Non-Structured Abstract
There have been many advances in the treatment of heart failure over the past several years. While these advancements have resulted in improved outcomes in adults with heart failure, these same treatments do not seem to be as efficacious in children with heart failure. Investigations of the failing pediatric heart suggest that there are unique phenotypic, pathologic and molecular differences that could influence how children with heart failure response to adult-based therapies. In this review, several recent studies and the potential implications of their findings on informing the future of the management of pediatric heart failure are discussed.
Keywords: Heart Failure, dilated cardiomyopathy, children
Introduction
Pediatric heart failure is rare but devastating diagnosis affecting approximately 1 in 100,000 children worldwide. The causes of heart failure in children are heterogenous ranging from primary cardiomyopathies to congenital heart defects. Unfortunately, there is very little evidence to support the optimal treatment of pediatric heart failure and therefore treatment recommendations are primarily extrapolated from the results of adult clinical trials. The purpose of this review is to summarize recent pre-clinical and clinical data demonstrating some of the unique attributes of the pediatric heart failure population, including outcomes, response to therapy and age-related differences in mechanisms of disease.
Outcomes in pediatric heart failure
Heart failure is a leading cause of death in the United States in adults. Many studies are devoted to improving our understanding of signaling mechanisms in adult heart failure. This concentrated research effort has resulted in the development of a wide range of evidence-based medical and device therapies which have significantly improved outcomes in the adult heart failure population [1].
While pediatric heart failure is far less prevalent, the outcome remains very poor, with a 5 year rate of death or transplant of 40–60% [2, 3]. Due to the rarity of pediatric heart failure, the challenges in performing invasive clinical studies in infants and children, and the heterogenous nature of the disease, there are very few clinical trials and dedicated research studies focused on this patient population. As a result, contemporary treatment of pediatric patients with heart failure is mostly extrapolated from the adult heart failure treatment guidelines [4, 5]. However, this approach may not be working as well as anticipated given that pediatric heart failure patients have not demonstrated the same improvement in outcomes as adult patients [1–3, 6, 7]. There is an evolving body of literature suggesting that differences in cellular and molecular signaling between failing pediatric and adult hearts could be playing an important role in the contrasting outcomes in these two patient populations. Improved understanding regarding the mechanisms underlying differential therapeutic response in pediatric heart failure patients could result in the identification of novel targeted therapies specific to this vulnerable population.
Age-specific responses to heart failure therapies
The Pediatric Carvedilol Treatment Trial, a landmark study in the field of pediatric heart failure was published in 2007 and remains the only multicenter, randomized, placebo-controlled clinical trial in pediatric heart failure to date [7, 8]. In this study, the effects of carvedilol, a non-selective β- and α1-blocker, was studied in a population of children with symptomatic systolic heart failure. While carvedilol was well tolerated by the enrolled children, there were no significant differences between placebo or carvedilol in the combined measures selected to indicate improved, unchanged, or worsened heart failure [8]. These findings were in direct contrast to the marked improvements in survival and cardiovascular related hospitalizations demonstrated in adult heart failure patients treated with carvedilol [9, 10]. However, there were limitations in the pediatric carvedilol study which challenged the ability to interpret the results; first, the primary end point was a composite clinical end point which included subjective components and may have been influenced by many factors, including achievement of developmental milestones as the children aged during the course of the study; second, children with varied etiologies of heart failure including those with single ventricle physiology, were included in the study; and third, the dosage of carvedilol might not have been sufficient as the trough levels of the drug at the doses used for the study were lower in children compared to adults. As a result of the limitations in the Pediatric Carvedilol Treatment Trial, the issue of whether carvedilol, or β-blockers in general, are efficacious in pediatric heart failure remains unresolved. In a post hoc analysis of the carvedilol trial subjects, Petko et al determined that echocardiogram parameters including systolic and diastolic sphericity index and TEI index in pediatric patients treated with carvedilol were improved when compared to placebo treated patients [11]. This analysis suggests that carvedilol might influence left ventricular reverse remodeling in pediatric heart failure patients, but it remains unclear why this was not associated with an improvement in outcome.
