regions are also conserved across all 14 currently known koala C. pecorum ompA genotypes.
As C. pecorum is a mucosal infection, it is essential that an anti-chlamydial vaccine elicits a strong mucosal IgA antibody response. Secretory IgA found in mucosal surfaces is the first line of defence against invading pathogens and works to prevent infection by blocking the attachment of pathogens to epithelial cells and by eliminating pathogens from the mucosal surface [40, 41]. Studies in mice have shown that mucosal IgA was responsible for protective immunity when challenged [42] and in another study, secretory IgA was shown to have an effect on reducing the chlamydial infectious load [43]. Furthermore, a recent study conducted by Desclozeaux et al. (2017) [20] also showed that IgA could possibly play a role in lowering the chlamydial burden in koalas. In our current study, we have shown a strong mucosal IgA antibody response by vaccinated koalas that recognises rMOMP (G) protein and peptide (P1 and P2) antigens. However, more importantly, this study has shown for the first time, that peptide vaccinated koalas are capable of producing a mucosal IgA immune response to whole C. pecorum EBs. This ability to identify the C. pecorum infectious agent is paramount to the successful development of an effective peptide-based anti-chlamydial vaccine. This demonstrates the specificity of our peptides to the key antigenic regions, required to stimulate a mucosal IgA response. Further showing that, despite any conformational 3D structures, our predicted epitopes of the target antigen molecule are surface exposed.
What was a surprising finding in our study, is that some of the peptide-vaccinated koalas had a stronger mucosal IgA antibody response to, rMOMP (G), peptides (P1 and P2) and whole chlamydial EBs, compared to MOMP-protein vaccinated koalas. One possible explanation for this could be the intracellular mechanisms by which antigens are processed within APCs, such as DCs. Synthetic peptides have been shown to follow a different intracellular pathway than proteins within DCs, with studies showing that DCs are more efficient at processing and directing synthetic peptides to MHC pathways, compared to whole proteins [23, 44, 45]. Furthermore, synthetic peptides have been shown to induce both CD4+ and CD8+ T cell immune responses in contrast to proteins, which have been shown to primarily induce CD4+ T cells and at lower levels [23, 45]. This further implies that synthetic peptides are more efficient at MHC class l cross-presentation, as outlined by Menager et al. (2014) [44].
To optimise immune responses and maximise DC activation and maturation, the addition of a Toll-like receptor (TLR) agonist is recommended for peptide based vaccines. Invading pathogens recognised by TLRs located on DCs trigger a response when bound, initiating DC maturation and immune responses [24, 46, 47]. To address this, we have combined our peptide vaccine with a Tri-Adjuvant containing Poly I:C, a known TLR agonist. Previously, it has been described that Poly I:C enhances immune responses, increases antigen uptake by DCs, [48, 49], and shows significant humoral and cellular immune responses [25]. Furthermore, a vaccine trial comparing adjuvants containing two different TLR agonists (Poly I:C and CpG), resulted in higher levels of IgA within the lungs of mice vaccinated with the Poly I:C adjuvant, and subsequently showed long lasting immunity [50]. This would suggest that poly I:C, combined with our peptide based vaccine, has contributed significantly to mucosal IgA antibody responses.
It has also been suggested that long lasting immunity could be attributed to the Tri-Adjuvant. Mice vaccinated with a live virus stimulated only short-lived immunity compared to mice vaccinated with a fusion protein of the virus combined with the Tri-Adjuvant, which demonstrated long term immunity [51]. They further showed that while both vaccinated groups of mice had similar responses to sera IgG, the fusion protein plus Tri-Adjuvant vaccinated group had a significantly higher IgA response than the live vaccinated group, in both the lung and lymph node [51]. The second component of the Tri-Adjuvant is a host defence synthetic peptide, innate defence regulator 1002 (IDR-1002), known for its ability to recruit immune cells, stimulate immature DCs, have antimicrobial activity, and anti-inflammatory properties [52, 53]. A recent study conducted to evaluate the ability of IDR-1002 demonstrated, both in-vitro and in-vivo, its ability to supress pro-inflammatory cytokines (tumor necrosis factor alpha and interleukin-6), and further showed a reduction in airway inflammation in IDR-1002 treated mice [54]. The third component of the Tri-Adjuvant is polyphosphazene, known for having immunostimulatory properties [55] and the ability to form non-covalent complexes with antigens enhancing uptake by APCs [56, 57]. Selecting an appropriate adjuvant to enhance the delivery and uptake of a peptide vaccine is crucial for optimal outcome. Here we have described the successful combination of a peptide vaccine antigen with a Tri-Adjuvant, resulting in strong mucosal IgA responses, necessary to combat a chlamydial infection.
