Abstract
Background: According to recent studies, reactive oxygen is the leader of human metabolic disease development. The use of natural antioxidants is the best way to stop or prevent this problem. Therefore, the aim of this study was to evaluate the antioxidant and anti-inflammatory activities and to determine the polyphenolic contents of the Bidens engleri and Boerhavia erecta fractions. Methods: Plant fractions were obtained using Soxhlet procedures with hexane, dichloromethane, acetonitrile, ethyl acetate, methanol, and butanol solvent, successively. The different fractions were compared according to their antioxidant, anti-inflammatory activities, total phenolic, and total flavonoid contents. The phenolic contribution to the biological activity was evaluated. Result: The Bidens engleri and Boerhavia erecta fractions showed the highest antioxidant abilities, notably the polar fractions, which inhibited significantly the radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-O-azinobis(3-ethylbenzoline-6-sulphonate) (ABTS). The butanol fraction from Bidens engleri and methanol fraction from Boerhavia erecta have presented the best iron (III) reduction power with 211.68 and 198.55 mgAAE/g, respectively. Butanol and acetonitrile were the best solvents for extracting phenolic compounds from Bidens engleri and Boerhavia erecta, respectively. In contrast, dichloromethane was the best solvent for extracting a flavonoid from two plants with anti-COX-2 and anti-LOX-15 active compounds. The phenolic compound contributed significantly to antioxidant activity (r > 0.80). Conclusion: The Bidens engleri and Boerhavia erecta fractions possessed a potential antioxidant for fighting oxidative stress and helping to prevent diabetes, hypertension, and cardiovascular diseases. The uses of this plant could be promoted in Burkina Faso.
Keywords: antioxidants, cyclooxygenase, lipoxygenase, phenolic, flavonoid, traditional medicine
1. Introduction
Oxidative stress is an inevitable consequence of life in an oxygen-rich atmosphere. The environment is filled with a lot of reactive oxygen species (ROS) and reactive nitrogen species (RNS). In recent data, it was demonstrated that ROS and RNS played an important role in human disease development [1]. In Burkina Faso, some recent studies have shown some alarming data concerning the prevalence of metabolic diseases [2]. The oxidative stress plays a direct or indirect role in the pathophysiology of diseases, such as cancer, diabetes, and cardiovascular diseases [3,4]. However, the intake of natural antioxidants has been reported to reduce the risk of cancer, cardiovascular diseases, diabetes, and other diseases that are associated with aging [5,6].
Plants, fruits, vegetables, and medicinal herbs possess a wide variety of free radical scavenging biomolecules, such as phenolic compounds, flavonoids, vitamins, terpenoids, and some other endogenous phytometabolites, which are rich in antioxidant capacity [7,8,9]. Bidens engleri and Boerhavia erecta were some well-known medicinal plants in the Central Plateau, because they were used in the treatment of diabetes mellitus, hypertension, and old wounds [10]. According to Nacoulma’s investigations, the traditional healers and herbalists used Bidens engleri and Boerhavia erecta in combination, for treating diabetes in Burkina Faso [10]. The same information was found in Cote d’Ivoire by ethnobotanical investigations [11].
The previous data demonstrated the antioxidant and anti-diabetic activities of Boerhavia erecta in India were associated with some polyphenolic compounds, such as phenolics and flavonoids [12,13]. Some antioxidant compounds, such as (+)-catechin (−)-epicatechin, quercetin, isorhamnetin, rutin, narcissin, isoquercitrin, and isorhamnetin 3-O-β-d-glucopyranoside, as well as other metabolites, were isolated from B. erecta leaves extract [14,15]. According to the medicinal importance of B. engleri and B. erecta in Burkina Faso, the present study aimed to highlight the potential of this plant by determining the antioxidant and anti-inflammatory activities, and the polyphenolic content of six organic fractions for identifying the type of metabolites that were responsible for biological activity.
2. Materials and Methods
2.1. Plant Material
Whole plants of Bidens engleri and Boerhavia erecta were taken from the Gampela region, which was situated in the mid-east of Kadiogo (central region), during the rainy season (August–September 2012). The sample was dried in the laboratory under ventilation. The sample was certified by Professor Jeanne MILLOGO, a botanist from the Laboratory of Plant Biology and Ecology (University of Ouagadougou). The herbaria were saved in the University Herbarium with numbers MC_501 and MC_502 for Bidens engleri and Boerhavia erecta, respectively.
