Abstract
An iron porphyrin catalyst, derived from the active center of proteins such as horseradish peroxidase and hemoglobin, was successfully used for the atom transfer radical polymerizations (ATRP) of methacrylic acid. ATRP of methacrylic acid and other acidic monomers is challenging due to Cu complexation by carboxylates, protonation of the ligand, and displacement of the halogen chain end. A robust mesohemin-based catalyst provided controlled ATRP of methacrylic acid, yielding poly(methacrylic acid) with Mn ≥ 20000 and dispersity Đ < 1.5. Retention of halogen chain end was confirmed by successful chain extension of a poly-(methacrylic acid)–Br macroinitiator.
Graphical abstract
Atom transfer radical polymerization (ATRP) is one of the most widely used reversible deactivation radical polymerization (RDRP) techniques that provide well-defined polymers with predetermined molecular weight, low dispersity, and precisely controlled architecture.1
ATRP can be carried out in organic solvents or in water under environmentally benign conditions. Moreover, water enables faster reactions,2 direct polymerization of water-soluble monomers, and grafting from biomolecules.3 Recently, various methods for Cu-based ATRP in aqueous media were developed, for a range of monomers, such as (meth)acrylates and (meth)acrylamides.4
Aqueous ATRP has been traditionally considered challenging due to several potential side reactions (Scheme 1). Most of them, however, can be prevented by adjusting experimental conditions. For example, dissociation of weak X–Cu(II) bonds in the deactivator is suppressed by adding an excess of halide salts (e.g., NaCl). Hydrolysis of the initiator or dormant chain-end is moderated when using C–Cl instead of C–Br initiators. Disproportionation of Cu(I) is slower at low catalyst concentration, which can also be achieved by regenerating the active Cu(I) catalyst by chemical, electrochemical, photo-chemical, or piezoelectric reduction.5 ATRP equilibrium constant, KATRP, is very large in water, which leads to high radical concentration. Radical concentration can be regulated by slow and continuous (re)generation of a small amount of active Cu(I) catalyst. On the other hand, high KATRP is beneficial because it results in fast polymerizations.6 In summary, aqueous ATRP has become well understood, and is now an established technique to prepare hydrophilic polymers.
Scheme 1.
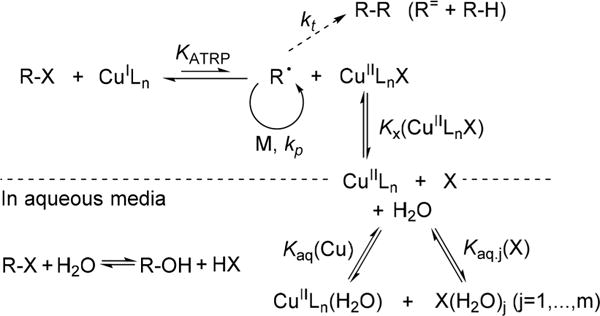
Mechanism of ATRP and Possible Side Reactions in Aqueous Media
Acidic monomers are still difficult to polymerize by ATRP. These monomers pose challenges such as protonation of ligands at low pH and displacement of Cu–Br and C–Br bonds by carboxylates.7 However, controlled ATRP of monomers containing carboxylic acid groups, such as methacrylic acid (MAA), is highly desired because it can pave the way to the development of new materials with unique complexing ability, pH responsiveness, and biocompatibility.
Direct controlled polymerization of MAA can be performed via RAFT8 or NMP.9 Alternative methods to synthesize well controlled poly(methacrylic acid) (PMAA) are ATRP of sodium methacrylate utilizing >10000 ppm of Cu versus monomer10 or ATRP of t-butyl methacrylate in organic solvents followed by deprotection and purification.11 Recently, homopolymerization of MAA was reported via electrochemically mediated ATRP and SARA ATRP under strong acidic conditions. High conversion (>90%) was reached in 4 h with experimental Mn values matching theoretical ones and with adequate control over dispersity (Đ < 1.5).12 Polymerization required pH = 0.9 and Cl-based initiators and catalysts. These harsh acidic conditions were needed to protonate all carboxylates to acidic groups. This transformation reduced the extent of an intramolecular side reaction: the penultimate carboxylate unit could displace the C–X end functionality, forming an inactive lactone (Scheme 2). The lactone was plausibly formed via a SN2-like intramolecular substitution at the chain-end in the presence of CuI species.12 This side reaction was so prominent that when ATRP of MAA was catalyzed by CuII/tris(2-pyridylmethyl)amine (TPMA) at pH 2.2, instead of 0.9, the reaction stopped at 7% conversion after complete loss of C–Br chain ends.