Another example of age-specific differences in response to heart failure therapies involves phosphodiesterase-3 inhibition (eg milrinone or enoximone). While treatment with phosphodiesterase-3 inhibitors has demonstrated acute beneficial hemodynamic alterations in adult heart failure patients [12–15]; chronic treatment is not well tolerated [16, 17]. Specifically, chronic treatment with phosphodiesterase-3 inhibitors in adults with heart failure leads to an increased incidence of arrhythmias and sudden death and is therefore not a recommended therapy [12, 18, 19]. In contrast, pediatric heart failure patients treated with phosphodiesterase-3 inhibitors demonstrate marked symptom improvement and no increase in the incidence of arrhythmias or sudden death [20, 21]. Children with heart failure listed for transplant are frequently sent home on milrinone as a bridge to heart transplant, with many tolerating several months of treatment without complication [22].
These differential clinical responses to medications such as carvedilol and phosphodiesterase-3 inhibitors suggest the possibility that adaptation of the pediatric and adult failing heart differs and that distinct cellular mechanisms may be involved.
Assessing age-specific cellular mechanism involved in HF
Because pediatric heart failure outcomes lag behind those of adults despite a similar therapeutic approach there is a need to identify targeted therapies for this special population. The first step in the identification and development of directed therapies is to enhance the currently limited understand of the underlying mechanisms and cellular signaling pathways that may be uniquely altered in the pediatric failing heart.
Outlined below are several recent examples of studies demonstrating potentially important differences in the adult and pediatric failing heart. These studies, while not comprehensive, represent a pre-clinical framework for ongoing pediatric focused research in this arena.
1. Investigations of the cardiac β-adrenergic and phosphodiesterase-3 systems
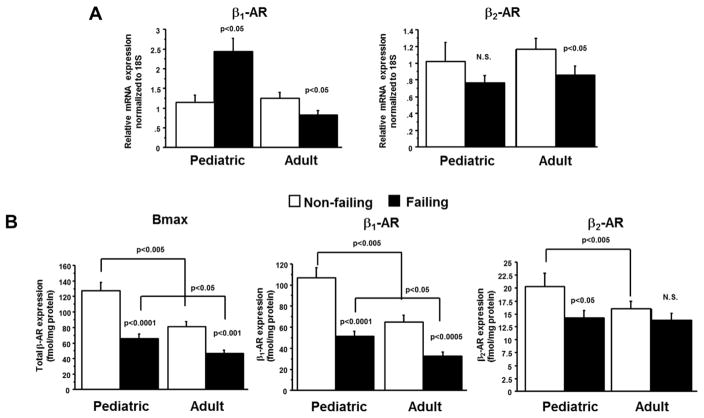
Explanted hearts from children and adults with idiopathic dilated cardiomyopathy demonstrate differences in distribution of β-adrenergic receptors [23]. Pediatric dilated cardiomyopathy patients have a unique pattern of β-adrenergic receptor adaptation compared to their adult counterparts. While both populations of patients demonstrated a decrease in total myocardial β-adrenergic receptor expression, in adult hearts this decrease was attributed entirely to downregulation of the β1-receptors, while children had downregulation of both the β1- and β2-receptors (Figure 1)[23]. While chronic β1-receptor activation in heart failure results in pathologic gene expression changes, dephosphorylation of calcium-handling proteins and ventricular remodeling, the β2-receptor seems to be mostly beneficial mediating a pro-survival, anti-apoptotic pathway [24, 25]. The resulting hypothesis from these human tissue studies was that perhaps some preservation of the β2-receptor is beneficial and that non-selective β-blockade with drugs such as carvedilol could be less efficacious in children due to their already down-regulated β2-receptor state.
Figure 1.
Down regulation of β1 and β2-adrenergic receptor levels in pediatric heart failure patients. A. mRNA levels of β1 and β2-adrenergic receptor demonstrate age-specific expression in heart failure patients. p-values correspond to failing-to-non-failing comparisons unless otherwise noted. B. β-Adrenergic receptor levels were determined by [ 125I]-iodocyanopindolol binding. AR = adrenergic receptor; F = failing; NF = non- failing; Bmax = β-Adrenergic receptor. Figure from Miyamoto et al (2014) [23].
In order to test this hypothesis, a young mouse model of acute β-adrenergic stimulation was developed [26]. Young mice treated with the β-adrenergic agonist isoproterenol demonstrated a decrease in both β1- and β2-adrenergic receptors, similar to the pediatric failing heart. When these mice were treated concomitantly with carvedilol (a non-selective β-blocker which blocks both β1- and β2-receptors), there was no attenuation of hypertrophy (as measured by heart weight to body weight ratio). However, when the young mice exposed to isoproterenol were treated with β1-specific blockers (eg metoprolol), the mice had an encouraging response with a decrease in heart weight/body weight ratio. Alternatively, adult aged mice demonstrated an improvement in isoproterenol-induced cardiac hypertrophy in response to treatment with both non-selective and β1-selective blockade. The results from the human tissue data and this mouse model suggest that perhaps differences in β2-receptor adaptation may contribute to different responses to medical therapies in children compared to adults [23, 26].