2.2. Reagents and Solvents
The Folin–Ciocalteu reagent, sodium phosphate mono- and di-basics, sodium tetraborate, potassium persulfate, aluminum trichloride, trolox, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-O-azinobis(3-ethylbenzoline-6-sulphonate) (ABTS), gallic acid, and trichloro acetic acid (TCA) were purchased from Sigma-Aldrich (Berlin, Germany). The sodium carbonate, potassium hexacyanoferrate, ascorbic acid, and ferric chloride were from Prolabo (Paris, France). The colorimetric COX (ovine) inhibitor screening assay kit, 15-lipoxygenase (soybean P1), linoleic, and arachidonic acids were purchased from Sigma-Aldrich, (New York, NY, USA).
2.3. Extraction Procedures
Of the sample, 20 g were successively extracted using hexane, dichloromethane, acetonitrile, ethyl acetate, methanol, and butanol in a Soxhlet system. The solvent was removed in a rotary evaporator system.
2.4. Antioxidant Effects Evaluation
2.4.1. Radical DPPH Inhibition Determination
The fractions’ capacities to inhibit radical DPPH were evaluated according to the method that was presented by Compaoré et al. [16]. In a 96 micro-well plate, 200 µL of DPPH (20 mg/L) and 100 µL of fraction were incubated in the dark for 10 min, and the absorbencies were read at 517 nm using a spectrophotometer (BioTek Instruments, New York, NY, USA). Quercetin was used to generate a standard curve (y = −27.94 + 8.15, r2 = 0.99, p < 0.0001). The results were expressed in milligram Quercetin equivalent per gram (mgQE/g).
2.4.2. Trolox Equivalent Antioxidant Capacity Assay
The method that was described by Compaoré et al. was used to evaluate the sample scavenging ABTS ability [16]. To 200 μL of diluted ABTS solution, 50 μL of fraction or trolox was added, with incubation in the dark for 5 min. The absorbance was read at 734 nm, with a microplate reader (BioTek Instruments, New York, NY, USA). Trolox was used to generate the standard curve (y = −72.38x + 54.57, r2 = 0.99, p < 0.001) and the results were expressed in millimole Trolox equivalent per gram (mMTE/g).
2.4.3. Ferric (Fe III) Reducing Antioxidant Power (FRAP) Assay
The reducing power of the extracts was determined according to the method that was presented by Compaoré et al. [16]. The data were transformed to mg of ascorbic acid per gram of fraction (mgAAE/g), because the standard curve was obtained with ascorbic acid (y = 105.9x, r2 = 0.99, p < 0.0001). The iron (III) reducing activity of each sample was obtained from two of the three independent determinations.
2.5. Anti-Inflammatory Tests
2.5.1. COX-1 and COX-2 Inhibition Assay
The inhibition of COXs was performed using a commercially available colorimetric COX (ovine) inhibitor screening assay kit (Cayman Chemical Company, New York, NY, USA). All of the inhibitors were dissolved in an appropriate solvent. The COX activity was evaluated using N,N,N’N’-tetramethyl-p-phenylenediamine (TMPD) as a co-substrate, with arachidonic acid. The TMPD oxidation was monitored spectrophotometrically at 590 nm (BioTek Instruments, New York, NY, USA). The inhibition percentage that was induced by 100 μg/mL of the sample was calculated.
2.5.2. Lipoxygenase 15 Inhibition Assay
The assay was performed according to the previous procedure that was presented by Compaoré et al. [17]. The incubation mixture consisted of the sample solution (100 μg/mL) in an appropriate solvent and 200 μL of the enzyme solution (167 U/mL) in a boric acid buffer (0.2 M, pH 9). After the incubation at room temperature for 5 min, the reaction was started by adding 250 μL of linoleic acid solution (250 mM in buffer). The conversion of linoleic acid to 13-hydroperoxylinoleic acid was recorded by measuring the samples’ absorbencies at 234 nm, during 3 min, and against the appropriate blank solutions, without extracts. The inhibition percentage was calculated.
