Abstract
The human fungal pathogen Candida glabrata is causing more and more problems in hospitals, as this species shows an intrinsic antifungal drug resistance or rapidly becomes resistant when challenged with antifungals. C. glabrata only grows in the yeast form, so it is lacking a yeast-to-hyphae switch, which is one of the main virulence factors of C. albicans. An important virulence factor of C. glabrata is its capacity to strongly adhere to many different substrates. To achieve this, C. glabrata expresses a large number of adhesin-encoding genes and genome comparisons with closely related species, including the non-pathogenic S. cerevisiae, which revealed a correlation between the number of adhesin-encoding genes and pathogenicity. The adhesins are involved in the first steps during an infection; they are the first point of contact with the host. For several of these adhesins, their importance in adherence to different substrates and subsequent biofilm formation was demonstrated in vitro or in vivo. In this review, we provide an overview of the role of C. glabrata adhesins during adhesion and biofilm formation both, under in vitro and in vivo conditions.
Keywords: Candida glabrata, adhesin, adhesion, biofilm
1. Introduction
Candida species pose a major problem in hospitals, as they are the most frequently isolated fungal microorganisms in Healthcare-Associated Infections (HAI) [1,2,3,4]. Major risk factors for Candida infections include the use of broad-spectrum antibiotics, immuno-suppression of the host, and the use of medical devices in surgery. While C. albicans is still the most common cause of HAI, the isolation rate of non-C. albicans Candida species has increased over the years [5]. C. glabrata is the second or third most frequently isolated Candida species, depending on the geographical area studied [6]. This high incidence can be partially explained by the inherent low susceptibility of C. glabrata to the most used class of antifungal drugs, the azoles, and consequently C. glabrata HAI are associated with high mortality rates [7].
The use of medical devices, such as catheters, dentures, and prostheses, has increased enormously over the last decades [8,9,10]. These surfaces serve as a substrate for cells to adhere and to form a microbial community called a biofilm. Cells inside a biofilm have an altered gene expression, which gives the biofilm distinct phenotypic properties, e.g., they are frequently highly resistant to antifungal treatment [11], and removal of these medical implants is often necessary to cure the patient, thereby extending the hospital stay and elevating medical costs [9,12]. Candida species are also able to form biofilms on medical devices [13]. It was shown that C. glabrata forms biofilms on urinary and vascular catheters, prosthetic valves, and pacemakers [9,14,15]. As biofilms are sessile, its formation starts when the cells attach to a surface (adherence), after which the cells divide (proliferation) and form an extracellular matrix (maturation). The cell-surface interaction is mediated by specific proteins in the cell wall, called adhesins, which are widespread across microorganisms [16,17]. A high number of adhesins was predicted to be present in the C. glabrata genome, and several were confirmed to be involved in adherence to a specific substrate [18,19]. Furthermore, C. glabrata is not polymorphic and only grows as a budding yeast, unlike C. albicans, in which the yeast-to-hyphal transition was shown to be one of the most important virulence factors [20]. This indicates that adhesion, and therefore biofilms, are important for virulence in C. glabrata. In this review, we will give an update on our current understanding of C. glabrata adhesion and its importance in virulence.
2. Identification of C. glabrata Adhesins from Genomic Data
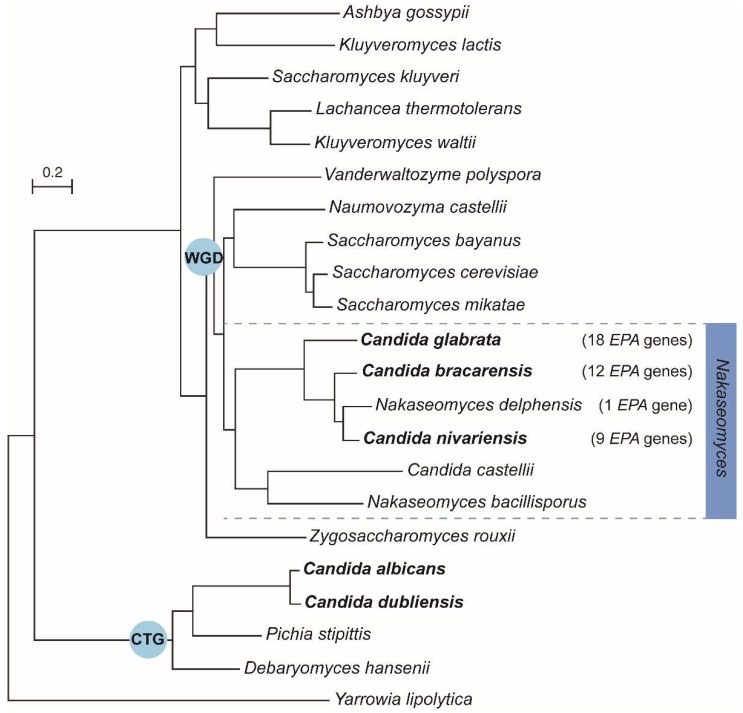
In 2004, the first complete genome sequences of C. glabrata, C. albicans and several other closely related fungal species were published [21,22,23]. Since then, several studies have been investigating genome evolution by the construction of phylogenetic trees based on different Candida species or with a more diverse set of fungal genomes including non-pathogenic species, with the main goal of identifying genes responsible for pathogenesis [23,24,25,26,27]. In these evolutionary trees, the C. glabrata genome is always positioned close to the non-pathogenic S. cerevisiae, in the clade of species that have undergone a whole genome duplication (WGD), rather than in the CTG clade (translating the CUG codon as serine instead of leucine) containing C. albicans and other fungal pathogens (Figure 1) [23,24,25].
Figure 1.
Phylogenetic tree of the subphylum Saccharomycotina, including several Candida species and Saccharomyces cerevisiae. C. glabrata is more closely related to the non-pathogenic S. cerevisiae than to the pathogen C. albicans, which belongs to the CTG clade. Gabaldon and co-workers found a correlation between the number of EPA genes (Epithelial adhesin) in the genome and pathogenicity in the Nakaseomyces clade (indicated in the figure). Pathogenic species are depicted in bold (Figure adapted from [28]).
A full comparison of the C. glabrata and S. cerevisiae genomes showed that 337 genes are specific to C. glabrata and may therefore provide information about its pathogenicity [29]. Further in depth in silico analysis revealed that a large number of these C. glabrata specific genes (51 or 67 genes [18,19]) encode for putative adhesins. 44 out of these 67 adhesin-like encoding genes identified by de Groot et al. [18] are located near the telomeres of all C. glabrata chromosomes. Because these subtelomeric regions contain sequence repeats, they are prone to rearrangements or non-allelic homologous recombination, which could explain the expansion of the adhesin-encoding gene families in C. glabrata. For some of these adhesins, it has been already shown that their expression is under control of subtelomeric silencing mediated by Rap1, the Sir-complex, and Ku70/80 [30,31,32,33,34]. An update on the effect of chromatin structure and pathways controlling this subtelomeric silencing on adherence in C. glabrata can be found in a separate review in this issue by López-Fuentes et al., 2018. Several adhesins also contain internal repetitive sequences (e.g., ‘VSHITT’ tandem repeats in PWP7 and AED1 [29,35,36]), which may allow for local chromosomal rearrangements to occur, resulting in different variants of the adhesins. For instance, an increase in the number of the repeats could result in an adhesin that may have its ligand-binding domain projected further out of the cell wall, thereby improving adherence to specific substrates [37,38].
In 2013, new genome sequences from the Nakaseomyces clade were published [26]. These species are more closely related to C. glabrata than to S. cerevisiae (Figure 1), including both pathogenic and non-pathogenic Candida and non-Candida species. The new genome comparisons of the Nakaseomyces species could expose new or confirm already identified factors important for pathogenesis. The latter is true, since Gabaldón and co-workers found a correlation between the number of EPA genes (Epithelial adhesin), the largest family of C. glabrata adhesins, and pathogenicity in the Nakaseomyces clade: the pathogenic C. bracarensis and C. nivariensis encode for 12 and 9 EPA adhesins, respectively, while only one EPA adhesin was found in the non-pathogenic Nakaseomyces delphensis [26]. This underscores that adhesins are important for the pathogenicity of fungal species.