Scheme 2.
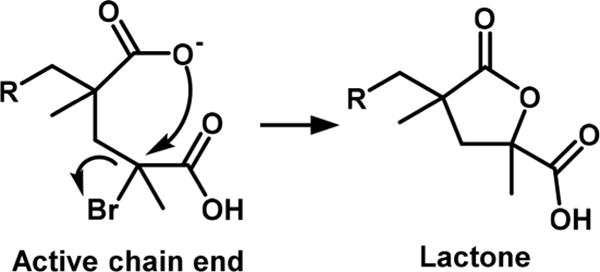
PMAA Chain-End Cyclization in the Presence of Cu Complexes
The use of iron complexes can diminish this undesired lactonization observed in the presence of Cu complexes. Fe porphyrins are good candidates for the ATRP of acidic monomers because they tolerate large pH changes.13 In this work, we present a Fe porphyrin catalyst that can polymerize MAA to high conversion by avoiding the chain-end lactonization and without requiring any pH adjustment to strongly acidic conditions.
Previously, porphyrin-based catalysts were used for the successful ATRP of neutral monomers.14 Hemin was modified with two methoxy poly(ethylene glycol) (MPEG550, Mn = 550) chains to enhance water solubility. Additionally, the vinyl groups of hemin were hydrogenated by Pd/C to prevent incorporation (copolymerization) of the catalyst into the polymer chains. The resulting mesohemin-(MPEG550)2 (MH-MPEG2) was used to polymerize oligo(ethylene oxide) methyl ether methacrylate (OEOMA475, Mn = 475) under benign aqueous conditions, generating polymers with well-defined molecular weight and low dispersity (Đ < 1.2) via activators regenerated by electron transfer (ARGET) ATRP. In this work, this mesohemin derivative was utilized as a catalyst for the direct polymerization of MAA under mild conditions via ARGET ATRP.
Under strongly acidic conditions, ATRP copper catalysts dissociate due to protonation of alkyl amine-based ligands.15 Some catalysts, such as CuII/TPMA, do not dissociate at low pH, but lose catalytic activity. Cyclic voltammetry (CV) in water showed that the redox potential of Br–CuIITPMA+ shifted by +0.12 V when the pH was changed from ∼7 to 2.2 by addition of 10 vol % MAA in water, indicating that the catalyst was much less reducing at low pH (Figure 1). This shift was correlated to a decrease in KATRP of 2 orders of magnitude.12 In contrast, the redox potential of the mesohemin catalyst was much less affected by pH. When the pH was changed from ∼7 to 2.2, the half-wave potential became only 0.01 V more positive, indicating that the mesohemin catalyst was unaffected by pH due to the high stability of the iron-porphyrin moiety.16
Figure 1.
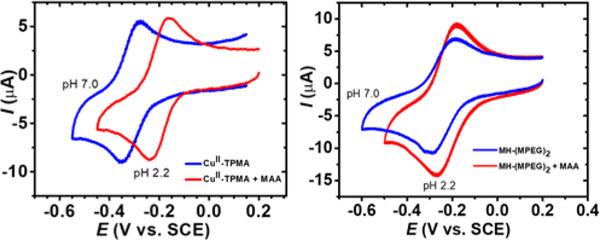
CV of 1 × 10−3 M Br–CuIITPMA+ (left) and 2 × 10−3 M MH-MPEG2 (right) in water and in 10% MAA in water. Supporting electrolyte = 0.1 M NaBr, scan rate = 0.1 V s−1, T = 25 °C.