Developing a peptide based vaccine is complex, with some challenges to consider. Although there are no commercially available peptide based vaccines as yet, there has been considerable progress towards this goal, for both the prophylactic and therapeutic effects [58, 59], particularly for the treatment of cancer [30, 60]. Progress towards the development of an anti-Chlamydia trachomatis peptide based vaccine have also been promising with results revealing strong humoral [42, 61] and cell mediated immune responses [61]. In considering future development, it has been suggested that the conjugation of both the antigen and adjuvant would ensure they are both delivered to the same APC, for optimal stimulation and maturation. Furthermore, having a better understanding about the functionality of the targeted epitope would also help in developing a more directed outcome specific to the required response.
In conclusion, we report for the first time, the success of a koala vaccine consisting of two synthetic peptides, derived from C. pecorum MOMP, in place of a previously used rMOMP protein based vaccine. We have shown that MOMP-peptide vaccinated koalas produced Chlamydia-specific antibodies that recognised not only rMOMP but importantly, also whole chlamydial EBs. We have shown that MOMP-peptide vaccinated koalas produced a Chlamydia-specific IgG and IgA immune response still seen at 26 weeks post-vaccination. Most importantly, this study has shown that MOMP-peptide vaccinated koalas can produce a stronger mucosal IgA response to whole EBs than MOMP-protein vaccinated koalas. Together, these results are promising and show the potential for the future development of a peptide based anti-C. pecorum vaccine that can be mass produced with precision for broader applications.
Acknowledgments
This project was financially supported by a grant from the Australian Research Council (ARC Linkage Scheme). Special thanks to Karen Nilsson and the rest of the team members at Lone Pine Koala Sanctuary for handling of the koalas and co-ordination of the vaccine trial. We thank the many groups that have supported the overall koala Chlamydia vaccine development work, including the Australian Research Council, the Queensland Government (Department of Transport and Main Roads, particularly the Moreton Bay Rail Project Team and Department of Environment and Heritage Protection), Moreton Bay Regional Council, Friends of Koala (Lismore), Koala Action Inc, Endeavour Veterinary Ecology, Australia Zoo Wildlife Hospital, Lone Pine Koala Sanctuary, City of Gold Coast, Redland City Council. We would also like to thank Dr Martina Jelocnik for her assistance with the ELISA assays and bioinformatics as well as Sankhya Bommana for her assistance with the ELISA assays.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Australian Research Council Linkage program received by PT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nyari S, Waugh CA, Dong J, Quigley BL, Hanger J, Loader J, et al. Epidemiology of chlamydial infection and disease in a free-ranging koala (Phascolarctos cinereus) population. PloS one. 2017;12(12):e0190114 doi: 10.1371/journal.pone.0190114 ; PubMed Central PMCID: PMCPMC5744985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanshard W, Bodley K. In 'Medicine of Australian Mammals' Vogelnest L, Woods R, editors. Collingwood: CSIRO; 2008. [Google Scholar]
- 3.McColl KA, Martin RW, Gleeson LJ, Handasyde KA, Lee AK. Chlamydia infection and infertility in the female koala (Phascolarctos cineteus). Veterinary record. 1984;115:25–6. [DOI] [PubMed] [Google Scholar]
- 4.Johnston SD, Deif HH, McKinnon A, Theilemann P, Griffith JE, Higgins DP. Orchitis and Epididymitis in Koalas (Phascolarctos cinereus) Infected With Chlamydia pecorum. Veterinary pathology. 2015;52(6):1254–7. doi: 10.1177/0300985815570069 . [DOI] [PubMed] [Google Scholar]
