Abstract
Background
A comprehensive assessment of cannabis use by patients with cancer has not previously been reported. In this study, we aimed to characterize patient perspectives about cannabis and its use.
Methods
An anonymous survey about cannabis use was offered to patients 18 years of age and older attending 2 comprehensive and 2 community cancer centres, comprising an entire provincial health care jurisdiction in Canada (ethics id: hreba-17011).
Results
Of 3138 surveys distributed, 2040 surveys were returned (65%), with 1987 being sufficiently complete for analysis (response rate: 63%). Of the respondents, 812 (41%) were less than 60 years of age; 45% identified as male, and 55% as female; and 44% had completed college or higher education.
Of respondents overall, 43% reported any lifetime cannabis use. That finding was independent of age, sex, education level, and cancer histology. Cannabis was acquired through friends (80%), regulated medical dispensaries (10%), and other means (6%). Of patients with any use, 81% had used dried leaves.
Of the 356 patients who reported cannabis use within the 6 months preceding the survey (18% of respondents with sufficiently complete surveys), 36% were new users. Their reasons for use included cancer-related pain (46%), nausea (34%), other cancer symptoms (31%), and non-cancer-related reasons (56%).
Conclusions
The survey demonstrated that prior cannabis use was widespread among patients with cancer (43%). One in eight respondents identified at least 1 cancer-related symptom for which they were using cannabis.
Keywords: Cannabis, marijuana, symptoms
INTRODUCTION
The frequency of cannabis use in cancer patient populations is not well-established. Uptake of its use or consensus about authorization practices in the medical community has been limited1–4. Anecdotally, cannabis is more commonly authorized for patients who have experience of previous use. It can be authorized for a variety of medical conditions3. Patients without authorization often acquire it by other means and use it either recreationally or for a variety of claimed medical benefits despite clinical trial data demonstrating efficacy or safety for smoked cannabis being limited5,6.
In response to patient demand, a growing number of Web sites have been devoted to the subject of medical cannabis (see, for example, http://phoenixtears.ca and http://www.medicalcannabis.com). Patients can access that information, but in pre-legalization environments might be hesitant to disclose use to their practitioners. Data about the use of cannabis in the general population are available, but information about use by oncology patients or the beliefs of oncology patients about cannabis are less well established6,7–10. The Canadian experience has yet to be described.
In the present study, we examined cannabis use in a North American multicentre outpatient cancer-centre population for whom possession for medical use is an exemption under the law. The survey explored the motivations of cannabis users for cannabis use, their willingness to discuss that use with their physicians, and general opinions about cannabis. The primary endpoint of the study was to determine the proportion of an unselected population of patients with cancer who would have consumed cannabis within 6 months of visiting a cancer centre.
METHODS
Survey Design
An anonymous survey was designed to solicit from patients their demographics, diagnosis, reason for cancer centre attendance, personal use of cannabis, opinions about cannabis, and comfort level discussing cannabis with their oncologists (supplemental Appendix a). The survey content was externally reviewed by the health care jurisdiction’s data integration, measurement, and reporting division to ensure that the questions were internally consistent. The survey cover letter and questions were then reviewed by 13 patient and family advisers through the involved centre’s patient advocacy program. Feedback was incorporated into the final questionnaire wording.
Data Collection
Patients eligible to receive a survey were those who were 18 years of age or older, who had a scheduled appointment at 1 of the 4 cancer centres in the province of Alberta, and who were checking in at a registration desk before their appointment. Those 4 centres administer 100% of the radiotherapy and 85% of the adult chemotherapy courses in the jurisdiction. Between 15 May and 19 May 2017, 2936 of 4784 patients with scheduled visits to 1 of 2 tertiary cancer centres serving rural and urban patient populations (centres 1 and 2) were approached to complete the survey by clerical staff at the time of registration. Then, between 17 July and 21 July 2017, 202 of 489 patients with scheduled visits to 1 of the 2 community cancer centres serving predominately rural patients (centres 3 and 4) in the jurisdiction were approached to complete the same survey. Use by the patients of family members as proxies to complete the survey was discouraged, but not prohibited. Completed surveys were returned by patients into confidential sealed boxes distributed at strategic locations throughout the cancer centres and were collected daily. Patients making multiple visits during the survey period were asked to complete the survey only once. For the duration of the study, the study authors did not directly contact patients, but were available to answer questions about the survey at patient or staff member request.
After the survey period ended, site-wide databases were interrogated to determine the number of patient visits during the study period at each centre and baseline demographic information for patients who had attended at least once. The surveys not distributed were then manually counted. The resulting information was used to determine response rates in a manner consistent with the principles espoused by the American Association for Public Opinion Research standards11.