The effect of pH was investigated in the polymerization of MAA with 500 ppm mesohemin-(MPEG550)2 as catalyst and α-bromoisobutyric acid (BiBA) as initiator (Scheme 3). An excess of bromide anions was added in the form of 0.1 M NaBr to provide a sufficient concentration of FeIII–Br deactivator (Table S1). Addition of 20% MAA to H2O/DMF 80/20 (v/v) established a pH = 2.7. Injection of ascorbic acid (AAc) as reducing agent triggered polymerization by reducing FeIII to FeII (Table 1, entry 1). The FeII catalyst could start the polymerization, but poor control of Mn and Đ were observed after 11% monomer conversion in 4 h. Increasing the pH to 5.0 by addition of NaOH solution gave no polymer (Table 1, entry 2). At this pH, MAA (pKa = 4.6) is present mostly as sodium carboxylate, which can either give lactonization of the chain end or bind to the iron center, deactivating the catalyst active site. Consequently, pH was decreased to 0.9 by addition of a small amount of HBr (Table 1, entry 3). A 25% conversion was obtained, which indicated that reactivity of the catalyst was maintained at low pH, as suggested by the CV measurements. Decreasing pH, however, did not improve the control of Fe-catalyzed polymerization of MAA, which gave high dispersity (Mw/Mn = 1.90) and low initiation efficiency (Ieff = Mn,app/Mn,th = 0.05). In comparison, decreasing pH drastically improved control in the presence of Cu catalyst, due to conversion of carboxylates to acids and consequent diminished chain-end lactonization. This suggested that lactonization of chain end might not be a critical side reaction in Fe-catalyzed ATRP of MAA. Diminished lactonization in the presence of mesohemin catalyst was also confirmed by 1H NMR of the obtained polymer (Figure S4).
Scheme 3.
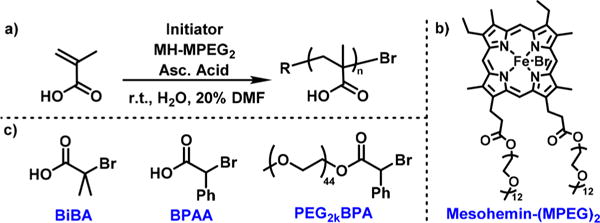
ATRP of MAA in Aqueous Solution (a) with Mesohemin-(MPEG550)2 Catalyst (b), and Structure of Polymerization Initiators (c)
Table 1.
Effect of pH on ATRP of MAA Initiated by BiBAa
| entry | pH | conv. (%) | Mn,th (×10−3) | Mn,app (×10−3) | Ieff | Đ |
|---|---|---|---|---|---|---|
| 1 | 2.7 | 11.1 | 2.1 | 12.6 | 0.20 | 1.71 |
| 2 | 5.0 | 0 | ||||
| 3 | 0.9 | 25.1 | 4.5 | 96.2 | 0.05 | 1.90 |
[M] = 20 vol % in water/DMF = 80/20, M/RBr/AAc/Cat. = 200/1/1/0.1, [RBr] = 12 mM, reaction time = 4 h, T = 30 °C; Mn,app measured by THF GPC using universal PMMA standards after methylation of reaction samples.
In order to increase Ieff, a more active initiator was selected, α-bromophenylacetic acid (BPAA). The more efficient initiator significantly increased both Ieff and polymerization rate (Table 2, entry 1). Conversion reached 71% within 4 h, which was approximately 7 times faster than the reaction with BiBA initiator under the same conditions. When the concentration of NaBr was doubled, Đ and reaction rate decreased, but the Mn,app was still twice larger than the theoretical value.
Table 2.
ATRP of MAA Initiated by α-Bromophenylacetic Acid (BPAA) and PEG2kBPAa
| entry | M/RBr/AAc/cat | initiator | added salt | conv. % | time (h) | Mn,th (×10−3) | Mn,app (×10−3) | Ieff | Mw/Mn |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 200:1:1:0.1 | BPAA | NaBr 0.1 M | 71 | 4 | 12.4 | 20.3 | 0.61 | 1.58 |
| 2 | 200:1:1:0.1 | BPAA | NaBr 0.2 M | 42 | 4 | 7.4 | 14.4 | 0.51 | 1.49 |
| 3 | 200:1:1:0.1 | BPAA | NaCl 0.1 M | 81 | 4 | 14.1 | 44.1 | 0.32 | 1.93 |
| 4 | 200:1:1:0.1 | BPAA | NaCl 0.2 M | 74 | 4 | 13.0 | 55.1 | 0.24 | 1.89 |
| 5 | 200:1:1:0.2 | BPAA | NaBr 0.2 M | 31 | 4 | 5.6 | 7.8 | 0.72 | 1.44 |
| 6 | 200:1:1:0.1 | PEG2kBPA | NaBr 0.1 M | 61 | 4 | 12.7 | 19.6 | 0.65 | 1.58 |
| 7 | 200:1:1:0.1 | PEG2kBPA | NaBr 0.2 M | 39 | 4 | 8.9 | 10.3 | 0.86 | 1.55 |
| 8b | 500:1:0.5:0.5 | PEG2kBPA | NaBr 0.1 M | 48 | 7 | 22.9 | 20.7 | 1.11 | 1.47 |
[M] = 20 vol % for entry 1–4, 10 vol % for entry 5–8, in water/DMF = 80/20, [RBr] = 12 mM, T = 30 °C; Mn,app measured by THF GPC using universal PMMA standards after methylation of reaction samples.