- 5.Wan C, Loader J, Hanger J, Beagley K, Timms P, Polkinghorne A. Using quantitative polymerase chain reaction to correlate Chlamydia pecorum infectious load with ocular, urinary and reproductive tract disease in the koala (Phascolarctos cinereus). Australian veterinary journal. 2011;89(10):409–12. doi: 10.1111/j.1751-0813.2011.00827.x . [DOI] [PubMed] [Google Scholar]
- 6.Black LA, McLachlan AJ, Griffith JE, Higgins DP, Gillett A, Krockenberger MB, et al. Pharmacokinetics of chloramphenicol following administration of intravenous and subcutaneous chloramphenicol sodium succinate, and subcutaneous chloramphenicol, to koalas (Phascolarctos cinereus). J Vet Pharmacol Ther. 2013;36(5):478–85. doi: 10.1111/jvp.12024 . [DOI] [PubMed] [Google Scholar]
- 7.Black LA, Higgins DP, Govendir M. In vitro activity of chloramphenicol, florfenicol and enrofloxacin against Chlamydia pecorum isolated from koalas (Phascolarctos cinereus). Australian veterinary journal. 2015;93(11):420–3. doi: 10.1111/avj.12364 . [DOI] [PubMed] [Google Scholar]
- 8.Griffith JE, Higgins DP, Li KM, Krockenberger MB, Govendir M. Absorption of enrofloxacin and marbofloxacin after oral and subcutaneous administration in diseased koalas (Phascolarctos cinereus). J Vet Pharmacol Ther. 2010;33(6):595–604. doi: 10.1111/j.1365-2885.2010.01169.x . [DOI] [PubMed] [Google Scholar]
- 9.Govendir M, Hanger J, Loader JJ, Kimble B, Griffith JE, Black LA, et al. Plasma concentrations of chloramphenicol after subcutaneous administration to koalas (Phascolarctos cinereus) with chlamydiosis. J Vet Pharmacol Ther. 2012;35(2):147–54. doi: 10.1111/j.1365-2885.2011.01307.x . [DOI] [PubMed] [Google Scholar]
- 10.Carey AJ, Timms P, Rawlinson G, Brumm J, Nilsson K, Harris JM, et al. A multi-subunit chlamydial vaccine induces antibody and cell-mediated immunity in immunized koalas (Phascolarctos cinereus): comparison of three different adjuvants. Am J Reprod Immunol. 2010;63(2):161–72. doi: 10.1111/j.1600-0897.2009.00776.x . [DOI] [PubMed] [Google Scholar]
- 11.Kollipara A, George C, Hanger J, Loader J, Polkinghorne A, Beagley K, et al. Vaccination of healthy and diseased koalas (Phascolarctos cinereus) with a Chlamydia pecorum multi-subunit vaccine: evaluation of immunity and pathology. Vaccine. 2012;30(10):1875–85. doi: 10.1016/j.vaccine.2011.12.125 . [DOI] [PubMed] [Google Scholar]
- 12.Kollipara A, Wan C, Rawlinson G, Brumm J, Nilsson K, Polkinghorne A, et al. Antigenic specificity of a monovalent versus polyvalent MOMP based Chlamydia pecorum vaccine in koalas (Phascolarctos cinereus). Vaccine. 2013;31(8):1217–23. doi: 10.1016/j.vaccine.2012.12.057 . [DOI] [PubMed] [Google Scholar]
- 13.Kollipara A, Polkinghorne A, Beagley K, Timms P. Vaccination of koalas with a recombinant Chlamydia pecorum Major Outer Membrane Protein induces antibodies of different specificity compared to those following a natural live infection. PlosOne. 2013;8(9):e74808 doi: 10.1371/10.1371/journal.pone.0074808.t001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan SA, Waugh C, Rawlinson G, Brumm J, Nilsson K, Gerdts V, et al. Vaccination of koalas (Phascolarctos cinereus) with a recombinant chlamydial major outer membrane protein adjuvanted with poly I:C, a host defense peptide and polyphosphazine, elicits strong and long lasting cellular and humoral immune responses. Vaccine. 2014;32(44):5781–6. doi: 10.1016/j.vaccine.2014.08.037 . [DOI] [PubMed] [Google Scholar]
- 15.Khan SA, Polkinghorne A, Waugh C, Hanger J, Loader J, Beagley K, et al. Humoral immune responses in koalas (Phascolarctos cinereus) either naturally infected with Chlamydia pecorum or following administration of a recombinant chlamydial major outer membrane protein vaccine. Vaccine. 2016;34(6):775–82. doi: 10.1016/j.vaccine.2015.12.050 . [DOI] [PubMed] [Google Scholar]