2.6. Polyphenolic Amount Quantification
2.6.1. Phenolic Content Determination
The total phenolic content was evaluated using a Folin–Ciocalteu colometric assay, as described by Compaoré et al. [16]. The sample was mixed with Folin-Ciocalteu Reagent (0.2N). After incubation in the dark, 100 µL of sodium carbonate was added. The absorbance (760 nm) was measured after a second incubation in the dark (2 h), using the Biotek equipment (BioTek Instruments, New York, NY, USA). Gallic acid was used to produce the standard curve (y = 201x − 21.22, r2 = 0.99, p < 0.0001) and the results were expressed in mg gallic acid, equivalent per gram (mgGAE/g) of extract.
2.6.2. Total Flavonoid Content Evaluation
The total flavonoid content was determined according to the previous method that was described by Compaoré et al. [16]. Then, 100 μm of sample and 100 μL of AlCl3 (2%) were mixed in 96 micro-wells and were incubated for 10 min. The absorbance was measured at 415 nm with a microplate reader (BioTek Instruments, New York, NY, USA). Quercetin was used to generate the standard curve (y = 39.8x − 3.5, r2 = 0.99, p < 0.0001) and the results were expressed at mg quercetin equivalent per gram (mgQE/g) of sample.
2.7. Statistical Analyses
Microsoft Excel was used to calculate the average and standard deviation of the repeated tests (n = 2 × 3). GraphPad Prism 6.01 (San Diego, CA, USA, 2012) and Xlstat Pro 7.5 (Paris, France, 2005) were used to produce the standard curve and to measure the statistical significant results, respectively (p < 5%).
3. Results and Discussion
3.1. Antioxidant Activities
The use of medicinal plants in Burkina Faso has been a current activity of the population [18]. However, the main role of the researchers was to promote the medicinal uses. The plant extracts’ antioxidant potential is shown in Table 1. The radical DPPH scavenging effect was decreased from 63.94 mgQE/g to 2.25 mgQE/g, and the radical ABTS scavenging power was decreased from 22.86 mMTE/g to 7.16 mMTE/g. The hexane fractions presented radical ABTS scavenging activities contrary to the anti-DPPH radical effect. The butanol fraction from Bidens engleri demonstrated the best antiradical possibility, similar to the acetonitrile from Boerhavia erecta. The ability of the fractions to reduce iron (III) were increased from 10.20 mgAAE/g to 211.68 mgAAE/g. In general, the B. erecta sample presented some antioxidant activity that was superior to the B. engleri samples. This data demonstrated the importance of these plant samples in stress oxidative management. In the previous data, it was demonstrated that B. erecta possessed some antioxidant activity that was supported by the flavonoid compounds [14,19]. The anti-DPPH, anti-ABTS, and iron (III) reduction abilities were evaluated [13,20]. However, it was the first antioxidant activity data from Bidens engleri, according to our bibliographic survey. According to previous antioxidant activities of similar fractions from Commifora africana (A. Rich.) Engl. (Burseraceae) and Loeseneriella africana (Willd.) (Celastraceae), which were from the same region, the present plants possessed a lowest antioxidant power [16].
Table 1.
Yield and antioxidant activity of fractions.