AAc was fed in the reaction mixture in 6 h.
Switching to NaCl instead of NaBr generated a C–Cl chain end after the first activation/deactivation cycles. However, the Mn,app was higher and the Đ increased (Table 2, entries 3 and 4). Increasing catalyst loading from 500 to 1000 ppm in the presence of 200 mM NaBr gave better control. The Mn,app was much closer to the theoretical value while the dispersity decreased to 1.44 (Table 2, entry 5).
The macroinitiator poly(ethylene glycol)2000 methyl ether α-bromophenylacetate (PEG2kBPA) was also used to form block copolymers. Due to the presence of the same initiating moiety as BPAA, the overall polymerization rate was similar, with well controlled Mn and Mw/Mn (Table 2, entry 6). Similarly, increasing NaBr or catalyst concentration resulted in improved control over molecular weight, while Đ decreased to 1.47 (Table 2, entries 7–8). The polymerization displayed linear semilogarithmic plot of monomer consumption (Figure 2), while MWs increased linearly with conversion.
Figure 2.
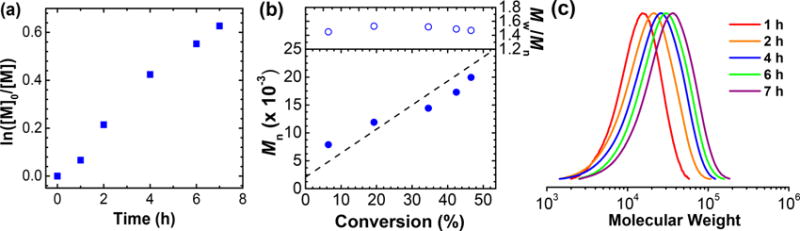
First-order kinetic plot (a), evolution of MW and D with conversion (b), GPC traces (c), for polymerization in Table 2, entry 8.
A chain extension experiment from PMAA was also performed. A sample of PEG2k-b-PMAA–Br (Mn = 22400) was synthesized (conditions as in entry 8, Table 2) and purified by dialysis in methanol using 5 kDa molar mass cut-off membrane (Figure 3). The PEG2k-b-PMAA-Br polymer was then used as a macroinitiator for ATRP of another water-soluble monomer, poly(ethylene oxide) methyl ether methacrylate (OEOMA500). The shift of GPC traces demonstrated retention of chain-end functionality. The MW increased from Mn = 22400 to 84600 with a small tailing, to indicate formation of a triblock copolymer PEG-b-PMAA-b-POEOMA500 (Scheme S2).
Figure 3.
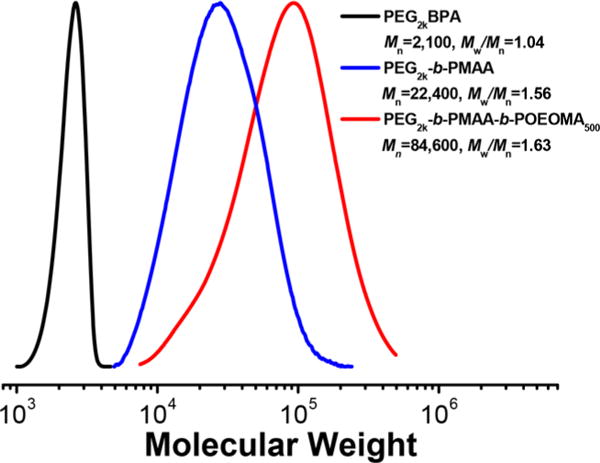
GPC traces of macroinitiator PEG2kBPA, PEG2k-b-PMAA, and triblock copolymer PEG-b-PMAA-b-POEOMA500.