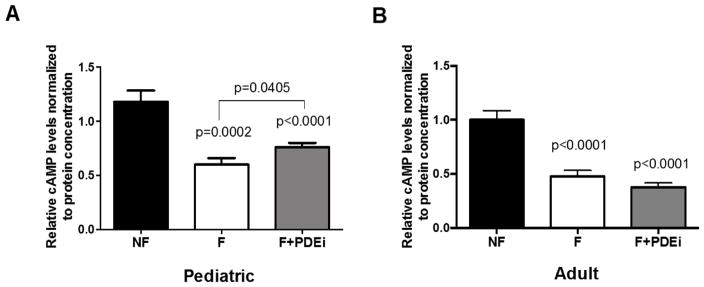
Using heart tissue from children and adults with idiopathic dilated cardiomyopathy, Nakano et al showed that children treated with the phosphodiesterase-3 inhibitor, milrinone, had an increase in myocardial cAMP levels, while adult cAMP levels remained low and were unchanged by phosphodiesterase-3 inhibitor therapy (Figure 2) [27]. Pediatric patients treated with milrinone also had an increase in phosphorylation of phospholamban (calcium handling protein), which was not demonstrated with phosphodiesterase-3 inhibitor therapy in adults [27]. In addition, expression of specific isoforms of adenylyl cyclases and phosphodiesterases differed between adults and children [28].
Figure 2.
cAMP levels in left ventricular adult and pediatric myocardium (quantitated by ELISA). A. Relative cAMP levels in pediatric myocardium. B. Relative cAMP levels in adult myocardium. p-values correspond to comparisons with non-failing unless otherwise noted in the figure. NF = non-failing; F = failing; FT = failing treated with PDE3i; cAMP = cyclic adenosine monophosphate. Figure from Nakano et al (2015) [27].
These studies demonstrate that the failing pediatric and adult heart differ in their adaptive responses to heart failure. While the findings are associative and not mechanistic, they support the importance of pre-clinical investigations focused on the pediatric heart failure population.
2. Fibrosis and cardiomyocyte hypertrophy is less prominent in pediatric failing hearts
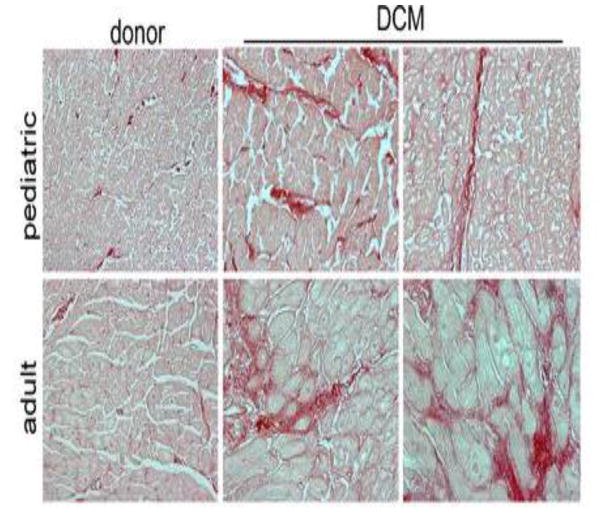
Certain hallmarks of pathologic responses in cardiomyocytes have been characterized in both pediatric and adult heart failure patients (such as upregulation of the fetal gene program [23], and increased circulation of catecholamines [29]). Similarly, it has been assumed that aspects of cardiac remodeling such as fibrosis and cardiomyocyte hypertrophy, nearly universal findings in adult heart failure patients, are also present in the failing pediatric heart. However, histologic analyses of patient samples in conjunction with molecular comparisons conclude that pediatric idiopathic dilated cardiomyopathy patients have less myocardial fibrosis than adult patients (Figure 3) [30, 31]. In these studies, there was no relationship between the development of fibrosis and the duration of heart failure and fibrosis related gene expression was distinct between the 2 populations [31]. Assessment of cardiomyocyte cell size in children with dilated cardiomyopathy also demonstrated a surprising lack of hypertrophy compared to age-matched non-failing controls [30, 32]. Importantly, many heart failure therapies target these markers of adverse remodeling. The implications of these human tissue findings on the clinical response to medications such as those targeting the renin-angiotensin-aldosterone system are unclear.
Figure 3.