| Fractions | Yield * (mg/g) | DPPH (mgQE/g) | ABTS (mMTE/g) | FRAP (mgAAE/g) | |
|---|---|---|---|---|---|
| B. engleri | Hexane fraction | 15.94 | Non-active | 10.06 ± 1.33 ef | 10.20 ± 0.61 j |
| Dichloromethane fraction | 12.11 | 2.25 ± 0.9 f | 8.67 ± 2.06 ef | 29.36 ± 0.75 hi | |
| Acetonitrile fraction | 19.17 | 17.88 ± 0.91 e | 11.42 ± 1.12 def | 64.34 ± 0.92 g | |
| Ethyl acetate fraction | 3.98 | 26.74 ± 1.84 d | 11.88 ± 0.91 cde | 74.61 ± 0.73 f | |
| Methanol fraction | 137.84 | 37.67 ± 0.88 c | 15.43 ± 1.04 bcd | 97.14 ± 3.55 e | |
| Butanol fraction | 1.23 | 51.88 ± 1.52 b | 17.10 ± 0.54 bcd | 211.68 ± 3.11 a | |
| B. erecta | Hexane fraction | 13.87 | Non-active | 7.16 ± 1.40 f | 24.56 ± 1.54 i |
| Dichloromethane fraction | 6.03 | 5.80 ± 0.18 f | 9.11 ± 0.96 ef | 37.02 ± 2.40 h | |
| Acetonitrile fraction | 20.79 | 64.14 ± 0.67 a | 22.86 ± 1.30 a | 174.16 ± 4.88 c | |
| Ethyl acetate fraction | 3.07 | 55.28 ± 3.46 b | 16.21 ± 1.75 bc | 126.65 ± 2.44 d | |
| Methanol fraction | 141.94 | 63.94 ± 0.78 a | 21.57 ± 1.82 a | 198.55 ± 4.54 b | |
| Butanol fraction | 1.67 | 41.40 ± 0.90 c | 14.86 ± 2.45 bcd | 92.02 ± 3.69 e |
Data in each column were statistically different letter (a–j) (p < 0.05) except data with same letters. The data were obtained in two independent triplate tests (n = 2 × 3). Aterisk (*) indicated data that were obtained by one procedure extraction. DPPH: 2,2-diphenyl-1-picrylhydrazyl, ABTS—2,2-O-azinobis(3-ethylbenzoline-6-sulphonate); FRAP—ferric (Fe III) reducing antioxidant power. mgQE/g: milligram quercetin equivalent per gram, mMTE/g: millimole Trolox equivalent per gram, mgAAE/g: milligram ascorbic acid equivalent per gram.
3.2. Anti-Inflammatory Activity
Table 2 presents the data concerning the inhibition of prostaglandin production from COXs and LOX-15 regular activities. COX-2 was more sensitive than COX-1 and LOX-15, which were not sensitive to the B. erecta fractions. The percentage inhibition of COX-2 was from 23.65% to 64.72% at 100 µg/mL, as the final concentration of the fraction. The LOX-15 inhibition percentage was increased from 36.76 to 64.90%. The maximal inhibition of COX-1 was obtained with ethyl acetate (42.51%) from B. engleri. Interestingly, the dichloromethane fractions from B. engleri and B. erecta were the active fractions for COX-2 (64.72 ± 2.13%) and LOX-15 (62.55 ± 5.09%), respectively, according to the enzyme activity classification scale [21]. According to the previous data, B. erecta possessed some anti-inflammatory activity in vivo [22], but in the present study, the B. erecta fractions could not significantly inhibit the prostaglandin production from the COXs and LOX-15 activity. It was suggested that the enzyme inhibition was not the method of action of this anti-inflammatory effect. This was the first study of the evaluation of the COXs and LOX-15 inhibition power of two plants. These enzyme inhibition activities of the B. engleri fractions were very little compared with the Commifora africana and Loeseneriella africana fractions inhibition effect [16]. In contrast, the butanol and dichloromethane fractions from B. engleri presented some interesting inhibition activity of LOX-15, compared with the Bauhinia rufescens extract, Lam. (Caesalpiniaceae) [17].
Table 2.
Anti-inflammatory activities of fractions.
| Fractions | COX-2 (%Inhibition) | COX-1 (%Inhibition) | LOX-15 (%Inhibition) | |
|---|---|---|---|---|
| Bidens engleri | Hexane fraction | 42.74 ± 6.23 b | Non active | Non active |
| Dichloromethane fraction | 64.72 ± 2.13 a | Non active | 62.55 ± 5.09 a | |
| Acetonitrile fraction | 31.17 ± 11.02 bc | 8.90 ± 1.15 c | 36.76 ± 3.59 b | |
| Ethyl acetate fraction | 37.39 ± 5.67 bc | 42.52 ± 0.90 a | 64.90 ± 4.78 a | |
| Methanol fraction | 32.31 ± 4.84 bc | 1.46 ± 0.46 d | 57.35 ± 0.01 a | |
| Butanol fraction | 37.87 ± 2.41 bc | 31.35 ± 2.30 b | 60.83 ± 0.80 a | |
| Boerhavia erecta | Hexane fraction | 23.65 ± 2.55 c | Non active | Non active |
| Dichloromethane fraction | 36.05 ± 2.19 bc | Non active | Non active | |
| Acetonitrile fraction | 31.10 ± 3.54 bc | Non active | Non active | |
| Ethyl acetate fraction | 26.44 ± 3.29 c | Non active | Non active | |
| Methanol fraction | 32.41 ± 3.08 bc | Non active | Non active | |
| Butanol fraction | 31.97 ± 6.07 bc | Non active | Non active |
Data in each column were statistically different letter (p < 0.05) except data with same letters (a–j). The data were obtained in triplate tests (n = 3).
