Abstract
In 2016, a severe leaf spot disease was found on Iris ensata Thumb. in Nanjing, China. The symptom was elliptical, fusiform, or irregularly necrotic lesion surrounded by a yellow halo, from which a small-spored Alternaria species was isolated. The fungus was identified as Alternaria iridiaustralis based on morphological characteristics. The pathogenicity tests revealed that the fungus was the causal pathogen of the disease. Phylogenic analyses using sequences of ITS, gpd, endoPG, and RPB2 genes confirmed the morphological identification. This study is the first report of A. iridiaustralis causing leaf spots on I. ensata in China.
Keywords: Alternaria iridiaustralis, Iris ensata Thumb, morphology, pathogenicity, sequence analysis
Plants of the genus Iris (Iridaceae) are popular in the horticultural trade, comprising six sub genera with approximately 260 species [1]. Iris is extensively grown as ornamental plants because its flowers have a wide variety of colors. Moreover, some species have been used as herbal medicine to treat various diseases and they are also used in perfume and cosmetic industries due to their sweet violet fragrance [2]. Diverse biological activities are reported among Iris plants, such as anticancer, cytotoxicity, insecticidal activities, and so on [3]. In China, there are more than 60 Iris species recorded mainly distributed in Southwest, Northwest, and Northeast [4]. Many different Iris plants are popular to decorate public parks of Nanjing city (China) and one of the perennial species, Iris ensata Thumb. grows in wetlands that flowers from June to July (Figure 1(A)).
Figure 1.
Plants of Iris ensata. (A) Plants in the field; (B–C) Symptoms; (D) Pathogenicity on detached leaves.
Alternaria is a ubiquitous fungal genus causing leaf spots or blight, which includes many pathogenic species associated with a wide variety of ornamental plants. Two Alternaria species (A. iridicola and A. iridiaustralis) have been reported from Iris plants [5–7]. During a field sampling of large-spored Alternaria species in China, a leaf spot disease was found on I. ensata Thumb. at Xuanwuhu Park in Nanjing in June 2016. The symptoms were elliptical, fusiform, or irregularly brown to black necrotic lesion surrounded by yellow halos (Figure 1(B)). Severely, infected leaves were blight, especially in leaf apex, and leaf margin (Figure 1(C)).
To isolate the pathogen, fresh samples with the typical symptoms were collected, cut, and placed in a moist Petri dish for conidial sporulation. After 1 d, single spore was collected using sterilized glass needle under a dissecting microscope and transferred to potato dextrose agar (PDA). Pure cultures were obtained by growing for 3–5 d at 25 °C in dark and deposited in the Culture Collection of Yangtze University (YZU) in Jingzhou, China. Four isolates with the same morphology were collected from leaves with symptoms. The isolate YZU 161003 was selected for further morphological and molecular classification.
To determine the cultural characteristics, mycelial plugs (6 mm in diameter) were taken from the edge of a colony grown on PDA and transferred onto the center of fresh PDA plates (90 mm). The plates were incubated for 7 d at 25 °C in the dark. Colonies effuse, felty, white grey to grey, 45–48 mm in diameter (Figure 2(A)). To observe the conidial morphology, mycelia were grown on potato carrot agar (PCA) media and inoculated at 22 °C with a light period of 8 h light/16 h dark according to Simmons [6]. After 7 d, sporulation patterns were observed. Meanwhile, conidia and conidiophores were mounted with lactophenol picric acid solution (Fluka, Washington, DC). Fifty randomly selected conidia were observed and measured under a light microscope. On PCA, crowed sporulation was found on abundant aerial mycelia. Conidia were normally solitary or two in a chain (Figure 2(B–D)), sometimes 3–5 conidia in a chain. Conidiophores were arising terminally or laterally from hyphae, single, dark brown, sub erect, 3–6 μm width, and with septa 2–10 (<10 occasionally) (Figure 2(B–D)). Solitary conidia were ellipsoid or long ellipsoid, beakless, normally 20–50 × 15–24 µm in size, and with 1–4 transverse septa. No longitudinal septum or with 1–2 septa formed in the transverse segments. A large portion of conidia could produce secondary conidia with beaks in chains, which were as large as 25–57 × 17–25 µm in size, with 3–5 transverse septa. Beaks developed from apical cell or internal cell of dark brown conidia body were variable, 15–100 (–133) × 3.5–4.5 µm in size with 1–11 septa. The ornamentation was smooth, punctuate, or granular in conidial wall (Figure 2(E)). Morphology of the present fungus is consistently identical to the small-spored A. iridiaustralis described by Simmons [6].