- 16.Khan SA, Desclozeaux M, Waugh C, Hanger J, Loader J, Gerdts V, et al. Antibody and Cytokine Responses of Koalas (Phascolarctos cinereus) Vaccinated with Recombinant Chlamydial Major Outer Membrane Protein (MOMP) with Two Different Adjuvants. PloS one. 2016;11(5):e0156094 doi: 10.1371/journal.pone.0156094 ; PubMed Central PMCID: PMCPMC4878773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waugh CA, Timms P, Andrew D, Rawlinson G, Brumm J, Nilsson K, et al. Comparison of subcutaneous versus intranasal immunization of male koalas (Phascolarctos cinereus) for induction of mucosal and systemic immunity against Chlamydia pecorum. Vaccine. 2015;33(7):855–60. doi: 10.1016/j.vaccine.2014.12.052 . [DOI] [PubMed] [Google Scholar]
- 18.Waugh C, Khan SA, Carver S, Hanger J, Loader J, Polkinghorne A, et al. A Prototype Recombinant-Protein Based Chlamydia pecorum Vaccine Results in Reduced Chlamydial Burden and Less Clinical Disease in Free-Ranging Koalas (Phascolarctos cinereus). PloS one. 2016;11(1):e0146934 doi: 10.1371/journal.pone.0146934 ; PubMed Central PMCID: PMCPMC4710501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waugh C, Austin R, Polkinghorne A, Timms P. Treatment of Chlamydia-associated ocular disease via a recombinant protein based vaccine in the koala (Phascolarctos cinereus). Biologicals. 2016;44(6):588–90. doi: 10.1016/j.biologicals.2016.09.006 . [DOI] [PubMed] [Google Scholar]
- 20.Desclozeaux M, Robbins A, Jelocnik M, Khan SA, Hanger J, Gerdts V, et al. Immunization of a wild koala population with a recombinant Chlamydia pecorum Major Outer Membrane Protein (MOMP) or Polymorphic Membrane Protein (PMP) based vaccine: New insights into immune response, protection and clearance. PloS one. 2017;12(6):e0178786 doi: 10.1371/journal.pone.0178786 ; PubMed Central PMCID: PMCPMC5456371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun G, Pal S, Sarcon AK, Kim S, Sugawara E, Nikaido H, et al. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. Journal of bacteriology. 2007;189(17):6222–35. doi: 10.1128/JB.00552-07 ; PubMed Central PMCID: PMCPMC1951919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skwarczynski M, Toth I. Peptide-based synthetic vaccines. Chem Sci. 2016;7(2):842–54. doi: 10.1039/c5sc03892h ; PubMed Central PMCID: PMCPMC5529997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosalia RA, Quakkelaar ED, Redeker A, Khan S, Camps M, Drijfhout JW, et al. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and T-cell activation. Eur J Immunol. 2013;43(10):2554–65. doi: 10.1002/eji.201343324 . [DOI] [PubMed] [Google Scholar]
- 24.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- 25.Cheng C, Jain P, Bettahi I, Pal S, Tifrea D, de la Maza LM. A TLR2 agonist is a more effective adjuvant for a Chlamydia major outer membrane protein vaccine than ligands to other TLR and NOD receptors. Vaccine. 2011;29(38):6641–9. doi: 10.1016/j.vaccine.2011.06.105 ; PubMed Central PMCID: PMCPMC3156873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002 ; PubMed Central PMCID: PMCPMC3420356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harandi AM, Davies G, Olesen O. Vaccine adjuvants: scientific challenges and strategic initiatives. Expert reviews vaccines. 2009;8(3):293–8. doi: 10.1586. [DOI] [PubMed] [Google Scholar]
- 28.Azmi F, Ahmad Fuaad AAH, Skwarczynski M, Toth I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Human Vaccines & Immunotherapeutics. 2013;10(3):778–96. doi: 10.4161/hv.27332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan K, Li R, Huang X, Liu Q. Outer Membrane Vesicles: Current Status and Future Direction of These Novel Vaccine Adjuvants. Front Microbiol. 2018;9:783 doi: 10.3389/fmicb.2018.00783 ; PubMed Central PMCID: PMCPMC5932156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elsawa SF, Rodeberg DA, Celis E. T-cell epitope peptide vaccines. Expert Rev Vaccines. 2004;3(5):563–75. doi: 10.1586/14760584.3.5.563 . [DOI] [PubMed] [Google Scholar]
- 31.Sioud M. Does our current understanding of immune tolerance, autoimmunity, and immunosuppressive mechanisms facilitate the design of efficient cancer vaccines? Scand J Immunol. 2009;70(6):516–25. doi: 10.1111/j.1365-3083.2009.02326.x . [DOI] [PubMed] [Google Scholar]