In conclusion, a direct controlled radical polymerization of methacrylic acid in the presence of mesohemin catalyst is reported. The mesohemin catalyst had some advantages over a traditional copper catalyst (i.e., Cu/TPMA). Addition of an excess of halide ions had a similar effect of slowing down the polymerization and improving control by increasing the concentration of FeIII–Br (or CuII–Br) deactivators. In contrast to Cu/L, decreasing pH only marginally influenced the robust mesohemin catalyst. Lactonization of PMAA–Br chain end was the major side reaction preventing polymerization of MAA in the presence of CuI/L, but the same reaction had much lower contribution in the presence of mesohemin catalyst. The relatively high dispersity with Fe-based catalyst is likely due to a slower activation/deactivation than for Cu catalysts. Also, while BiBA was a good initiator for copper catalysts in water, it was a less efficient initiator for the Fe-based catalyst. This suggests that penultimate-unit effect is stronger for Fe-based catalyst.17
With the mesohemin catalyst and active initiators such as BPAA and PEG2kBPA, the molecular weights of the obtained poly(methacrylic acid) agreed with theoretical values and displayed low dispersity. The synthesis of a PEG-b-PMAA-b-POEOMA500 block copolymer by chain-extension confirmed good retention of chain-end functionality. The use of this bioinspired hemin derivative catalyst under biocompatible conditions indicates that acidic polymers can be successfully synthesized and potentially could be used for grafting from proteins or other biologically active molecules.
Supplementary Material
Acknowledgments
The financial support from NSF (CHE 1707490) and NIH (R01DE020843) is gratefully acknowledged. Helpful discussions with Dr. Sangwoo Park and Sipei Li are appreciated.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmacrolett.7b00909.
Experimental details and supporting figures, tables, and schemes (PDF).
ORCID
Yi Wang: 0000-0002-4002-9516
Krzysztof Matyjaszewski: 0000-0003-1960-3402
Notes
The authors declare no competing financial interest.
References
- 1.(a) Matyjaszewski K, Xia J. Atom Transfer Radical Polymerization. Chem Rev. 2001;101:2921–2990. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]; (b) Matyjaszewski K, Tsarevsky NV. Nanostructured functional materials prepared by atom transfer radical polymerization. Nat Chem. 2009;1(4):276–288. doi: 10.1038/nchem.257. [DOI] [PubMed] [Google Scholar]; (c) Matyjaszewski K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules. 2012;45(10):4015–4039. [Google Scholar]; (d) Kamigaito M, Ando T, Sawamoto M. Metal-catalyzed living radical polymerization. Chem Rev. 2001;101(12):3689–3745. doi: 10.1021/cr9901182. [DOI] [PubMed] [Google Scholar]
- 2.Krys P, Fantin M, Mendonça PV, Abreu CMR, Guliashvili T, Rosa J, Santos LO, Serra AC, Matyjaszewski K, Coelho JFJ. Mechanism of supplemental activator and reducing agent atom transfer radical polymerization mediated by inorganic sulfites: experimental measurements and kinetic simulations. Polym Chem. 2017;8(42):6506–6519. doi: 10.1039/c7py01319a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Wang XS, Armes SP. Facile Atom Transfer Radical Polymerization of Methoxy-Capped Oligo(ethylene glycol) Methacrylate in Aqueous Media at Ambient Temperature. Macromolecules. 2000;33:6640–6647. [Google Scholar]; (b) Nicolas J, Guillaneuf Y, Lefay C, Bertin D, Gigmes D, Charleux B. Nitroxide-mediated polymerization. Prog Polym Sci. 2013;38(1):63–235. [Google Scholar]; (c) Azuma Y, Terashima T, Sawamoto M. Precision Synthesis of Imine-Functionalized Reversible Microgel Star Polymers via Dynamic Covalent Cross-Linking of Hydrogen-Bonding Block Copolymer Micelles. Macromolecules. 