Recently, C. glabrata gene annotation was updated [39], and previously annotated pseudogenes were corrected by splitting the open reading frame (ORF) into two or more new ORFs. Among these, genes were repeatedly predicted as adhesin-like genes, demonstrating that updating and functional analysis of the genome sequence is important. For example, a new gene encoding for a putative GPI-anchored adhesin was identified (CgNP25) and probably is part of the EPA family [39].
Since sequencing of fungal genomes became affordable, more studies have shown interest in comparing the genomes of clinical isolates with the genome of a reference lab strain [40,41,42,43,44]. All these studies have in common that they show that the C. glabrata genome is highly dynamic. This genome plasticity was also found in other pathogens, both prokaryotes and eukaryotes, and allows the pathogen to adapt to environmental changes [45,46,47]. Because C. glabrata is considered to be asexual, its genome plasticity is advantageous for its evolution as a pathogen.
Recently, one study sequenced the genomes of two isolates from one patient suffering from oropharyngeal candidiasis [43]. Both clinical isolates had a large number of nucleotide changes compared to the CBS138 reference sequence, and between these two clinical isolates there is a difference of 1024 nucleotides. In depth genome analysis showed that 6 adhesin-like genes of CBS138 were missing in the two clinical isolates and, interestingly, new predicted adhesin-like encoding genes were found exclusively in the clinical isolates. The two clinical isolates contained 101 and 107 adhesin-like genes, which is almost twice the number found in the CBS138 reference strain. Similar to the reference strain, half of the adhesin-like encoding genes were located close to the telomeres [18]. A closer inspection of the predicted adhesin-like encoding genes identified a duplication of several EPA genes and also of PWP and AWP genes [43]. The number of adhesin-like encoding genes present in clinical isolates is significantly higher, which suggests, again, that adhesins play an important role in infection.
In summary, genomic data strongly suggest that the adhesin-like encoding genes present in the C. glabrata genome are important for its pathogenicity. As new Candida species are identified in clinic, analysing their genomes is extremely relevant and can provide insights into their potential to be a pathogen, as was the case for C. glabrata. As a perspective, we think that further genomic analysis of C. glabrata clinical isolates and identification of their adhesin-like genes, followed by in-depth expression analysis and functional characterisation, could further increase our knowledge about the mechanism of pathogenesis in C. glabrata.
3. Adhesin Families and Ligand Binding Specificity
The C. glabrata cell wall structure and composition is largely similar to S. cerevisiae, but contains significantly more proteins and mannan, probably due to glycosylation of the cell wall proteins (CWPs). The majority of C. glabrata CWPs, among which glycosylphosphatidylinositol (GPI) anchored proteins, is cross-linked to 1,3-β-glucan, while a minority is bound to 1,6-β-glucan via an alkali-sensitive bond [18].
Assuming that adhesins are GPI-anchored proteins, Weig and co-workers used an optimized algorithm on the C. glabrata proteome to identify all putative GPI-CWPs [19]. The structural requirements of GPI-CWPs include a N-terminal hydrophobic signal sequence to target the protein to the endoplasmic reticulum, a C-terminal consensus sequence for GPI anchoring and the lack of internal transmembrane domains [48]. In addition, GPI-CWPs have a modular structure with the N-terminal ligand binding domain followed by a low-complexity and usually highly repetitive region with a high percentage of serine and threonine residues that is heavily glycosylated [18]. Out of 106 predicted GPI proteins, 51 were identified as adhesin-like proteins, because they contained adhesin-like structural features [19]; additional putative adhesins were identified by scanning for the conserved ‘VSHITT’ repeat sequences, a typical adhesin structure [18]. Therefore, a total of 67 C. glabrata predicted adhesins were identified, and these were classified into several subclasses based on their N-terminal substrate-binding domain [18,19]. The first group includes the Epa (Epithelial adhesion) protein family that contains a ligand-binding domain of approximately 300 amino acids that was identified as the conserved anthrax protective antigen (PA14) domain, suggesting a carbohydrate-binding function [49,50]. This N-terminal ligand binding domain is projected out of the cell through a long and highly glycosylated serine/threonine-rich region. This serine/threonine-rich region is essential for the adherence function of the Epa proteins [37,51]. A second subgroup also has a N-terminal PA14 domain and were therefore named Pwps (PA14 containing wall proteins). The remaining predicted adhesins were organised into five different subgroups, which have more distantly related ligand binding domains [18]. These subgroups are poorly characterized, since most studies have focussed on the Epa family of adhesins. De Groot et al. published that the low-complexity sequence repeats C-terminal of the ligand binding domain, which provides the flexibility to project the substrate-binding domain outside of the cell, is widespread across the different adhesin families [18]. The presence of these adhesins in the C. glabrata cell wall was confirmed by the identification of CWPs by mass spectrometry: Awp1, Awp2, Awp3, Awp4, and Epa6 [18], while in another study Epa3, Epa6, Awp2, and Awp4 plus Awp5 and Awp6 (two new adhesin-like cell wall proteins) and several non-unique peptides of other adhesins were identified [52].
The ligand binding specificities of C. glabrata adhesins have been determined by modelling of the N-terminal ligand binding domain [51], glycan interaction studies [53,54,55,56], mutagenesis [54], inhibition experiments [57,58], and atomic force microscopy (AFM) studies [59,60]. These studies focused on the Epa family of CWPs, as they were the first C. glabrata adhesins characterized (Table 1).
Table 1.
Literature overview of expression data (RNA or protein level) of C. glabrata adhesins in cells harvested during planktonic growth, during the adhesion phase or during biofilm formation.