3.3. Total Phenolic and Total Flavonoid Contents
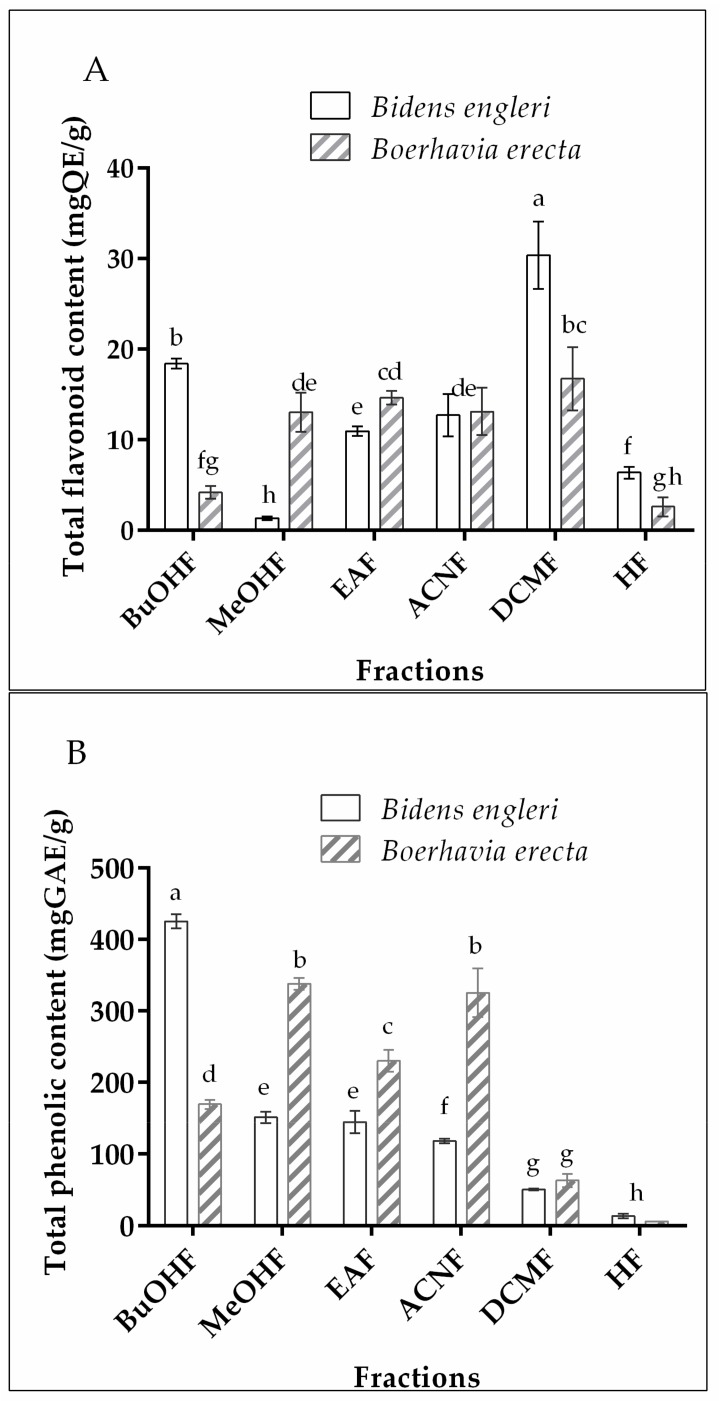
As the metabolites were the main contributor to the antioxidant and anti-inflammatory powers, the phenolic and flavonoid contents were evaluated [16,23]. The yield of extraction is shown in Table 1. The methanol was the best solvent for extracting some metabolites from two plants, with a yield that was superior to 100 mg/g. Figure 1 shows the amount of flavonoid and phenolic in all of the fractions from B. erecta and B. engleri. The phenolic content was decreased from 425.12 to 5.92 mgGAE/g, and the flavonoid amount was increased from 2.62 to 30.38 mgQE/g. A notable variable distribution of polyphenolic compounds was found in concordance with the solvent polarities. Notably, B. engleri contained some non-polar flavonoids in the major compound that were extracted in dichloromethane, in contrast to B. erecta, which presented some polar compounds that were extractible by acetonitrile, ethyl acetate, and methanol. In previous phytochemical investigations, the flavonoid and phenolic contents were evaluated in the extracts from B. erecta. It was found that the ethanol and phosphate buffer were able to extract the flavonoid and phenolic compounds [13,14]. The flavonoid and phenolic individual compounds, with a radical scavenging activity and iron (III) reduction ability, were previously detected in the B. erecta extracts [24,25,26]. It was quercetin and isorhamnetin and their glycosides, rutin, narcissin, isoquercitrin, and isorhamnetin 3-O-β-d-glucopyranoside, as well as the two flavan-3-ols, [(+)-catechin] and [(−)-epicatechin], that are well known antioxidant phytometabolites [24,25,26]. These compounds showed anti-COX and anti-LOX properties [27,28].
Figure 1.