2017;50(2):587–596. [Google Scholar]; (d) Bai J, Yin H, Zhang Y, Zhang C, Liu L, Xu X. A chiral polymer as chromo-fluorescence and CD response sensor for specific recognition of fluoride. Eur Polym J. 2017;87:380–388. [Google Scholar]; (e) Peeler JC, Woodman BF, Averick S, Miyake-Stoner SJ, Stokes AL, Hess KR, Matyjaszewski K, Mehl RA. Genetically Encoded Initiator for Polymer Growth from Proteins. J Am Chem Soc. 2010;132:13575–13577. doi: 10.1021/ja104493d. [DOI] [PubMed] [Google Scholar]; (f) Averick S, Mehl RA, Das SR, Matyjaszewski K. Well-defined biohybrids using reversible-deactivation radical polymerization procedures. J Controlled Release. 2015;205:45–57. doi: 10.1016/j.jconrel.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 4.(a) Konkolewicz D, Magenau AJD, Averick SE, Simakova A, He H, Matyjaszewski K. ICAR ATRP with ppm Cu Catalyst in Water. Macromolecules. 2012;45(11):4461–4468. [Google Scholar]; (b) Simakova A, Averick SE, Konkolewicz D, Matyjaszewski K. Aqueous ARGET ATRP. Macromolecules. 2012;45(16):6371–6379. [Google Scholar]; (c) Zhang Q, Wilson P, Li Z, McHale R, Godfrey J, Anastasaki A, Waldron C, Haddleton David M. Aqueous Copper-Mediated Living Polymerization: Exploiting Rapid Disproportionation of CuBr with Me6TREN. J Am Chem Soc. 2013;135(19):7355–63. doi: 10.1021/ja4026402. [DOI] [PubMed] [Google Scholar]; (d) Wever DAZ, Raffa P, Picchioni F, Broekhuis AA. Acrylamide Homopolymers and Acrylamide-N-Isopropylacrylamide Block Copolymers by Atomic Transfer Radical Polymerization in Water. Macromolecules. 2012;45(10):4040–4045. [Google Scholar]; (e) Teodorescu M, Matyjaszewski K. Atom transfer radical polymerization of (meth)acrylamides. Macromolecules. 1999;32(15):4826–4831. [Google Scholar]
- 5.(a) Min K, Matyjaszewski K. Atom transfer radical polymerization in microemulsion. Macromolecules. 2005;38(20):8131–8134. [Google Scholar]; (b) Jakubowski W, Min K, Matyjaszewski K. Activators Regenerated by Electron Transfer for Atom Transfer Radical Polymerization of Styrene. Macromolecules. 2006;39(1):39–45. doi: 10.1002/anie.200600272. [DOI] [PubMed] [Google Scholar]; (c) Matyjaszewski K, Jakubowski W, Min K, Tang W, Huang J, Braunecker WA, Tsarevsky NV. Diminishing catalyst concentration in atom transfer radical polymerization with reducing agents. Proc Natl Acad Sci U S A. 2006;103(42):15309–15314. doi: 10.1073/pnas.0602675103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Bortolamei N, Isse AA, Magenau AJD, Gennaro A, Matyjaszewski K. Controlled Aqueous Atom Transfer Radical Polymerization with Electrochemical Generation of the Active Catalyst. Angew Chem Int Ed. 2011;50(48):11391–11394. doi: 10.1002/anie.201105317. [DOI] [PubMed] [Google Scholar]; (e) Pan X, Malhotra N, Simakova A, Wang Z, Konkolewicz D, Matyjaszewski K. Photoinduced Atom Transfer Radical Polymerization with ppm-Level Cu Catalyst by Visible Light in Aqueous Media. J Am Chem Soc. 2015;137(49):15430–15433. doi: 10.1021/jacs.5b11599. [DOI] [PubMed] [Google Scholar]; (f) Wang Z, Pan X, Yan J, Dadashi-Silab S, Xie G, Zhang J, Wang Z, Xia H, Matyjaszewski K. Temporal Control in Mechanically Controlled Atom Transfer Radical Polymerization Using Low ppm of Cu Catalyst. ACS Macro Lett. 2017;6(5):546–549. doi: 10.1021/acsmacrolett.7b00152. [DOI] [PubMed] [Google Scholar]