Absence of myocardial fibrosis in pediatric DCM. Picrosirius red staining (red) demonstrating that, compared with donor controls, pediatric DCM specimens display minimal interstitial or perivascular fibrosis. In contrast, adult DCM patients display increased interstitial and perivascular fibrosis compared with donor controls. Magnification 200×. Figure from Patel et al (2017) [30].
3. Transcriptomic analysis of pediatric hearts with heart failure compared to pediatric non-failing hearts
Identifying the factors that govern differences in the mechanisms of heart failure between pediatric and adult patients is critically important, but is also extremely challenging. Recent publications describing transcriptome-based analysis of hearts from age-matched non-failing adult and pediatric controls and dilated cardiomyopathy subjects are beginning to address this question [30, 32]. The pathways dysregulated in the pediatric heart are distinct from those regulated in the adult failing heart. The pediatric failing heart is maintained in an undifferentiated state [32], while the adult heart shows enrichment of genes associated with activation of the innate immune system, fatty acid and oxidative metabolism and metabolite signaling [30].
Together, the above findings suggest that pediatric dilated cardiomyopathy may represent a distinct disease entity from adult dilated cardiomyopathy. This premise challenges the current pediatric heart failure treatment paradigm which is based primarily on extrapolation of adult guidelines and supports the concept that investigations of pediatric-specific therapies are needed.
Challenges and Future Directions
In the past 10 years, a growing body of literature has enhanced our understanding of the unique nature of pediatric heart failure. However, there is still a long way to go and there are several challenges that limit the progress of pediatric heart failure research endeavors. First, and most importantly, children are a vulnerable population so invasive and interventional studies are difficult to perform. Pediatric heart failure is rare making it nearly impossible to conduct well-designed and appropriately powered clinical trials. Disease etiology is diverse and includes not only cardiomyopathies, but also congenital and other acquired forms of heart failure. Finally, there are very few animal models that recapitulate the phenotypes seen in pediatric heart failure patients. This lack of appropriate animal models is particularly problematic for the study of heart failure secondary to congenital heart disease. Therefore, innovative disease model development [32, 33] and performance of comparative effectiveness research, development of a body of evidence through the incorporation of results from pre-clinical investigations, observational registry based studies as well as retrospective and prospective clinical studies, is needed if outcomes for pediatric heart failure are to improve [34].
Highlights.
Pediatric patients with heart failure have not had the same improvement in outcomes over the past several years as adults with heart failure
Children with heart failure respond differently to heart failure medications and this may be due to differential adaptation of the failing pediatric heart
Pediatric and adult dilated cardiomyopathy are distinct diseases with the pediatric failing heart demonstrating differing pathology and gene expression changes compared to the failing adult heart
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cubbon RM, et al. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail. 2011;4(4):396–403. doi: 10.1161/CIRCHEARTFAILURE.110.959882. [DOI] [PubMed] [Google Scholar]
- 2.Kantor PF, et al. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol. 2010;55(13):1377–84. doi: 10.1016/j.jacc.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Towbin JA, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296(15):1867–76. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 4.Kantor PF, et al. Presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2013;29(12):1535–52. doi: 10.1016/j.cjca.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Kirk R, et al. The International Society for Heart and Lung Transplantation Guidelines for the management of pediatric heart failure: Executive summary. [Corrected] J Heart Lung Transplant. 2014;33(9):888–909. doi: 10.1016/j.healun.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Daubeney PE, et al. Clinical features and outcomes of childhood dilated cardiomyopathy: results from a national population-based study. Circulation. 2006;114(24):2671–8. doi: 10.1161/CIRCULATIONAHA.106.635128. [DOI] [PubMed] [Google Scholar]
- 7.Tsirka AE, et al. Improved outcomes of pediatric dilated cardiomyopathy with utilization of heart transplantation. J Am Coll Cardiol. 2004;44(2):391–7. doi: 10.1016/j.jacc.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Shaddy RE, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298(10):1171–9. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 9.Packer M, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334(21):1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 10.Poole-Wilson PA, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362(9377):7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 11.Petko C, et al. Echocardiographic evaluation of children with systemic ventricular dysfunction treated with carvedilol. Pediatr Cardiol. 2010;31(6):780–4. doi: 10.1007/s00246-010-9700-2. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JL. Hemodynamic and clinical benefits with intravenous milrinone in severe chronic heart failure: results of a multicenter study in the United States. Am Heart J. 1991;121(6 Pt 2):1956–64. doi: 10.1016/0002-8703(91)90832-3. [DOI] [PubMed] [Google Scholar]