Polyphenolic content of fractions. A—total flavonoid contents; B—total phenolic contents. The data were obtained in two independent triplate tests (n = 2 × 3). The data in each histogram were statistically different (p < 0.05), except for the data with the same letters (a–h). BuOHF: butanol Fraction, MeOHF: methanol fraction, EAF: ethyl acetate fraction, ACNF: acetonitrile fraction, DCMF: dichloromethane fraction, HF: hexane fraction, mgQE/g: milligram quercetin equivalent per gram, mgGAE milligram gallic acid equivalent per gram.
The correlation analysis showed that phenolic contributed significantly to the radical scavenging and iron (III) reduction. The contribution to the anti-DPPH, anti-ABTS, and iron (III) reduction were 0.91, 0.86, and 0.99, respectively (p < 0.0001). Similar findings were shown in a previous study [16,29,30]. Additionally, it was found in this study that there was an insignificant correlation between the COX-2 and phenolic compound, contrary to a previous study [16].
4. Conclusions
This study highlighted the antioxidant, anti-inflammatory, and the phytochemical potential of six fractions from B. engleri and B. erecta, well-known medicinal plants of Burkina Faso. Their utilization could be supported partially by antiradical scavenging and iron (III) reduction. According to the interesting biological activity of B. engleri, the next step would be to isolate the anti-radical flavonoid from butanol, ethyl acetate, and acetonitrile fractions, as well as the anti-COX-2 and anti-LOX-15 compounds from the dichloromethane in vitro model.
Acknowledgments
Supported by International Foundation for Sciences (grant No. AF/20286). We also thanked Professor Jeanne MILLOGO for the botanical authentication of two plants.
Author Contributions
M.C. corresponding author, sampling, conception of protocol, redaction of paper, and data analysis; R.N.T.M. conception of protocol, redaction, and correction; S.B. redaction and correction of the article; and O.G.N. laboratory headmaster, validation of plant list, and correction.