Statistical Methods
All surveys were scanned into an electronic database for analysis. All text responses were manually verified, and 20% of the source data for multiple-choice responses was then randomly verified by independent reviewers for the accuracy of data entry. The overall response rate and response rates by treatment centre were calculated. Proportions were then calculated for survey responses based on the total number of surveys completed. In cases in which patients were asked to skip questions based on a previous response, the calculation was based on the actual number of respondents. Comparisons between group responses used the chi-square or Fisher exact test, as appropriate.
Logistic regression was used to determine the dependencies of lifetime cannabis use and cannabis use within the preceding 6 months by respondent factors. For the logistic regression analyses, surveys without responses to the questions about lifetime cannabis use or the time since last use were excluded as appropriate. For lifetime use, the independent variables included age, education level, sex, and cancer type as categorical ordinal variables. For use within the preceding 6 months, the independent variables included age groupings, education level (divided into high school or less, diploma or degree, and master’s degree or higher), sex, and cancer type as categorical ordinal variables; current use of chemotherapy or immunotherapy or targeted therapy, current use of hormonal therapy, current use of radiotherapy, and current or recently planned surgery were included as dichotomous categorical variables.
For ordinal regression, all surveys with missing data (n = 163) were excluded. In the included surveys, data were ranked as 1 (strongly agree or agree), 2 (unsure or don’t know), and 3 (disagree or strongly disagree). The independent variables examined were age groupings and highest achieved education level (high school or less, diploma or degree, and master’s degree or higher) as categorical ordinal variables, and sex and any lifetime use of cannabis as dichotomous ordinal variables. On ordinal regression modelling, only surveys with complete data were included. Two surveys in which sex was designated as “other” were excluded. All data were analyzed using the R programming language (version 3.1.3: The R Foundation, Vienna, Austria).
Ethics Considerations
Before survey distribution, the project was reviewed and approved by the Health Research Ethics Board of Alberta responsible for the 4 institutions (hreba–Cancer Committee id: 17011).
Role of the Funding Source
The project was supported in part by funds from the University of Calgary Department of Oncology and in part by research grant funding from Alberta Health Services. The funders had no participation in study design, data interpretation, or manuscript preparation. The corresponding author had full access to all data and final responsibility for the decision to submit for publication.
RESULTS
Response Rate
Table i outlines the demographics for all patients visiting the cancer centres at the time of survey administration and all respondents to the survey. Of 3138 surveys distributed, 2040 were returned (return rate: 65%), and 1987 were more than 50% complete (response rate: 63%). Response rates for centres 1, 2, 3, and 4 were 57%, 54%, 100%, and 100% respectively (p < 0.001 favouring rural centres). In general, the cohort of respondents appeared to be a representative sample of the patients with a planned cancer centre appointment during the study interval. Very elderly patients (>80 years) and patients with skin, gynecologic, and hematologic malignancies were either less likely to have been approached or to respond to the questionnaire (p < 0.001).
TABLE I.
Baseline characteristics of all patients with visits scheduled at the study centres and of patients who completed the cannabis questionnaire
| Characteristic | Patient group [n (%)] | Exposure | p Value | |
|---|---|---|---|---|
|
| ||||
| Visiting (n=5273) | Respondents (n=1987) | |||
| Age group | ||||
| <30 Years | 131 (2) | 47 (2) | 36 | <0.001a |
| 30–39 Years | 286 (5) | 102 (5) | 36 | |
| 40–49 Years | 556 (11) | 217 (11) | 39 | |
| 50–59 Years | 1139 (22) | 446 (22) | 39 | |
| 60–69 Years | 1591 (30) | 639 (32) | 40 | |
| 70–79 Years | 1149 (22) | 436 (22) | 38 | |
| ≥80 Years | 421 (8) | 84 (4) | 20 | |
| Unknown | 16 | |||
| Sex | ||||
| Men | 2328 (44) | 874 (45) | 38 | NS |
| Women | 2945 (56) | 1078 (55) | 37 | |
| Otherb | 0 (0) | 2 (0) | — | |
| Unknown | 33 | |||
| Primary cancer site | ||||
| Breast | 1107 (21) | 428 (22) | 39 | <0.001 |
| Genitourinary | 704 (13) | 286 (15) | 41 | |
| Gynecologic | 404 (8) | 129 (7) | 32 | |
| Skin | 99 (2) | 28 (1) | 28 | |
| Lung | 385 (7) | 171 (18) | 44 | |
| Gastrointestinal | 808 (15) | 345 (17) | 43 | |
| Hematologic | 962 (18) | 290 (15) | 30 | |
| Other | 804 (15) | 240 (13) | 30 | |
| Unknown | 70 | |||
| Completed education | ||||
| ≤High school | — | 1079 (55) | — | NA |
| Diploma or bachelor’s | — | 691 (35) | — | |
| ≥Master’s | — | 182 (9) | — | |
| Unknown | 5273 | 35 | ||
| Time from diagnosis | ||||
| <6 Months | 1531 (29) | 570 (29) | 37 | NS |
| ≥6 Months | 3630 (68) | 1369 (71) | 38 | |
| Unknown | 111 | 48 | ||
| On active treatment | ||||
| Yes | — | 1199 (64) | NA | |
| No | — | 687 (36) | ||
| Unknown | 5273 | 101 | ||
Omitting the ≥80 group, the p value is nonsignificant.