- 32.Sultan H, Trillo Tinoco J, Rodriguez P, Celis E. Effective antitumor peptide vaccines can induce severe autoimmune pathology. Impact journals. 2017;8(41):70317–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toes R, Blom R, Offringa R, Kast W, Melief C. Enhanced tumor outgrowth after peptide vaccination. Functional deletion of tumor-specific CTL induced by peptide vaccination can lead to the inability to reject tumors. Journal of Immunology. 1996;156(10):3911–8. [PubMed] [Google Scholar]
- 34.Toes R, Offringa R, Blom R, Melief C, Kast W. Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Immunology. 1996;93:7855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kollipara A, Polkinghorne A, Wan C, Kanyoka P, Hanger J, Loader J, et al. Genetic diversity of Chlamydia pecorum strains in wild koala locations across Australia and the implications for a recombinant C. pecorum major outer membrane protein based vaccine. Veterinary microbiology. 2013;167(3–4):513–22. doi: 10.1016/j.vetmic.2013.08.009 . [DOI] [PubMed] [Google Scholar]
- 36.Garg R, Babiuk L, van Drunen Littel-van den Hurk S, Gerdts V. A novel combination adjuvant platform for human and animal vaccines. Vaccine. 2017;35(35 Pt A):4486–9. doi: 10.1016/j.vaccine.2017.05.067 . [DOI] [PubMed] [Google Scholar]
- 37.Bommana S, Walker E, Desclozeaux M, Timms P, Polkinghorne A. Humoral immune response against two surface antigens of Chlamydia pecorum in vaccinated and naturally infected sheep. PloS one. 2017;12(11):e0188370 doi: 10.1371/journal.pone.0188370 ; PubMed Central PMCID: PMCPMC5708773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polkinghorne A, Hanger J, Timms P. Recent advances in understanding the biology, epidemiology and control of chlamydial infections in koalas. Veterinary microbiology. 2013;165(3–4):214–23. doi: 10.1016/j.vetmic.2013.02.026 . [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Astudillo V, Allavena R, McKinnon A, Larkin R, Henning J. Decline causes of Koalas in South East Queensland, Australia: a 17-year retrospective study of mortality and morbidity. Sci Rep. 2017;7:42587 doi: 10.1038/srep42587 ; PubMed Central PMCID: PMCPMC5316976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woof JM, Kerr MA. IgA function—variations on a theme. Immunology. 2004;113(2):175–7. doi: 10.1111/j.1365-2567.2004.01958.x ; PubMed Central PMCID: PMCPMC1782559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corthesy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol. 2013;4:185 doi: 10.3389/fimmu.2013.00185 ; PubMed Central PMCID: PMCPMC3709412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taha MA, Singh SR, Hulett K, Pillai SR, Agee R, Dennis VA. A Peptide Containing T-Cell Epitopes of Chlamydia trachomatis Recombinant MOMP Induces Systemic and Mucosal Antibody Responses in Mice. World Journal of Vaccines. 2011;01(04):138–47. doi: 10.4236/wjv.2011.14014 [Google Scholar]
- 43.Armitage CW, O'Meara CP, Harvie MC, Timms P, Wijburg OL, Beagley KW. Evaluation of intra- and extra-epithelial secretory IgA in chlamydial infections. Immunology. 2014;143(4):520–30. doi: 10.1111/imm.12317 ; PubMed Central PMCID: PMCPMC4253500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menager J, Ebstein F, Oger R, Hulin P, Nedellec S, Duverger E, et al. Cross-presentation of synthetic long peptides by human dendritic cells: a process dependent on ERAD component p97/VCP but Not sec61 and/or Derlin-1. PloS one. 2014;9(2):e89897 doi: 10.1371/journal.pone.0089897 ; PubMed Central PMCID: PMCPMC3937416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H, Hong H, Li D, Ma S, Di Y, Stoten A, et al. Comparing pooled peptides with intact protein for accessing cross-presentation pathways for protective CD8+ and CD4+ T cells. J Biol Chem. 2009;284(14):9184–91. doi: 10.1074/jbc.M809456200 ; PubMed Central PMCID: PMCPMC2666570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medzhitov R. Toll like receptors and innate immunity. Nature Reviews Immunology. 2001;1:135–45. doi: 10.1038/35100529 [DOI] [PubMed] [Google Scholar]