Funding
This research was funded by the International Foundation for Sciences grant number AF/20286
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Rajendran P., Nandakumar N., Rengarajan T., Palaniswami R., Gnanadhas E.N., Lakshminarasaiah U., Gopas J., Nishigaki I. Antioxidants and human diseases. Clin. Chim. Acta. 2014;436:332–347. doi: 10.1016/j.cca.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Sagna Y., Yanogo D.A.R., Tiéno H., Guira O., Bagbila A. Nutritional Disorders & Therapy Obesity and Metabolic Syndrome in a Burkina Faso Urban Area: Prevalence, Associated Factors and Comorbidities. J. Nutr. Disord. Ther. 2014;4:2–7. doi: 10.4172/2161-0509.1000141. [DOI] [Google Scholar]
- 3.Marie D., Ateba G., Felicité K.D., Fernando K.L., Chia M., Henry L.N. Oxidative Stress in Patients with Chronic Inflammatory Diseases in a Tertiary Health Care Setting in Africa. J. Autoimmun. Disord. 2017;3:47. [Google Scholar]
- 4.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganjifrockwala F.A., Joseph J.T., George G. Decreased total antioxidant levels and increased oxidative stress in South African type 2 diabetes mellitus patients Decreased total antioxidant levels and increased oxidative stress in South African type 2 diabetes mellitus patients. J. Endocrinol. Metab. Diabetes S. Afr. 2017;22:21–25. doi: 10.1080/16089677.2017.1324590. [DOI] [Google Scholar]
- 6.Cosme F., Pinto T., Vilela A. Phenolic Compounds and Antioxidant Activity in Grape Juices: A Chemical and Sensory View. Beverages. 2018;4:22. doi: 10.3390/beverages4010022. [DOI] [Google Scholar]
- 7.Gullo G., Dattola A., Liguori G., Vonella V. Evaluation of fruit quality and antioxidant activity of kiwifruit during ripening and after storage. J. Berry Res. 2016;6:25–35. doi: 10.3233/JBR-150111. [DOI] [Google Scholar]
- 8.Pistollato F., Battino M., Cliniche S., Biochimica S. Role of plant-based diets in the prevention and regression of metabolic syndrome and neurodegenerative diseases. Trends Food Sci. Technol. 2014;40:62–81. doi: 10.1016/j.tifs.2014.07.012. [DOI] [Google Scholar]
- 9.Zhang Y., Gan R., Li S., Zhou Y., Li A., Xu D. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules. 2015;20:21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nacoulma O.G. Ph.D. Thesis. University Ouagadougou; Ouagadougou, Burkina Faso: 1996. Plantes Médicinales et Pratiques Médicales Traditionnelles au Burkina Faso: Cas du Plateau Central; p. 303. [Google Scholar]
- 11.Konkon N.G., Ouatara D., Kpan W.B., Kouakou T.H. Medicinal plants used for treatment of diabetes by traditional practitioners in the markets of Abidjan district in Côte d’Ivoire. J. Med. Plants Stud. 2017;5:39–48. [Google Scholar]
- 12.Nisha M., Vinod B.N., Sunil C. Evaluation of Boerhavia erecta L. for potential antidiabetic and antihyperlipidemic activities in streptozotocin-induced diabetic Wistar rats. Futur. J. Pharm. Sci. 2018 doi: 10.1016/j.fjps.2017.12.001. in press. [DOI] [Google Scholar]
- 13.Govindan P., Muthukrishnan S. Evaluation of total phenolic content and free radical scavenging activity of Boerhavia erecta. J. Acute Med. 2013;3:103–109. doi: 10.1016/j.jacme.2013.06.003. [DOI] [Google Scholar]
- 14.Petrus A.J.A., Hemalatha S.S., Suguna G. Isolation and Characterisation of the Antioxidant Phenolic Metabolites of Boerhaavia erecta L. leaves. J. Pharm. Sci. Res. 2012;4:1856–1861. [Google Scholar]
- 15.Nugraha A.S., Hilou A., Vandegraaff N., David I., Haritakun R., Keller P.A. Bioactive glycosides from the African medicinal plant Boerhavia erecta L. Nat. Prod. Res. 2015;29:37–41. doi: 10.1080/14786419.2015.1013470. [DOI] [PubMed] [Google Scholar]
- 16.Compaoré M., Meda R.N.-T., Bakasso S., Vlase L., Kiendrebeogo M. Antioxidative, anti-inflammatory potentials and phytochemical profile of Commiphora africana (A. Rich.) Engl. (Burseraceae) and Loeseneriella africana (Willd.) (Celastraceae) stem leaves extracts. Asian Pac. J. Trop. Biomed. 2016;6:665–670. doi: 10.1016/j.apjtb.2016.06.001. [DOI] [Google Scholar]