- 13.Baim DS, et al. Evaluation of a new bipyridine inotropic agent--milrinone--in patients with severe congestive heart failure. N Engl J Med. 1983;309(13):748–56. doi: 10.1056/NEJM198309293091302. [DOI] [PubMed] [Google Scholar]
- 14.Grose R, et al. Systemic and coronary effects of intravenous milrinone and dobutamine in congestive heart failure. J Am Coll Cardiol. 1986;7(5):1107–13. doi: 10.1016/s0735-1097(86)80231-5. [DOI] [PubMed] [Google Scholar]
- 15.Narahara KA. Oral enoximone therapy in chronic heart failure: a placebo-controlled randomized trial. The Western Enoximone Study Group. Am Heart J. 1991;121(5):1471–9. doi: 10.1016/0002-8703(91)90154-a. [DOI] [PubMed] [Google Scholar]
- 16.Cuffe MS, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287(12):1541–7. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 17.Felker GM, et al. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol. 2003;41(6):997–1003. doi: 10.1016/s0735-1097(02)02968-6. [DOI] [PubMed] [Google Scholar]
- 18.Packer M, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991;325(21):1468–75. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 19.Seino Y, et al. Multicenter, double-blind study of intravenous milrinone for patients with acute heart failure in Japan. Japan Intravenous Milrinone Investigators. Crit Care Med. 1996;24(9):1490–7. doi: 10.1097/00003246-199609000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Berg AM, Snell L, Mahle WT. Home inotropic therapy in children. J Heart Lung Transplant. 2007;26(5):453–7. doi: 10.1016/j.healun.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Price JF, et al. Outpatient continuous parenteral inotropic therapy as bridge to transplantation in children with advanced heart failure. J Card Fail. 2006;12(2):139–43. doi: 10.1016/j.cardfail.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Birnbaum BF, et al. Intravenous home inotropic use is safe in pediatric patients awaiting transplantation. Circ Heart Fail. 2015;8(1):64–70. doi: 10.1161/CIRCHEARTFAILURE.114.001528. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto SD, et al. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J. 2014;35(1):33–41. doi: 10.1093/eurheartj/ehs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chesley A, et al. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3'-kinase. Circ Res. 2000;87(12):1172–9. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 25.Communal C, et al. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis : role of a pertussis toxin-sensitive G protein. Circulation. 1999;100(22):2210–2. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 26.Sucharov CC, et al. beta-Adrenergic receptor antagonism in mice: a model for pediatric heart disease. J Appl Physiol (1985) 2013;115(7):979–87. doi: 10.1152/japplphysiol.00627.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano SJ, et al. Age-related differences in phosphodiesterase activity and effects of chronic phosphodiesterase inhibition in idiopathic dilated cardiomyopathy. Circ Heart Fail. 2015;8(1):57–63. doi: 10.1161/CIRCHEARTFAILURE.114.001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano SJ, et al. Cardiac Adenylyl Cyclase and Phosphodiesterase Expression Profiles Vary by Age, Disease, and Chronic Phosphodiesterase Inhibitor Treatment. J Card Fail. 2017;23(1):72–80. doi: 10.1016/j.cardfail.2016.07.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venugopalan P, Agarwal AK. Plasma catecholamine levels parallel severity of heart failure and have prognostic value in children with dilated cardiomyopathy. Eur J Heart Fail. 2003;5(5):655–8. doi: 10.1016/s1388-9842(03)00109-0. [DOI] [PubMed] [Google Scholar]
- 30.Patel MD, et al. Pediatric and adult dilated cardiomyopathy represent distinct pathological entities. JCI Insight. 2017;2(14) doi: 10.1172/jci.insight.94382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woulfe KC, et al. Fibrosis and Fibrotic Gene Expression in Pediatric and Adult Patients With Idiopathic Dilated Cardiomyopathy. J Card Fail. 2017;23(4):314–324. doi: 10.1016/j.cardfail.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tatman PD, et al. Pediatric dilated cardiomyopathy hearts display a unique gene expression profile. JCI Insight. 2017;2(14) doi: 10.1172/jci.insight.94249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang X, et al. Exosomes from pediatric dilated cardiomyopathy patients modulate a pathological response in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2017;312(4):H818–H826. doi: 10.1152/ajpheart.00673.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tunis SR, Pearson SD. US moves to improve health decisions. BMJ. 2010;341:c3615. doi: 10.1136/bmj.c3615. [DOI] [PubMed] [Google Scholar]