Patients were given the option of identifying their gender as “other” on the survey, but all patients are registered as “male” or “female” in the electronic health tracking record.
NS = nonsignificant; NA = not applicable.
Lifetime Cannabis Use
Of 1928 respondents, 834 reported any lifetime cannabis use (43%), and 59 chose not to complete this question. Cannabis use within the preceding week, 6 months, or 5 years was reported by 241 (13%), 356 (18%), and 471 (24%) respondents respectively. On logistic regression, younger age showed a trend to be predictive for lifetime cannabis use [50–59 years vs. 70–79 years; odds ratio (or): 1.06; 95% confidence interval (ci): 1.00 to 1.12; chi-square: 3.37; p = 0.07], but lifetime use was not associated with education level, sex, or type of cancer diagnosis.
Among respondents reporting any lifetime cannabis use, 119 (14%) reported holding an authorization, and 670 (80%) reported acquiring cannabis through friends or acquaintances. Ever-acquisition from a medical dispensary was reported by 79 respondents (9%), and 50 (6%) reported acquisition by other means.
Of lifetime users, 672 (81%) reported having used dry leaves; 402 (48%), oils or edibles; 234 (28%), hashish; and 52 (6%), some other form of cannabis.
Cannabis Use in the Preceding Six Months
Of the 356 respondents (18%) who reported cannabis use within the 6 months preceding survey completion, 239 (67%) indicated they were currently receiving treatment for cancer, including 192 (54%) receiving systemic therapy, 28 (8%) receiving hormonal therapy, 58 (16%) receiving radiotherapy, and 15 (4%) having recent or upcoming surgery. When considered independently on logistic regression, current systemic therapy use was predictive of cannabis use within the preceding 6 months (or: 1.6; 95% ci: 1.3 to 2.0; chi-square: 15; p < 0.001). Age, sex, education level, type of malignancy, use of hormonal therapy, use of radiotherapy, and use of surgery were not associated with the likelihood of cannabis use in the preceding 6 months.
Of respondents reporting cannabis use within the preceding 6 months, 75 (21%), 74 (21%), 80 (22%), and 65 (18%) reported spending less than $100, $100–$200, $200–$500, and more than $500 respectively during that period. The question about expenditure for cannabis was not answered by 62 (17%) of the respondents who had indicated use during that period.
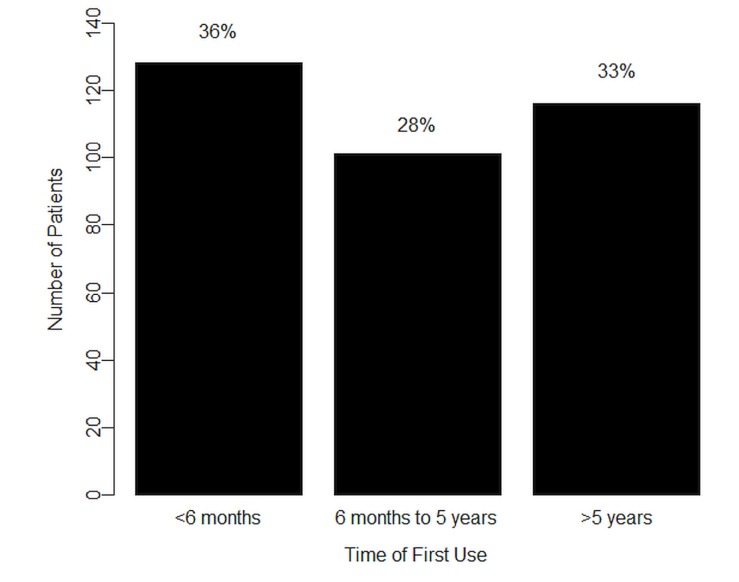
As Figure 1 shows, when the 6-months-preceding users were asked about when they had started using cannabis, 128 (36%) reported starting within that period, with 101 (28%) indicating having started more than 6 months but less than 5 years earlier, 116 (33%) indicating having started more than 5 years earlier, and 11 (3%) choosing not to answer the question.
FIGURE 1.
Time from first use of cannabis in respondents with any cannabis use in the past six months.