| Process | Adhesin-Encoding Gene | Substrate | Evidence | Conditions | Read Out | Strains | Reference |
|---|---|---|---|---|---|---|---|
| Planktonic | AWP1-4; EPA6 | Peptides of Awp1-4 and Epa6 were found in at least one condition tested. Peptides of Awp4 and Epa6 were identified only in stationary-phase cells, while Awp3 was only found in log-phase cells. Awp1 was not identified in the ATCC2001 strain | Cells were grown in YPD or SC medium + 2% glucose and harvested at log phase (OD600nm of 2) or at stationary phase (24 h incubation) | LC-MS/MS on extracted cell walls | ATCC 90876 and ATCC2001 | [18] | |
| AWP2; AWP4; EPA3/EPA2; EPA6 | Awp2, Awp4, Epa6, and Epa3/Epa22 peptide were identified in YPD grown stationary phase cell walls. Awp5 was identified in SdmYg-cultured stationary phase cells | Cells inoculated at OD600nm of 0.1, incubation (16 h, 37 °C, 160 rpm) | LC-MS/MS on extracted cell walls | CBS138 | [52] | ||
| AWP1-7; EPA1; EPA3; EPA6; EPA7; EPA22 | Relative mRNA expression of clinical isolates to CBS138 reference reveals EPA1; EPA6 and AWP4 in PEU427 and EPA6 in PEU382 had an elevated expression. Other adhesins tested showed even lower expression compared to reference strain | Cells from overnight cultures (stationary phase) | RT-PCR from total RNA extraction | CBS138; PEU382; PEU427 | [63] | ||
| Adhesion | EPA1 | Epithelial cells | epa1∆ shows 95% reduced adhesion compared to wild type | Exponential phase cells in RPMI medium, added to Lec2 or HEp2 cells, briefly centrifugated (1–2 min, 500 g), 60 min incubation | Colony Forming Units (CFU) | BG2; epa1∆;BY4741; BY4741 + pEPA1 | [61] |
| 99% of S. cerevisiae adhere to Lec2 cells by heterologous expression of Epa1 | |||||||
| EPA1 | Macrophage-like cells | epa1∆ shows reduced adherence to THP-1 or PBMC cells | Cells in HBSS medium were added (MOI 3:1) to mammalian macrophage-like cells (106 cells/mL) in 96-well plates, 45 min, 37 °C, 5% CO2 | Fluorescent emission or flow cytometry | BG2; epa1∆; S288C; S288C + pEPA1 | [58] | |
| S. cerevisiae adheres to THP-1 or PBMC cells by heterologous expression of Epa1 | |||||||
| Macrophage-like cells | Strains expressing PDR1L280F hyperactive allele adhere less to PBMCs | C. glabrata cells were added to THP-1 cells treated with cytochalasin D (inhibition of phagocytosis), incubated (30 min, 37 °C, 5% CO2) | Colony Forming Units (CFU) | DSY562 (PDR1WT); DSY562 (PDR1L280F); DSY565 (PDR1L280F) | [64] | ||
| EPA1 | Epithelial cells | PDR1L280F strains show increased adherence to epithelial cells, concomitant with elevated EPA1 expression | C. glabrata cells were added to Lec2, HeLa or Caco-2 cell lines, centrifuated (1 min, 200 g), incubated (30 min, 37 °C, 5% CO2) | Colony Forming Units (CFU) | CBS138; BG2; epa1∆; DSY562; DSY565 with either PDR1WT or PDR1L280F | [64,65] | |
| EPA6 | Polystyrene (96-well plate) | epa6∆ shows significant lower in vitro adhesion | 106 cells/mL in RPMI 1640 (pH 7.0), 90 min, 37 °C, static; | XTT formazan production | ATCC2001; epa6∆ | [59] | |
| Hydrophobic groups | epa6∆ has smaller adhesion forces and shorter ruptures to CH3 than wild type | Probing of single C. glabrata cells with hydrophobic group (CH3) | AFM interaction forces | ||||
| AED1; PWP7 | Endothelial cells | pwp7∆ and aed1∆ strains had 66% and 50% reduced adhesion respectively, while aed2∆ strain showed wild type adherence | C. glabrata was added to human umbilical vein endothelial cells and incubated (15–60 min, 37 °C) | Colony Forming Units (CFU) | BG14; pwp7∆; aed1∆; aed2∆ | [29] | |
| C. albicans hyphae | EPA8, EPA19, AWP2, AWP7 and CAGL0F0018g expression were induced upon incubation with C. albicans hyphae | C. albicans germinated or yeast cells and C. glabrata (1:1 ratio), incubated (60 min) | Scanning electron microscopy | BG2; DSY562; VSY55 | [66] | ||
| Biofilm | EPA6 | Polystyrene (96-well plate) | epa6∆ shows 30% reduced biofilm formation, while superbiofilms were formed when EPA6 was overexpressed | Cells in SC medium, overnight incubation at 37 °C | XTT formazan production | BG2; epa6∆ | [67] |
| EPA6 | Polystyrene (96-well plate) | epa6∆ shows significant lower in vitro biofilm formation | After adherence, washed cells were submerged in fresh RPMI 1640 medium, 24 h, 37 °C | XTT formazan production | ATCC2001; epa6∆ | [59] | |
| EPA1, EPA6/7, EAP1 and CAGL0G04125g | Polystyrene (petri plate) | EPA1, EPA6/7, EAP1 and CAGL0G04125g expression was upregulated in 24 h biofilms compared to 6 h biofilms | cells in RPMI 1640 (pH 4) at OD600nm of 0.05, incubation (6 or 24 h, 30 °C, 30 rpm) | RT-PCR from total RNA extraction | CBS138 | [68] | |
| AWP6 | Polystyrene (petri plate) | Awp6 peptides were identified only in biofilm cell walls Epa3 peptides were found in both planktonic and SdmYg biofilms | Biofilms: Cells at OD600nm of 0.2, incubation (24 h, 37 °C) Planktonic: Cells at OD600nm of 0.1, incubation (16 h, 37 °C, 160 rpm) |
LC-MS/MS on extracted cell walls | CBS138 | [52] | |
| AWP1-7; EPA1, EPA3, EPA6, EPA7, EPA22 | AWP1, AWP3, AWP5, AWP7, EPA3 expression upregulated in biofilms (YPD and SdmYg medium); AWP4 and AWP6 expression upregulated, AWP2 and EPA7downregulated in YPD biofilms; EPA1 and EPA22 expression upregulated in SdmYg biofilms | Biofilms: Cells at OD600nm of 0.2, incubation (24 h, 37 °C) Planktonic: Cells at OD600nm of 0.1, incubation (37 °C, 160 rpm) to 0D600nm = 1.0 |
RT-PCR from total RNA extraction | ||||
| EC21:I21PA3-7; AWP2; AWP4; AWP6; AWP8-13EPA3-7; AWP2; AWP4; AWP6; AWP8-13 | Polystyrene (petri plate) | Peptides of Epa3, Epa6, Awp2, Awp4, Awp6, and Awp12 were identified in the CBS138 strain. Epa3, Epa6, Epa7, Awp2, Awp4, Awp6, Awp8 were identified in both PEU382 and PEU427 clinical isolates. Awp9, Awp10, Awp12, and Awp13, were unique to PEU427 while Awp11 was only found in PEU382. Epa4 and Epa5 peptides were found in PEU427, while absent in in CBS138 strain. | Cells inoculated in YPD medium, incubation (37 °C) to logarithmic phase and seeded in Petri dishes, incubation (24 h, 37 °C) | LC-MS/MS on extracted cell walls | CBS138; PEU382; PEU427 | [63] | |
| EPA1; EPA3; EPA6; AWP1-7 | Polyurethane (catheter) | Expression of EPA3, EPA6, AWP2, AWP3, and AWP5 was significantly higher in in vivo biofilms compared to in vitro biofilms | In vitro biofilms: Cells were added to catheters in RPMI 1640 medium and grown for 6 days in vivo biofilms. Cells were added to catheters in RPMI 1640 medium, and, after the period of adhesion (90 min, 37 °C), the catheters were washed and implanted in the back of the animals for 6 days | RT-PCR from total RNA extraction | ATCC2001 | [69] |
Already in 1999, Cormack and co-workers found that galactose, lactose (galactose β1-4 linked to glucose), and some other glycoconjugates were able to inhibit adhesion of C. glabrata to epithelial cells, suggesting a lectin binding function [61]. This theory was supported by the structural analysis of the N-terminal Epa1 binding domain (N-Epa1), co-crystallized with lactose [51]. Later on, a glycan interaction assay using the N-Epa1 showed a strong preference of N-Epa1 to bind glycans containing a terminal galactose residue β1-3 or β1-4 linked to galactose, glucose, N-acetylgalactosamine, or N-acetylglucosamine [53]. N-Epa1 also bound weakly to a terminal galactose α1-3 or α1-4 linked to galactose, N-acetylgalactosamine, or N-acetylglucosamine [53]. The same study investigated the N-terminal ligand binding domains of Epa6 and Epa7, which are highly homologous [62]. It was shown that N-Epa7 has the narrowest ligand specificity, only binding to Galβ1-3Gal or Galβ1-4Glc, while Epa6 does not have any preference and binds to both α- and β-linked glycosides with a terminal galactose residue [53]. These interactions were later confirmed and extended in a study with all 17 Epa proteins of the CBS138 reference strain. Analysis of these 17 proteins resulted in three functional binding classes: class I proteins (Epa1,3,7,9,10) prefer a β1-3 or β1-4 linked galactoside; class II proteins show a preference to β1-3 or β1-6 linked (Epa6,13,22) or sulfated galactosides (Epa12,15,23); and class III proteins prefer to bind acidic sugars (Epa2,19,20,21), sulfated galactosamines (Epa8), or α1-3 linked galactosides (Epa11) [56]. In the human body, these Epa proteins may interact with carbohydrates present in human cells. Indeed, N-Epa1 was found to bind to mucin (the main constituent of mucus and the glycocalyx), fibronectin (major component blood plasma and extracellular matrix), and tumor necrosis factor (TNF-α) on peripheral blood mononuclear cells (PBMCs), and both Epa1 and Epa7 were found to interact with N-glycan structures of kidney and brain tissue [55], while in a competitive binding assay, Epa6 was found to bind to fibronectin [57]. Based on the previous described glycan interaction studies, a lectin-glycan interaction network was constructed that is able to predict a correlation between certain Epas and severe diseases, such as cystic fibrosis or adenocarcinoma [55].