- 6.(a) Tsarevsky NV, Pintauer T, Matyjaszewski K. Deactivation efficiency and degree of control over polymerization in ATRP in protic solvents. Macromolecules. 2004;37:9768–9778. [Google Scholar]; (b) Bortolamei N, Isse AA, Magenau AJD, Gennaro A, Matyjaszewski K. Controlled Aqueous Atom Transfer Radical Polymerization with Electrochemical Generation of the Active Catalyst. Angew Chem Int Ed. 2011;50:1–5. doi: 10.1002/anie.201105317. [DOI] [PubMed] [Google Scholar]; (c) Billing M, Festag G, Bellstedt P, Schacher FH. Amphiphilic and double hydrophilic block copolymers containing a polydehydroalanine block. Polym Chem. 2017;8(5):936–945. [Google Scholar]
- 7.(a) Mori H, Mueller AHE. New polymeric architectures with (meth)acrylic acid segments. Prog Polym Sci. 2003;28(10):1403–1439. [Google Scholar]; (b) Fantin M, Isse AA, Gennaro A, Matyjaszewski K. Understanding the Fundamentals of Aqueous ATRP and Defining Conditions for Better Control. Macromolecules. 2015;48(19):6862–6875. [Google Scholar]
- 8.Chaduc I, Lansalot M, D’Agosto F, Charleux B. RAFT Polymerization of Methacrylic Acid in Water. Macromolecules. 2012;45(3):1241–1247. [Google Scholar]
- 9.Couvreur L, Lefay C, Belleney J, Charleux B, Guerret O, Magnet S. First Nitroxide-Mediated Controlled Free-Radical Polymerization of Acrylic Acid. Macromolecules. 2003;36(22):8260–8267. [Google Scholar]
- 10.Ashford EJ, Naldi V, O’Dell R, Billingham NC, Armes SP. First example of the atom transfer radical polymerisation of an acidic monomer: direct synthesis of methacrylic acid copolymers in aqueous media. Chem Commun. 1999;14:1285–1286. [Google Scholar]
- 11.Burguiere C, Pascual S, Bui C, Vairon JP, Charleux B, Davis KA, Matyjaszewski K, Betremieux I. Block Copolymers of Poly(styrene) and Poly(acrylic acid) of Various Molar Masses, Topologies, and Compositions Prepared via Controlled/Living Radical Polymerization. Application as Stabilizers in Emulsion Polymerization. Macromolecules. 2001;34(13):4439–4450. [Google Scholar]
- 12.Fantin M, Isse AA, Venzo A, Gennaro A, Matyjaszewski K. Atom Transfer Radical Polymerization of Methacrylic Acid: A Won Challenge. J Am Chem Soc. 2016;138(23):7216–7219. doi: 10.1021/jacs.6b01935. [DOI] [PubMed] [Google Scholar]
- 13.Ding H, Zhong M, Wu H, Park S, Mohin JW, Klosterman L, Yang Z, Yang H, Matyjaszewski K, Bettinger CJ. Elastomeric Conducting Polyaniline Formed Through Topological Control of Molecular Templates. ACS Nano. 2016;10(6):5991–5998. doi: 10.1021/acsnano.6b01520. [DOI] [PubMed] [Google Scholar]
- 14.Simakova A, Mackenzie M, Averick SE, Park S, Matyjaszewski K. Bioinspired Iron-Based Catalyst for Atom Transfer Radical Polymerization. Angew Chem Int Ed. 2013;52(46):12148–12151. doi: 10.1002/anie.201306337. [DOI] [PubMed] [Google Scholar]
- 15.Fantin M, Isse AA, Gennaro A, Matyjaszewski K. Understanding the Fundamentals of Aqueous ATRP and Defining Conditions for Better Control. Macromolecules. 2015;48(19):6862–6875. [Google Scholar]
- 16.Khabibullin A, Kopec M, Matyjaszewski K. Modification of Silica Nanoparticles with Miktoarm Polymer Brushes via ATRP. J Inorg Organomet Polym Mater. 2016;26(6):1292–1300. [Google Scholar]
- 17.(a) Nanda AK, Matyjaszewski K. Effect of penultimate unit on the activation process in ATRP. Macromolecules. 2003;36(22):8222–8224. [Google Scholar]; (b) Lin CY, Coote ML, Petit A, Richard P, Poli R, Matyjaszewski K. Ab initio study of the penultimate effect for the ATRP activation step using propylene, methyl acrylate, and methyl methacrylate monomers. Macromolecules. 2007;40(16):5985–5994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