Figure 2.
Morphological features of the fungus described in this work. (A) Colony characterization on PDA after 7 d. (B–D) Sporulation patterns on PCA. (E) Conidia (scale bars: B–E = 20 µm).
To fulfill the Koch’s postulate and pathogenicity tests were carried out on detached leaves of I. ensata Thumb. Healthy leaves (7 cm in length) were cut and surface sterilized with 1% sodium hypochlorite (NaOCl) for 2 min, washed three times with sterilized distilled water and dried on sterile tissue paper. Conidia of all isolates formed on PCA plates (described before) were flooded with distilled sterile water. Spore suspension was concentrated to 105 spores per mL with the help of a hemocytometer. Leaves were placed into a wet container, inoculated with the spore suspension (20 µL) and incubated at 25 °C for 5 d. Small brown necrotic spots appeared after 3 d and showed symptoms of 8–16 cm diameter with fungal mycelia after 5 d (Figure 1(D)). No symptoms were observed on control leaves inoculated with distilled water. The same fungus was successfully re-isolated from the lesions. The experiment was repeated for three times with the same results indicating that the fungus is the causal pathogen of leaf spot disease on I. ensata Thumb.
To confirm the identification based on the morphology, genomic DNA of isolate YZU 161003 was extracted from 7-d-old mycelia grown on PDA using a modified CTAB method [8]. Four genes, internal transcribed spacer rDNA regions (ITS), glycerol-3-phosphate dehydrogenase gene (gpd), endopolygalacturonase gene (endoPG), and RNA polymerase second largest subunit gene (RPB2) were amplified using the primers ITS4 and ITS5 [9], gpd1 and gpd2 [10], EPG-specific and EPG-3 b [11] and RPB2-6 F, and RPB2-7cR [12], respectively. A 40-µL PCR mixture of 2 × Taq PCR Starmix with Loading Dye was used for amplification following the manufacturer’s protocol (Genstar, Beijing, China). The amplified PCR products were sequenced by BGI (Wuhan, China) using two specific primers for each gene. The resulting sequences were compared with the sequences deposited in the GenBank (NCBI, http//www.ncbi.nlm.nih.gov/BLAST/). Sequences from the Sect. Alternata of the Alternaria genus complex [13,14] were downloaded from the database. All the sequences were edited and aligned by using the Clustal X version 2.0 program (Bioinformatics Institute, Singapore) [15]. Four gene sequences were successively combined in the Mega version 5.05 program (Pennsylvania State University, State College, PA) [16]. A maximum likelihood analysis using the combined dataset was performed with GTRCAT model of nucleotide substitution in the RAxML program (Bioinformatics Institute, Singapore) [17]. Bootstrap analysis was performed with 1000 replications.
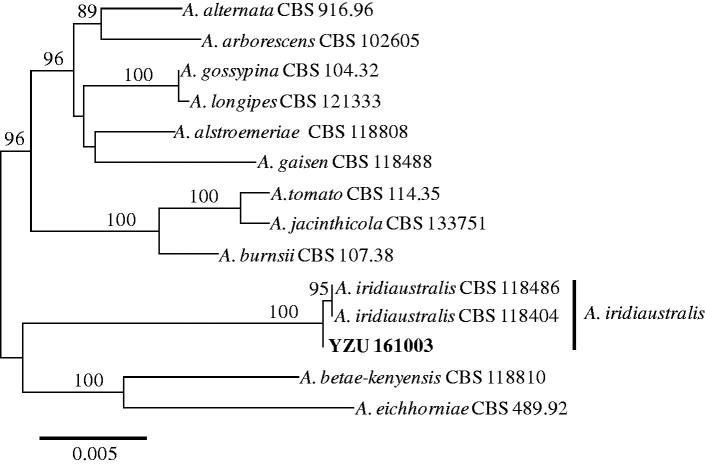
The comparison of sequences showed that the isolate YZU 161003 were 100% identical to the type strain CBS 118486 of A. iridiaustralis based on ITS, gpd, and RPB2 genes [14]. However, there was one nucleotide difference based on endoPG gene. The four gene sequences obtained in this work were deposited in the GenBank and assigned with the accession numbers of MF414189 (ITS), MF414192 (gpd), MF414194 (endoPG), and MF414193 (RPB2). In total 2175 bp were obtained, 515 bp from ITS, 576 bp from gpd, 444 bp from endoPG, and 640 bp from RPB2. The resulting phylogenetic tree is shown in Figure 3. The present isolate YZU 161003 were clustered with two stains (CBS 118486 and CBS 118404) of A. iridiaustralis from Iris plants in Australia and New Zealand supported with 100% bootstrap values. Thus, the molecular analysis confirmed the results of morphological identification. In summary, based on the morphology, pathogenicity, and molecular characteristics, the fungus causing leaf spot disease on I. ensata Thumb. characterized in this work has been identified as A. iridiaustralis. To our knowledge, this is the first report of A. iridiaustralis on I. ensata in China.