- 47.Shizuo A, Kiyoshi T, Tsuneyasu K. Toll-like receptors: critical proteins linking innate and aquired immunity. Nature Immunology 2001;2(8):675–90. doi: 10.1038/90609 [DOI] [PubMed] [Google Scholar]
- 48.Garg R, Latimer L, Simko E, Gerdts V, Potter A, van den Hurk S. Induction of mucosal immunity and protection by intranasal immunization with a respiratory syncytial virus subunit vaccine formulation. The Journal of general virology. 2014;95(Pt 2):301–6. doi: 10.1099/vir.0.058461-0 . [DOI] [PubMed] [Google Scholar]
- 49.Trumpfheller C, Longhi MP, Caskey M, Idoyaga J, Bozzacco L, Keler T, et al. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Intern Med. 2012;271(2):183–92. doi: 10.1111/j.1365-2796.2011.02496.x ; PubMed Central PMCID: PMCPMC3261312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garg R, Latimer L, Gerdts V, Potter A, van Drunen Littel-van den Hurk S. Vaccination with the RSV fusion protein formulated with a combination adjuvant induces long-lasting protective immunity. The Journal of general virology. 2014;95(Pt 5):1043–54. doi: 10.1099/vir.0.062570-0 . [DOI] [PubMed] [Google Scholar]
- 51.Garg R, Theaker M, Martinez EC, van Drunen Littel-van den Hurk S. A single intranasal immunization with a subunit vaccine formulation induces higher mucosal IgA production than live respiratory syncytial virus. Virology. 2016;499:288–97. doi: 10.1016/j.virol.2016.09.023 . [DOI] [PubMed] [Google Scholar]
- 52.Mansour SC, Pena OM, Hancock RE. Host defense peptides: front-line immunomodulators. Trends Immunol. 2014;35(9):443–50. doi: 10.1016/j.it.2014.07.004 . [DOI] [PubMed] [Google Scholar]
- 53.Hancock RE, Haney EF, Gill EE. The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol. 2016;16(5):321–34. doi: 10.1038/nri.2016.29 . [DOI] [PubMed] [Google Scholar]
- 54.Wuerth KC, Falsafi R, Hancock REW. Synthetic host defense peptide IDR-1002 reduces inflammation in Pseudomonas aeruginosa lung infection. PloS one. 2017;12(11):e0187565 doi: 10.1371/journal.pone.0187565 ; PubMed Central PMCID: PMCPMC5673212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Awate S, Wilson HL, Lai K, Babiuk LA, Mutwiri G. Activation of adjuvant core response genes by the novel adjuvant PCEP. Mol Immunol. 2012;51(3–4):292–303. doi: 10.1016/j.molimm.2012.03.026 . [DOI] [PubMed] [Google Scholar]
- 56.Andrianov AK, Marin A, BE R. Polyphosphazene Polyelectrolytes: A Link between the Formation of Noncovalent Complexes with Antigenic Proteins and Immunostimulating Activity. Biomacromolecules. 2005;6:1375–9. doi: 10.1021/bm049329t [DOI] [PubMed] [Google Scholar]
- 57.Kovacs-Nolan J, Latimer L, Landi A, Jenssen H, Hancock RE, Babiuk LA, et al. The novel adjuvant combination of CpG ODN, indolicidin and polyphosphazene induces potent antibody- and cell-mediated immune responses in mice. Vaccine. 2009;27(14):2055–64. doi: 10.1016/j.vaccine.2009.01.118 . [DOI] [PubMed] [Google Scholar]
- 58.Li W, Joshi MD, Singhania S, Ramsey KH, Murthy AK. Peptide Vaccine: Progress and Challenges. Vaccines (Basel). 2014;2(3):515–36. doi: 10.3390/vaccines2030515 ; PubMed Central PMCID: PMCPMC4494216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu T, Hussein W M, Toth I, Skwarczynski M. Advances in peptide-based human papillomavirus therapeutic vaccines. Current topics in medicinal chemistry. 2012;12:1581–92. [DOI] [PubMed] [Google Scholar]
- 60.Kametani Y, Miyamoto A, Tsuda B, Tokuda Y. B Cell Epitope-Based Vaccination Therapy. Antibodies. 2015;4(4):225–39. doi: 10.3390/antib4030225 [Google Scholar]
- 61.Tu J, Hou B, Wang B, Lin X, Gong W, Dong H, et al. A multi-epitope vaccine based on Chlamydia trachomatis major outer membrane protein induces specific immunity in mice. Acta Biochim Biophys Sin (Shanghai). 2014;46(5):401–8. doi: 10.1093/abbs/gmu016 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.