Other studies mutagenized specific residues in the Epa N-terminal domains in order to alter their binding affinities, which gives a good indication of the residues that are involved in substrate binding [53,54,56]. Kuhn and co-workers used inhibition experiments by addition of different carbohydrates to S. cerevisiae cells expressing the C. glabrata Epa1 adhesin [58]. The results of this study showed that the addition of lactose could only inhibit Epa1-mediated binding to THP-1 cells (monocytes from patient with acute monocytic leukemia), while no effect was seen for other cell lines such as U937 (lymphocytes from histiocytic lymphoma patient) and PBMC (monocytes and lymphocytes of healthy donors). This suggests that the specificity of Epa1 may play a variable role in adhesion or that several adhesions are involved in binding to the substrates tested [58] (Table 1).
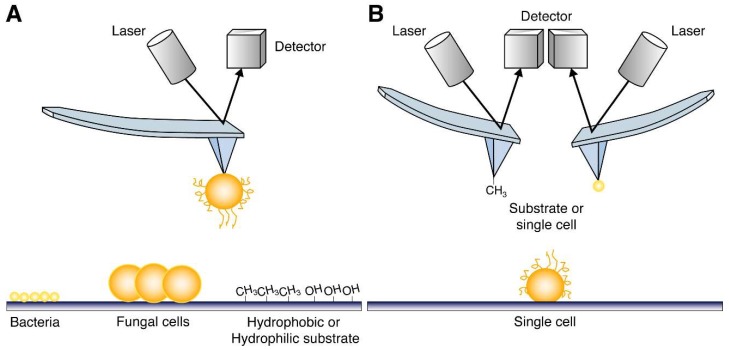
Atomic force microscopy (AFM) was used to investigate the adhesion profiles of C. glabrata cells. Using AFM, adhesion forces of single fungal cells towards different substrates can be measured, providing information about the kind of interaction, e.g., hydrophobic or hydrophilic (Figure 2A) [60]. Using this approach, it was shown that C. glabrata single cells have a high adhesive force towards a hydrophobic surface, which was confirmed by probing C. glabrata single cells with a CH3-bound AFM tip (Figure 2B). In contrast, when an epa6∆ strain was used in the same experiments, a significantly lower adhesion force and less frequent adhesion events were found, indicating that Epa6 mediates this hydrophobic interaction [59].
Figure 2.
Schematic representation of different AFM strategies used to probe ligand binding specificities of adhesins. (A) A single C. glabrata cell can be put on the AFM cantilever to probe a certain surface, which can be made of any (hydrophobic or hydrophilic) material or coated with specific biotic substrates, such as bacterial cells or other fungal cells or even other cell types (e.g., human cell lines). (B) The cell surface of a single C. glabrata cell can be probed in three dimensions using an AFM cantilever tip to which any substrate or other single cell can be attached (Figure based on [60]). Because of all these possibilities to adapt the system, AFM is very attractive to be used in adhesion research.
4. Surface Hydrophobicity and Adherence
C. glabrata is able to adhere to a diverse set of surfaces, which can be biotic, as well as abiotic. Adherence to abiotic surfaces is frequently tested in vitro using polystyrene or polypropylene multiwell plates because of the similarity to the Clinical & Laboratory Standards Institute (CLSI) susceptibility tests and because of the simple high throughput screening capacity [70]. Other abiotic surfaces tested include more clinically relevant materials such as polyurethane catheter pieces, denture material, or silicone pieces.
Plastic surfaces, as well as yeast cells, possess a negative surface charge, suggesting a repulsive force [71]. However, C. glabrata was found to adhere well to abiotic surfaces. The adhesion force of C. glabrata cells is much lower towards a hydrophilic surface compared to a hydrophobic surface [59], and consistent with these results, several studies found a good correlation between an increased adherence capacity of C. glabrata cells to abiotic surfaces and a high Cell Surface Hydrophobicity (CSH) [18,63,72]. Thus, adherence to an inert material is mediated by hydrophobic interactions, also called London van der Waals forces [71]. The relative CSH of C. glabrata was found to be significantly higher than C. albicans, although both show intra-species variation [72,73].
As CSH was found to be correlated to adherence to plastics; one would expect that this interaction is not mediated by specific receptor/adhesin ligand interactions. However, El Kirat-Chatel and co-workers found that adherence to polystyrene was significantly lower in an epa6∆ strain [59]; the hydrophobic adhesion forces were low in this strain. This indicates that Epa6, which is rich in hydrophobic residues, is, at least partially, responsible for providing the CSH under the conditions tested [59] (Table 1).
5. Adherence to Substrates and Biofilm Formation
Adherence is the first step in the formation of a biofilm, which was defined by Donlan and Costerton as a multi-layered structure consisting of a community of microorganisms irreversibly attached to a surface, embedded in an exopolymeric matrix and exhibiting distinctive phenotypic properties [11]. Biofilms are a major concern now in hospitals, as colonization on indwelling medical devices (e.g., urinary catheters) may require removal of the implant, as proper treatment is not available because of different physiology of the biofilm cells compared to planktonic cells, resulting in altered sensitivity towards antimicrobial drugs [1,2,11]. These biofilms can go undetected in the body for years or be life-threatening, depending on the microorganism and host environment [74]. The main problem is that biofilms can serve as a reservoir for seeding infections.
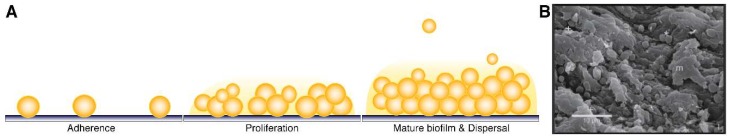
A biofilm is formed in different stages [75,76]: First, yeast cells attach to a surface, which can be abiotic or biotic. Second, the adhered cells proliferate on the surface to form microcolonies, after which extracellular matrix is produced. In the final stage, some cells will detach from the biofilm to disperse to other body sites. Because C. glabrata only grows by budding, C. glabrata mature biofilms are characterized by a dense network of yeast cells embedded in an extracellular matrix (Figure 3), in contrast to C. albicans, which forms hyphae during the proliferation phase [69,76,77,78,79]. This is also reflected in the thickness of the biofilms: mature C. glabrata biofilms are approximately half as thick (75–90 µm) as C. albicans biofilms [69].
Figure 3.
(A) Schematic overview of the different stages of biofilm formation in C. glabrata. (B) Scanning electron microscopy picture of an in vivo mature C. glabrata biofilm on a catheter piece, which was recovered from an Intensive Care Unit (ICU) patient. The biofilm is composed of yeast cells (asterix) embedded in extracellular matrix material (m) (Figure from [76]).
Several studies have investigated the expression of C. glabrata adhesins, at either transcriptional or protein level. However, it is difficult to compare between studies because of the variation in using different C. glabrata strains and the experimental conditions used. For example, the growth medium used to grow the biofilms, or even variations in the pH, changed the expression of adhesins, thus affecting the biofilm morphology [52,80]. Linde et al. even identified that some adhesins, including EPA3, EPA6, and EPA20, are expressed as different isoforms depending on the growth medium [39].