Figure 3.
Maximum likelihood tree of A. iridiaustralis, generated from a combined analysis of ITS, gpd, endoPG, and RPB2 gene datasets. Numbers above the branches indicate bootstrap values (≥60%) obtained for 1000 replicates. The scale bar indicates the number of substitutions per position.
Funding Statement
The work was supported by the National Natural Science Foundation of China [31400014], by the Young Scientist Foundation of Yangtze University [2016cqr08], and by the National Research Foundation of Korea [NRF-2014R1A1A2057824].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Wilson CA.Subgeneric classification in Iris re-examined using chloroplast sequence data. Taxon. 2011;60:27–35. [Google Scholar]
- 2.Wang H, Cui Y, Zhao C. Flavonoids of the genus Iris (Iridaceae)). Mini Rev Med Chem. 2010;10:643–661. [DOI] [PubMed] [Google Scholar]
- 3.Orhan I, Nasim S, Sener B, et al. Two isoflavones and bioactivity spectrum of the crude extracts of Iris germanica rhizomes. Phytother Res. 2003;17:575–577. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y, Meng T, Bi X, et al. Investigation and evaluation of wild Iris resources in Liaoning Province, China. Genet Resour Crop Evol. 2017;64:967–978. [Google Scholar]
- 5.Zhang TY.Flora fungorum sinicorum, vol. 16 Alternaria. Beijing: Science Press; 2003. [Google Scholar]
- 6.Simmons EG.Alternaria: an identification manual, CBS biodiversity series 6. Utrecht: Centraalbureau voor Schimmelcultures; 2007. [Google Scholar]
- 7.Yu SH.Alternaria and allied genaera. Suwon: National Institute of Biological Resources Ministry of Environment; 2015. [Google Scholar]
- 8.Wu FH, Huang DY, Huang XL, et al. Comparing study on several methods for DNA extraction from endophytic fungi. Chin Agric Sci Bull. 2009;25:62–64. [Google Scholar]
- 9.White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: Innis MA, Gelfand DH, Sninsky JJ, et al., editors. PCR protocols: a guide to methods and applications. San Diego (CA): Academic Press; 1990. p. 315–322. [Google Scholar]
- 10.Berbee ML, Pirseyedi M, Hubbard S.. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia. 1999;91:964–977. [Google Scholar]
- 11.Andrew M, Peever TL, Pryor BM. An expanded multilocus phylogeny does not resolve morphological species within the small-spored Altemrnaria species complex. Mycologia. 2009;101:95–109. [DOI] [PubMed] [Google Scholar]
- 12.Liu YJ, Whelen S, Hall BD.. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol. 1999; 16:1799–1808. [DOI] [PubMed] [Google Scholar]
- 13.Woudenberg JH, Groenewald JZ, Binder M, et al. Alternaria redefined. Stud Mycol. 2013;75:171–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woudenberg JH, Seidl MF, Groenewald JZ, et al. Alternaria section Alternaria: species, formae speciales or pathotypes? Stud Mycol. 2015;82:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K, Peterson D, Peterson N, et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2007;28:2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamatakis A.RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. [DOI] [PubMed] [Google Scholar]