- 17.Compaoré M., Lamien C.E., Vlase L., Kiendrébéogo M., Ionescu C., Nacoulma O. Antioxidant, xanthine oxidase and lipoxygenase inhibitory activities and phenolics of Bauhinia rufescens Lam. (Caesalpiniaceae) Nat. Prod. Res. 2012;26:1069–1074. doi: 10.1080/14786419.2011.559948. [DOI] [PubMed] [Google Scholar]
- 18.Zizka A., Thiombiano A., Dressler S., Nacoulma B.M., Ouédraogo A., Ouédraogo I., Ouédraogo O., Zizka G., Hahn K., Schmidt M. Traditional plant use in Burkina Faso (West Africa): A national-scale analysis with focus on traditional medicine. J. Ethnobiol. Ethnomed. 2015;11:11–19. doi: 10.1186/1746-4269-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajeswari P., Krishnakumari S. Boerhaavia erecta—A potential source for phytochemicals and antioxidants. J. Pharm. Sci. Res. 2010;2:728–733. [Google Scholar]
- 20.Shareef M.I., Gopinath S.M., Gupta A., Gupta S. Antioxidant and Anticancer Study of Boerhavia erecta. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:879–885. [Google Scholar]
- 21.Fawole O.A., Amoo S.O., Ndhlala A.R., Light M.E., Finnie J.F., Van Staden J. Anti-inflammatory, anticholinesterase, antioxidant and phytochemical properties of medicinal plants used for pain-related ailments in South Africa. J. Ethnopharmacol. 2010;127:235–241. doi: 10.1016/j.jep.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Muthumani P., Meera R., Devi P., Arabath S.A., Jeyasundari K., Babmanaban R. Phytochemical investigation, diuretic and anti-inflammatory activity of root and stem extracts of Boerhaavia erecta Linn in experimental animals. Int. J. Appl. Biol. Pharm. Technol. 2010;1:1285–1292. doi: 10.21276/Ijabpt. [DOI] [Google Scholar]
- 23.Yi Y., Sun J., Xie J., Min T., Wang L.-M., Wang H.-X. Phenolic Profiles and Antioxidant Activity of Lotus Root Varieties. Molecules. 2016;21:863. doi: 10.3390/molecules21070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seyoum A., Asres K., El-Fiky F.K. Structure–radical scavenging activity relationships of flavonoids. Phytochemistry. 2006;67:2058–2070. doi: 10.1016/j.phytochem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Cai Y., Sun M., Xing J., Luo Q., Corke H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006;78:2872–2888. doi: 10.1016/j.lfs.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Bubols B.G., da Rocha Vianna D., Medina-Remón A., von Poser G., Maria Lamuela-Raventos R., Lucia Eifler-Lima V., Cristina Garcia S. The Antioxidant Activity of Coumarins and Flavonoids. Mini-Rev. Med. Chem. 2013;13:318–334. doi: 10.2174/1389557511313030002. [DOI] [PubMed] [Google Scholar]
- 27.Sadik C.D., Sies H., Schewe T. Inhibition of 15-lipoxygenases by flavonoids: Structure-activity relations and mode of action. Biochem. Pharmacol. 2003;65:773–781. doi: 10.1016/S0006-2952(02)01621-0. [DOI] [PubMed] [Google Scholar]
- 28.Aravindaram K., Yang N. Anti-Inflammatory Plant Natural Products for Cancer Therapy. Planta Med. 2010;76:1103–1117. doi: 10.1055/s-0030-1249859. [DOI] [PubMed] [Google Scholar]
- 29.Agregán R., Munekata P.E.S., Franco D., Carballo J., Barba F.J., Lorenzo J.M. Antioxidant Potential of Extracts Obtained from Macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and Micro-Algae (Chlorella vulgaris and Spirulina platensis) Assisted by Ultrasound. Medecines. 2018;5:33. doi: 10.3390/medicines5020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamien-Meda A., Lamien C.E., Compaoré M.M.Y., Meda R.N., Kiendrebeogo M., Zeba B., Millogo J.F., Nacoulma O.G. Polyphenol Content and Antioxidant Activity of Fourteen Wild Edible Fruits from Burkina Faso. Molecules. 2008;13:581–594. doi: 10.3390/molecules13030581. [DOI] [PMC free article] [PubMed] [Google Scholar]