EPA1 (Epithelial adhesin 1) was the first C. glabrata CWP identified to be important for adhesion in an insertional mutant library screen [61] (Table 1). Epa1 was shown to be largely responsible for in vitro adherence of C. glabrata to epithelial cells, since epa1∆ strains showed a 95 percent reduced adherence, while S. cerevisiae becomes highly adherent by heterologous expression of Epa1 [61]. The presence of Ca2+ was required for adhesion, and adherence could be inhibited by addition of galactose or lactose (see above). Epa1 is also involved in adherence to human macrophage-like cells: C. glabrata or S. cerevisiae cells expressing EPA1 showed a great in vitro adherence to THP-1 cells, and this interaction was inhibited by adding lactose [58]. A similar high adherence was seen with matured human PBMC-derived macrophages, but strikingly, this interaction could not be inhibited by addition of lactose. While the presence of Epa1 was sufficient for binding macrophage-like cells, C. glabrata was able to avoid subsequent phagocytosis, whereas S. cerevisiae cells expressing Epa1 were phagocytosed by the macrophages after adhesion [58]. Vale-Silva et al. found that the transcription factor Pdr1, known to control the expression of ABC transporters, also regulates EPA1 expression. Interestingly, several Pdr1 gain of function (GOF) mutants were found to adhere less to THP-1 macrophage-like cells and mouse BMDM macrophages, while adherence to epithelial cells in vitro was increased compared to the wild type strain [64]. A Pdr1 binding site was found in the EPA1 promoter and the Pdr1L280F GOF mutant leads to EPA1 overexpression, which was eliminated by deletion of this Pdr1 binding site. Furthermore, introducing Pdr1 GOF mutations did not affect in vitro adherence to epithelial cells in an epa1∆ strain, indicating that Pdr1 regulates EPA1 expression [65].
In vivo, there was no difference in virulence between an epa1∆ or wild type strain in a murine vaginal model and in a gastrointestinal tract infection model [61]. In a murine model of urinary tract infection (UTI), overexpression of EPA1 leads to significant elevated colonization of bladder and kidneys [65]. Interestingly, deletion of EPA1 alone caused a small decrease in organ colonization, although not significantly [65]. This is probably due to the involvement of other adhesins, as it was shown by Domergue et al. that the triple mutant strain (epa1∆ epa6∆ epa7∆) showed a significant decreased colonization in the bladder in the murine UTI model [31]. This study also showed that the expression of these adhesins was induced by removing nicotinic acid (NA) from the medium. Consistently, in vitro adherence to uroepithelial cells was significantly lower in presence of excess NA [31].
The insertional mutant library of Cormack and co-workers was used to search for aberrant in vitro biofilm formation in polystyrene multi-well plates. epa6∆ strains had significant reduced biofilms, while epa1-5∆ strains were only slightly affected. The expression of EPA6 and EPA7 was also shown to be induced in biofilms [67,80]. EPA6 and EPA7 have a highly homologous sequence and are both located near the telomeres, where their expression is regulated by subtelomeric silencing [62]. Other mutants that showed poor biofilms were in genes encoding for the chromatin remodelling Swi/Snf complex and a protein kinase YAK1. The possible reason for the defect in biofilm formation in strains mutated for these genes is the fact that they are required for the expression of EPA6 and EPA7, possibly by affecting subtelomeric-silencing [67,81]. On the other hand, overproducing biofilm strains were isolated: deletion of CST6 resulted in a strain with strong biofilm formation capacity and consistently, and the transcription factor Cst6 was shown to be a negative regulator of EPA6 expression independent of subtelomeric silencing [81]. Another overproducing biofilm strain had an insertion between the two EPA-like genes CAGL0I10147g and CAGL0I10200g [81]. Furthermore, the multidrug resistance transporter Tpo1_2 was also found to affect in vitro biofilm formation on polystyrene plates, as its deletion strain showed a 40 percent reduction in biofilm formation. The expression of several adhesins, including EPA1, was repressed in the tpo1_2∆ strain [68].
C. glabrata biofilm formation on polystyrene was found to be significantly higher when C. albicans was present [66]. In dynamic flow conditions, C. glabrata is unable to form a biofilm unless C. albicans hyphae are present. Fluorescence microscopy pictures showed that the C. glabrata yeast cells were tightly associated along the C. albicans hyphae. This association was significantly reduced when the C. albicans ALS1 and/or ALS3 genes were deleted. The C. glabrata adhesins EPA8, EPA19, AWP2, AWP7, and CAGL0F0018g were found to mediate this adherence, and their expression was induced upon incubation with C. albicans hyphae [66].
Furthermore, Pwp7 and Aed1 (Adherence to endothelial cells) were found to play a role in adherence to endothelial cells in vitro, as their deletion strains show a significant reduced adherence compared to the wild type strain [29]. Deletion of AED2, located next to AED1 at the end of chromosome K, did not alter endothelial adherence.
Other studies analyzed the expression of adhesins during biofilm formation. Santos et al. showed that the expression of EPA1, EPA6/7, EAP1, and CAGL0G04125g was upregulated in a 24-h in vitro biofilm compared to 6-h biofilm [68]. Another study compared the adhesin expression of in vitro biofilms to planktonic grown cells: a unique peptide of Awp6 was found exclusively in biofilm cell walls, while peptides of Epa3 were found in the cell wall of biofilm cells and planktonic cells grown in semi-defined yeast growth (SdmYg) medium [52]. At the transcriptional level, the expression of AWP adhesins, except AWP2, was increased in biofilms compared to planktonic cells. EPA3 expression was elevated in YPD cultured biofilms, while EPA7 expression was lower. The expression of EPA1, EPA3, EPA7, and EPA22 was only induced in SdmYg medium-cultured biofilms [52]. Gómez-Molero et al. compared the CWP profiles of two clinical isolates, which showed an increased in vitro adherence to polystyrene, silicone, and denture material. Mass spectrometry of the isolated CWPs led to the identification of 12 adhesin-like proteins, including Epa3, Epa6, Epa7, and several Awp adhesins, of which 6 were newly identified. Most of the adhesins were shared between the two isolates [63].
Kucharíková and co-workers compared the expression of several adhesins in mature in vitro biofilms formed on polyurethane catheter pieces to mature biofilms isolated from a rat subcutaneous biofilm model. Major differences were found between the in vitro and in vivo biofilms, which was expected, since the growth conditions in vitro can be tightly controlled, while in vivo there is variation originating from the environment (different nutrient states, the state of the immune system, mouse variability, etc.). The expression of EPA3, EPA6, AWP2, AWP3, and AWP5 were significantly higher under the in vivo conditions than the in vitro biofilms, while there was no difference in EPA1 expression [69].
To determine the expression of specific adhesin-encoding genes under in vivo conditions, Domergue and co-workers made use of recombinant in vivo expression technology (IVET) [31]. C. glabrata cells were engineered to become auxotroph for tryptophan and resistant to hygromycin when EPA6 was expressed. In this way, the percentage of hygromycin-resistant and tryptophan auxotroph colonies can be assessed for the C. glabrata recovered from in vivo models of infection. Using IVET, one can determine whether an adhesin was expressed in the tested condition, but it is not possible to address the specific moment of induction or expression. It was shown that EPA6 was not expressed during in vitro growth or during intravenous infection of mice, while a significant portion of C. glabrata cells recovered from an in vivo UTI model was hygromycin-resistant [31]. Using IVET, EPA2 was found to be expressed in a small but significant part of C. glabrata recovered from the liver in a murine model of systemic infection [82].
6. Concluding Remarks
Because of its increasing incidence, it is important to investigate the main virulence factors of this pathogen. For many years, it is known that C. glabrata encodes for a high number of adhesins, which are considered as one of the main virulence factors of this pathogen. The importance of several C. glabrata adhesins in adherence to both biotic and abiotic substrates, as well as for biofilm formation, was demonstrated as presented in this review. Yet, the function for virulence of many other adhesin-encoding genes is still unknown, and, as is clear from recent work, the adhesin-encoding gene family seems to rapidly adapt, as strains with over 100 adhesin genes have now been described [43]. It will be important to continue to study these adhesins in all their aspects: On the one hand, the analysis of newly sequenced genomes of clinical isolates can provide new insights into recent evolutionary events. On the other hand, understanding the environmental conditions that are involved in the regulation of gene expression and posttranscriptional control of specific CWPs could uncover which are the more relevant adhesins to use as targets for antifungal drugs. Yet, it is necessary to complement these expression studies with interaction studies or experiments using mutants to confirm the actual binding of an adhesin to a certain substrate. As several of the C. glabrata adhesins are part of protein families, having a similar adhesin structure, redundancy is possible so that the effect of a single deletion can be underestimated due to compensation by other adhesins. With the introduction of CRISPR-Cas9 in C. glabrata [83,84], it will be interesting to investigate the functional analysis of a strain containing deletions of several or even all adhesin-encoding genes. This will shed light on their role in virulence in the coming years, as up until now, the in vivo studies published, in which an adhesin deletion mutant was used, did not show differences in virulence.
Acknowledgments
This work was supported by the grant bilateral project FWO-Flanders (VS.036.14N)/CONACYT-Mexico (219669). We would like to thank Nico Vangoethem (VIB-KU Leuven) for assisting in the production of the figures.
Author Contributions
B.T. and P.V.D. designed the review, B.T. wrote the review and generated the figures, B.T., A.D.L.P., I.C. and P.V.D. were involved in the preparation of the final version of the review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.European Centre for Disease Prevention and Control . Annual Epidemiological Report 2016—Surgical Site Infections. European Centre for Disease Prevention and Control; Solna City, Sweden: 2016. [Google Scholar]
- 2.European Centre for Disease Prevention and Control Healthcare-Associated Infections Acquired in Intensive Care Units. [(accessed on 2 April 2018)]; Available online: https://ecdc.europa.eu/en/publications-data/healthcare-associated-infections-acquired-intensive-care-units-annual.
- 3.Zarb P., Coignard B., Griskeviciene J., Muller A., Vankerckhoven V., Weist K., Goossens M., Vaerenberg S., Hopkins S., Catry B., et al. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Eurosurveillance. 2012;17:20316. doi: 10.2807/ese.17.46.20316-en. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller M.A., Diekema D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfaller M.A., Diekema D.J., Gibbs D.L., Newell V.A., Ellis D., Tullio V., Rodloff A., Fu W., Ling T.A. Global Antifungal Surveillance Group. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: A 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 2010;48:1366–1377. doi: 10.1128/JCM.02117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014;20(Suppl. 6):5–10. doi: 10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- 7.Choi H.K., Jeong S.J., Lee H.S., Chin B.S., Choi S.H., Han S.H., Kim M.S., Kim C.O., Choi J.Y., Song Y.G., et al. Blood stream infections by Candida glabrata and Candida krusei: A single-center experience. Korean J. Intern. Med. 2009;24:263–269. doi: 10.3904/kjim.2009.24.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Eiff C., Jansen B., Kohnen W., Becker K. Infections associated with medical devices: Pathogenesis, management and prophylaxis. Drugs. 2005;65:179–214. doi: 10.2165/00003495-200565020-00003. [DOI] [PubMed] [Google Scholar]
- 9.Kojic E.M., Darouiche R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004;17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawser S.P., Douglas L.J. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 1994;62:915–921. doi: 10.1128/iai.62.3.915-921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donlan R.M., Costerton J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darouiche R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 13.Kumamoto C.A. Candida biofilms. Curr. Opin. Microbiol. 2002;5:608–611. doi: 10.1016/S1369-5274(02)00371-5. [DOI] [PubMed] [Google Scholar]
- 14.Richards M.J., Edwards J.R., Culver D.H., Gaynes R.P. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 2000;21:510–515. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 15.Coco B.J., Bagg J., Cross L.J., Jose A., Cross J., Ramage G. Mixed Candida albicans and Candida glabrata populations associated with the pathogenesis of denture stomatitis. Oral. Microbiol. Immunol. 2008;23:377–383. doi: 10.1111/j.1399-302X.2008.00439.x. [DOI] [PubMed] [Google Scholar]
- 16.Kline K.A., Falker S., Dahlberg S., Normark S., Henriques-Normark B. Bacterial adhesins in host-microbe interactions. Cell Host Microbe. 2009;5:580–592. doi: 10.1016/j.chom.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 17.de Groot P.W., Bader O., de Boer A.D., Weig M., Chauhan N. Adhesins in human fungal pathogens: Glue with plenty of stick. Eukaryot. Cell. 2013;12:470–481. doi: 10.1128/EC.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Groot P.W., Kraneveld E.A., Yin Q.Y., Dekker H.L., Gross U., Crielaard W., de Koster C.G., Bader O., Klis F.M., Weig M. The cell wall of the human pathogen Candida glabrata: Differential incorporation of novel adhesin-like wall proteins. Eukaryot. Cell. 2008;7:1951–1964. doi: 10.1128/EC.00284-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weig M., Jansch L., Gross U., De Koster C.G., Klis F.M., De Groot P.W. Systematic identification in silico of covalently bound cell wall proteins and analysis of protein-polysaccharide linkages of the human pathogen Candida glabrata. Microbiology. 2004;150:3129–3144. doi: 10.1099/mic.0.27256-0. [DOI] [PubMed] [Google Scholar]
- 20.Biswas S., Van Dijck P., Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol. Mol. Biol. Rev. 2007;71:348–376. doi: 10.1128/MMBR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dujon B., Sherman D., Fischer G., Durrens P., Casaregola S., Lafontaine I., De Montigny J., Marck C., Neuveglise C., Talla E., et al. Genome evolution in yeasts. Nature. 2004;430:35–44. doi: 10.1038/nature02579. [DOI] [PubMed] [Google Scholar]
- 22.Jones T., Federspiel N.A., Chibana H., Dungan J., Kalman S., Magee B.B., Newport G., Thorstenson Y.R., Agabian N., Magee P.T., et al. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. USA. 2004;101:7329–7334. doi: 10.1073/pnas.0401648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler G., Rasmussen M.D., Lin M.F., Santos M.A., Sakthikumar S., Munro C.A., Rheinbay E., Grabherr M., Forche A., Reedy J.L., et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–662. doi: 10.1038/nature08064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzpatrick D.A., Logue M.E., Stajich J.E., Butler G. A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol. Biol. 2006;6:99. doi: 10.1186/1471-2148-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcet-Houben M., Gabaldon T. The tree versus the forest: The fungal tree of life and the topological diversity within the yeast phylome. PLoS ONE. 2009;4:e4357. doi: 10.1371/journal.pone.0004357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabaldon T., Martin T., Marcet-Houben M., Durrens P., Bolotin-Fukuhara M., Lespinet O., Arnaise S., Boisnard S., Aguileta G., Atanasova R., et al. Comparative genomics of emerging pathogens in the Candida glabrata clade. BMC Genom. 2013;14:623. doi: 10.1186/1471-2164-14-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabaldon T., Naranjo-Ortiz M.A., Marcet-Houben M. Evolutionary genomics of yeast pathogens in the Saccharomycotina. FEMS Yeast Res. 2016;16 doi: 10.1093/femsyr/fow064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabaldon T., Carrete L. The birth of a deadly yeast: Tracing the evolutionary emergence of virulence traits in Candida glabrata. FEMS Yeast Res. 2016;16:fov110. doi: 10.1093/femsyr/fov110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desai C., Mavrianos J., Chauhan N. Candida glabrata Pwp7p and Aed1p are required for adherence to human endothelial cells. FEMS Yeast Res. 2011;11:595–601. doi: 10.1111/j.1567-1364.2011.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Las Peñas A., Pan S.J., Castaño I., Alder J., Cregg R., Cormack B.P. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev. 2003;17:2245–2258. doi: 10.1101/gad.1121003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domergue R., Castaño I., De Las Peñas A., Zupancic M., Lockatell V., Hebel J.R., Johnson D., Cormack B.P. Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science. 2005;308:866–870. doi: 10.1126/science.1108640. [DOI] [PubMed] [Google Scholar]
- 32.Gallegos-Garcia V., Pan S.J., Juarez-Cepeda J., Ramirez-Zavaleta C.Y., Martin-del-Campo M.B., Martinez-Jimenez V., Castaño I., Cormack B., De Las Peñas A. A novel downstream regulatory element cooperates with the silencing machinery to repress EPA1 expression in Candida glabrata. Genetics. 2012;190:1285–1297. doi: 10.1534/genetics.111.138099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halliwell S.C., Smith M.C., Muston P., Holland S.L., Avery S.V. Heterogeneous expression of the virulence-related adhesin Epa1 between individual cells and strains of the pathogen Candida glabrata. Eukaryot. Cell. 2012;11:141–150. doi: 10.1128/EC.05232-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juarez-Reyes A., De Las Peñas A., Castaño I. Analysis of subtelomeric silencing in Candida glabrata. Methods Mol. Biol. 2011;734:279–301. doi: 10.1007/978-1-61779-086-7_14. [DOI] [PubMed] [Google Scholar]
- 35.Thierry A., Bouchier C., Dujon B., Richard G.F. Megasatellites: A peculiar class of giant minisatellites in genes involved in cell adhesion and pathogenicity in Candida glabrata. Nucleic Acids Res. 2008;36:5970–5982. doi: 10.1093/nar/gkn594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thierry A., Dujon B., Richard G.F. Megasatellites: A new class of large tandem repeats discovered in the pathogenic yeast Candida glabrata. Cell. Mol. Life Sci. 2010;67:671–676. doi: 10.1007/s00018-009-0216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frieman M.B., McCaffery J.M., Cormack B.P. Modular domain structure in the Candida glabrata adhesin Epa1p, a beta1,6 glucan-cross-linked cell wall protein. Mol. Microbiol. 2002;46:479–492. doi: 10.1046/j.1365-2958.2002.03166.x. [DOI] [PubMed] [Google Scholar]
- 38.Verstrepen K.J., Jansen A., Lewitter F., Fink G.R. Intragenic tandem repeats generate functional variability. Nat. Genet. 2005;37:986–990. doi: 10.1038/ng1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linde J., Duggan S., Weber M., Horn F., Sieber P., Hellwig D., Riege K., Marz M., Martin R., Guthke R., et al. Defining the transcriptomic landscape of Candida glabrata by RNA-Seq. Nucleic Acids Res. 2015;43:1392–1406. doi: 10.1093/nar/gku1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polakova S., Blume C., Zarate J.A., Mentel M., Jorck-Ramberg D., Stenderup J., Piskur J. Formation of new chromosomes as a virulence mechanism in yeast Candida glabrata. Proc. Natl. Acad. Sci. USA. 2009;106:2688–2693. doi: 10.1073/pnas.0809793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller H., Thierry A., Coppee J.Y., Gouyette C., Hennequin C., Sismeiro O., Talla E., Dujon B., Fairhead C. Genomic polymorphism in the population of Candida glabrata: Gene copy-number variation and chromosomal translocations. Fungal Genet. Biol. 2009;46:264–276. doi: 10.1016/j.fgb.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad K.M., Ishchuk O.P., Hellborg L., Jorgensen G., Skvarc M., Stenderup J., Jorck-Ramberg D., Polakova S., Piskur J. Small chromosomes among Danish Candida glabrata isolates originated through different mechanisms. Anton. Leeuwenhoek J. Microbiol. 2013;104:111–122. doi: 10.1007/s10482-013-9931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vale-Silva L., Beaudoing E., Tran V.D.T., Sanglard D. Comparative Genomics of Two Sequential Candida glabrata Clinical Isolates. Genes Genomes Genet. 2017;7:2413–2426. doi: 10.1534/g3.117.042887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bader O., Schwarz A., Kraneveld E.A., Tangwattanachuleeporn M., Schmidt P., Jacobsen M.D., Gross U., De Groot P.W., Weig M. Gross karyotypic and phenotypic alterations among different progenies of the Candida glabrata CBS138/ATCC2001 reference strain. PLoS ONE. 2012;7:e52218. doi: 10.1371/journal.pone.0052218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thieme F., Koebnik R., Bekel T., Berger C., Boch J., Buttner D., Caldana C., Gaigalat L., Goesmann A., Kay S., et al. Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J. Bacteriol. 2005;187:7254–7266. doi: 10.1128/JB.187.21.7254-7266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selmecki A., Forche A., Berman J. Genomic plasticity of the human fungal pathogen Candida albicans. Eukaryot. Cell. 2010;9:991–1008. doi: 10.1128/EC.00060-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobrindt U., Hacker J. Whole genome plasticity in pathogenic bacteria. Curr. Opin. Microbiol. 2001;4:550–557. doi: 10.1016/S1369-5274(00)00250-2. [DOI] [PubMed] [Google Scholar]
- 48.De Groot P.W., Hellingwerf K.J., Klis F.M. Genome-wide identification of fungal GPI proteins. Yeast. 2003;20:781–796. doi: 10.1002/yea.1007. [DOI] [PubMed] [Google Scholar]
- 49.Rigden D.J., Mello L.V., Galperin M.Y. The PA14 domain, a conserved all-beta domain in bacterial toxins, enzymes, adhesins and signaling molecules. Trends Biochem. Sci. 2004;29:335–339. doi: 10.1016/j.tibs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Maestre-Reyna M., Diderrich R., Veelders M.S., Eulenburg G., Kalugin V., Bruckner S., Keller P., Rupp S., Mosch H.U., Essen L.O. Structural basis for promiscuity and specificity during Candida glabrata invasion of host epithelia. Proc. Natl. Acad. Sci. USA. 2012;109:16864–16869. doi: 10.1073/pnas.1207653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ielasi F.S., Decanniere K., Willaert R.G. The epithelial adhesin 1 (Epa1p) from the human-pathogenic yeast Candida glabrata: Structural and functional study of the carbohydrate-binding domain. Acta Crystallogr. Sect. D. 2012;68:210–217. doi: 10.1107/S0907444911054898. [DOI] [PubMed] [Google Scholar]
- 52.Kraneveld E.A., de Soet J.J., Deng D.M., Dekker H.L., de Koster C.G., Klis F.M., Crielaard W., de Groot P.W. Identification and differential gene expression of adhesin-like wall proteins in Candida glabrata biofilms. Mycopathologia. 2011;172:415–427. doi: 10.1007/s11046-011-9446-2. [DOI] [PubMed] [Google Scholar]
- 53.Zupancic M.L., Frieman M., Smith D., Alvarez R.A., Cummings R.D., Cormack B.P. Glycan microarray analysis of Candida glabrata adhesin ligand specificity. Mol. Microbiol. 2008;68:547–559. doi: 10.1111/j.1365-2958.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- 54.Ielasi F.S., Verhaeghe T., Desmet T., Willaert R.G. Engineering the carbohydrate-binding site of Epa1p from Candida glabrata: Generation of adhesin mutants with different carbohydrate specificity. Glycobiology. 2014;24:1312–1322. doi: 10.1093/glycob/cwu075. [DOI] [PubMed] [Google Scholar]
- 55.Ielasi F.S., Alioscha-Perez M., Donohue D., Claes S., Sahli H., Schols D., Willaert R.G. Lectin-Glycan Interaction Network-Based Identification of Host Receptors of Microbial Pathogenic Adhesins. MBio. 2016;7:e00584-16. doi: 10.1128/mBio.00584-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diderrich R., Kock M., Maestre-Reyna M., Keller P., Steuber H., Rupp S., Essen L.O., Mosch H.U. Structural Hot Spots Determine Functional Diversity of the Candida glabrata Epithelial Adhesin Family. J. Biol. Chem. 2015;290:19597–19613. doi: 10.1074/jbc.M115.655654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zajac D., Karkowska-Kuleta J., Bochenska O., Rapala-Kozik M., Kozik A. Interaction of human fibronectin with Candida glabrata epithelial adhesin 6 (Epa6) Acta Biochim. Pol. 2016;63:417–426. doi: 10.18388/abp.2016_1328. [DOI] [PubMed] [Google Scholar]
- 58.Kuhn D.M., Vyas V.K. The Candida glabrata adhesin Epa1p causes adhesion, phagocytosis, and cytokine secretion by innate immune cells. FEMS Yeast Res. 2012;12:398–414. doi: 10.1111/j.1567-1364.2011.00785.x. [DOI] [PubMed] [Google Scholar]
- 59.El-Kirat-Chatel S., Beaussart A., Derclaye S., Alsteens D., Kucharikova S., Van Dijck P., Dufrene Y.F. Force nanoscopy of hydrophobic interactions in the fungal pathogen Candida glabrata. ACS Nano. 2015;9:1648–1655. doi: 10.1021/nn506370f. [DOI] [PubMed] [Google Scholar]
- 60.El-Kirat-Chatel S., Beaussart A., Boyd C.D., O’Toole G.A., Dufrene Y.F. Single-cell and single-molecule analysis deciphers the localization, adhesion, and mechanics of the biofilm adhesin LapA. ACS Chem. Biol. 2014;9:485–494. doi: 10.1021/cb400794e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cormack B.P., Ghori N., Falkow S. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science. 1999;285:578–582. doi: 10.1126/science.285.5427.578. [DOI] [PubMed] [Google Scholar]
- 62.Castaño I., Pan S.J., Zupancic M., Hennequin C., Dujon B., Cormack B.P. Telomere length control and transcriptional regulation of subtelomeric adhesins in Candida glabrata. Mol. Microbiol. 2005;55:1246–1258. doi: 10.1111/j.1365-2958.2004.04465.x. [DOI] [PubMed] [Google Scholar]
- 63.Gomez-Molero E., de Boer A.D., Dekker H.L., Moreno-Martinez A., Kraneveld E.A., Chauhan N., Weig M., de Soet J.J., de Koster C.G., et al. Proteomic analysis of hyperadhesive Candida glabrata clinical isolates reveals a core wall proteome and differential incorporation of adhesins. FEMS Yeast Res. 2015;15 doi: 10.1093/femsyr/fov098. [DOI] [PubMed] [Google Scholar]
- 64.Vale-Silva L., Ischer F., Leibundgut-Landmann S., Sanglard D. Gain-of-function mutations in PDR1, a regulator of antifungal drug resistance in Candida glabrata, control adherence to host cells. Infect. Immun. 2013;81:1709–1720. doi: 10.1128/IAI.00074-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vale-Silva L.A., Moeckli B., Torelli R., Posteraro B., Sanguinetti M., Sanglard D. Upregulation of the Adhesin Gene EPA1 Mediated by PDR1 in Candida glabrata Leads to Enhanced Host Colonization. mSphere. 2016;1:e00065-15. doi: 10.1128/mSphere.00065-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tati S., Davidow P., McCall A., Hwang-Wong E., Rojas I.G., Cormack B., Edgerton M. Candida glabrata Binding to Candida albicans Hyphae Enables Its Development in Oropharyngeal Candidiasis. PLoS Pathog. 2016;12:e1005522. doi: 10.1371/journal.ppat.1005522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iraqui I., Garcia-Sanchez S., Aubert S., Dromer F., Ghigo J.M., d’enfert C., Janbon G. The Yak1p kinase controls expression of adhesins and biofilm formation in Candida glabrata in a Sir4p-dependent pathway. Mol. Microbiol. 2005;55:1259–1271. doi: 10.1111/j.1365-2958.2004.04475.x. [DOI] [PubMed] [Google Scholar]
- 68.Santos R., Costa C., Mil-Homens D., Romao D., de Carvalho C.C., Pais P., Mira N.P., Fialho A.M., Teixeira M.C. The multidrug resistance transporters CgTpo1_1 and CgTpo1_2 play a role in virulence and biofilm formation in the human pathogen Candida glabrata. Cell. Microbiol. 2017;19 doi: 10.1111/cmi.12686. [DOI] [PubMed] [Google Scholar]
- 69.Kucharikova S., Neirinck B., Sharma N., Vleugels J., Lagrou K., Van Dijck P. In vivo Candida glabrata biofilm development on foreign bodies in a rat subcutaneous model. J. Antimicrob. Chemother. 2015;70:846–856. doi: 10.1093/jac/dku447. [DOI] [PubMed] [Google Scholar]
- 70.Krom B.P., Willems H.M. In Vitro Models for Candida Biofilm Development. Humana Press; New York, NY, USA: 2016. [DOI] [PubMed] [Google Scholar]
- 71.Klotz S.A., Drutz D.J., Zajic J.E. Factors governing adherence of Candida species to plastic surfaces. Infect. Immun. 1985;50:97–101. doi: 10.1128/iai.50.1.97-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luo G., Samaranayake L.P. Candida glabrata, an emerging fungal pathogen, exhibits superior relative cell surface hydrophobicity and adhesion to denture acrylic surfaces compared with Candida albicans. APMIS. 2002;110:601–610. doi: 10.1034/j.1600-0463.2002.1100902.x. [DOI] [PubMed] [Google Scholar]
- 73.Hazen K.C., Plotkin B.J., Klimas D.M. Influence of growth conditions on cell surface hydrophobicity of Candida albicans and Candida glabrata. Infect. Immun. 1986;54:269–271. doi: 10.1128/iai.54.1.269-271.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vertes A., Hitchins V., Phillips K.S. Analytical challenges of microbial biofilms on medical devices. Anal. Chem. 2012;84:3858–3866. doi: 10.1021/ac2029997. [DOI] [PubMed] [Google Scholar]
- 75.Nobile C.J., Fox E.P., Nett J.E., Sorrells T.R., Mitrovich Q.M., Hernday A.D., Tuch B.B., Andes D.R., Johnson A.D. A Recently Evolved Transcriptional Network Controls Biofilm Development in Candida albicans. Cell. 2012;148:126–138. doi: 10.1016/j.cell.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paulitsch A.H., Willinger B., Zsalatz B., Stabentheiner E., Marth E., Buzina W. In-vivo Candida biofilms in scanning electron microscopy. Med. Mycol. 2009;47:690–696. doi: 10.3109/13693780802635237. [DOI] [PubMed] [Google Scholar]
- 77.Nett J., Lincoln L., Marchillo K., Andes D. Beta-1,3 glucan as a test for central venous catheter biofilm infection. J. Infect. Dis. 2007;195:1705–1712. doi: 10.1086/517522. [DOI] [PubMed] [Google Scholar]
- 78.Seneviratne C.J., Wang Y., Jin L., Abiko Y., Samaranayake L.P. Proteomics of drug resistance in Candida glabrata biofilms. Proteomics. 2010;10:1444–1454. doi: 10.1002/pmic.200900611. [DOI] [PubMed] [Google Scholar]
- 79.Kuhn D.M., Chandra J., Mukherjee P.K., Ghannoum M.A. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect. Immun. 2002;70:878–888. doi: 10.1128/IAI.70.2.878-888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kucharikova S., Tournu H., Lagrou K., Van Dijck P., Bujdakova H. Detailed comparison of Candida albicans and Candida glabrata biofilms under different conditions and their susceptibility to caspofungin and anidulafungin. J. Med. Microbiol. 2011;60:1261–1269. doi: 10.1099/jmm.0.032037-0. [DOI] [PubMed] [Google Scholar]
- 81.Riera M., Mogensen E., d’Enfert C., Janbon G. New regulators of biofilm development in Candida glabrata. Res. Microbiol. 2012;163:297–307. doi: 10.1016/j.resmic.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 82.Juarez-Cepeda J., Orta-Zavalza E., Canas-Villamar I., Arreola-Gomez J., Perez-Cornejo G.P., Hernandez-Carballo C., Gutierrez-Escobedo G., Castaño I., De Las Peñas A. The EPA2 adhesin encoding gene is responsive to oxidative stress in the opportunistic fungal pathogen Candida glabrata. Curr. Genet. 2015;61:529–544. doi: 10.1007/s00294-015-0473-2. [DOI] [PubMed] [Google Scholar]
- 83.Enkler L., Richer D., Marchand A.L., Ferrandon D., Jossinet F. Genome engineering in the yeast pathogen Candida glabrata using the CRISPR-Cas9 system. Sci. Rep. 2016;6:35766. doi: 10.1038/srep35766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cen Y., Timmermans B., Souffriau B., Thevelein J.M., Van Dijck P. Comparison of genome engineering using the CRISPR-Cas9 system in C. glabrata wild-type and lig4 strains. Fungal Genet. Biol. 2017;107:44–50. doi: 10.1016/j.fgb.2017.08.004. [DOI] [PubMed] [Google Scholar]