Table ii shows the reasons given for cannabis use within the preceding 6 months. Notably, 70% of the 6-months-preceding respondents reported a cancer-related reason for use. Of the 241 respondents who reported having used cannabis within the preceding week, 171 (71%) reported use for at least 1 cancer-related reason.
TABLE II.
Reasons for usea given by 356 respondents reporting cannabis use within the preceding 6 months
| Reason | Active users [n (%)] |
|---|---|
| Any cancer symptom (combined) | 250 (70) |
| Cancer-related pain | 165 (46) |
| Cancer-related nausea | 122 (34) |
| Other cancer symptoms | 110 (31) |
| Any non-cancer reason (combined) | 199 (56) |
| Non-cancer symptoms or illness | 76 (21) |
| Other non-cancer reasons | 157 (44) |
Respondents were allowed to select more than one reason for having used cannabis.
Thoughts About Cannabis Use
Table iii shows respondent opinions about cannabis use. In 1823 surveys, no variables were missing, and those surveys were included in the ordinal regression (descriptions for surveys with missing variables are available in supplemental Table 1). Table iv shows the ordinal regression outcomes for all questions asked. Notably, younger respondents and respondents who had previously used cannabis were less likely to agree with the statements “cannabis is harmful to the body,” “cannabis interferes with other medications,” and “cannabis should be used only under guidance of a doctor.” Respondents with prior cannabis use were more likely to believe that cannabis should be legalized, that it helped to treat nausea, and that it helped to cure cancer.
TABLE III.
Opinions about cannabis use from 1987 respondents
| Statement | Opinion [n (%)] |
|---|---|
| Cannabis is harmful to the body | |
| Strongly agree or agree | 743 (37) |
| Unsure or no response | 703 (35) |
| Disagree or strongly disagree | 541 (27) |
| Cannabis helps cure cancer | |
| Strongly agree or agree | 326 (16) |
| Unsure or no response | 945 (48) |
| Disagree or strongly disagree | 716 (36) |
| Cannabis interferes with other medications | |
| Strongly agree or agree | 297 (15) |
| Unsure or no response | 1307 (66) |
| Disagree or strongly disagree | 383 (19) |
| Cannabis helps treat cancer symptoms | |
| Strongly agree or agree | 1087 (55) |
| Unsure or no response | 812 (41) |
| Disagree or strongly disagree | 88 (4) |
| Cannabis should be used only under guidance of a doctor | |
| Strongly agree or agree | 1162 (58) |
| Unsure or no response | 418 (21) |
| Disagree or strongly disagree | 407 (20) |
| Cannabis should be legalized for recreational use | |
| Strongly agree or agree | 651 (33) |
| Unsure or no response | 504 (25) |
| Disagree or strongly disagree | 832 (42) |
TABLE IV.
Ordinal regressiona of strong agreement or agreement with survey statements
| Statement and comparator | OR | 95% CI |
|---|---|---|
| Cannabis is harmful to the body | ||
| Age group (years): 50–59 vs. 70–79 | 0.7 | 0.6 to 0.8b |
| Education: ≤high school vs. diploma/degree | 0.6 | 0.5 to 0.8b |
| Sex: men vs. women | NA | |
| Lifetime use: yes vs. no | 0.3 | 0.2 to 0.3b |
| Cannabis helps cure cancer | ||
| Age group (years): 50–59 vs. 70–79 | 1.3 | 1.1 to 1.5c |
| Education: ≤high school vs. diploma/degree | 2.8 | 2.1 to 3.6b |
| Sex: men vs. women | NA | |
| Lifetime use: yes vs. no | 1.4 | 1.2 to 1.7b |
| Cannabis interferes with other medications | ||
| Age group (years): 50–59 vs. 70–79 | 0.7 | 0.6 to 0.8b |
| Education: ≤high school vs. diploma/degree | 0.6 | 0.4 to 0.7b |
| Sex: men vs. women | NA | |
| Lifetime use: yes vs. no | 0.3 | 0.2 to 0.4b |
| Cannabis helps treat cancer symptoms | ||
| Age group (years): 50–59 vs. 70–79 | 1.4 | 1.2 to 1.7b |
| Education: ≤high school vs. diploma/degree | NA | |
| Sex: men vs. women | NA | |
| Lifetime use: yes vs. no | 4.6 | 3.7 to 5.8b |
| Cannabis should be used only under guidance of a doctor | ||
| Age group (years): 50–59 vs. 70–79 | 0.7 | 0.6 to 0.8b |
| Education: ≤high school vs. diploma/degree | NA | |
| Sex: men vs. women | NA | |
| Lifetime use: yes vs. no | 0.3 | 0.2 to 0.4b |
| Cannabis should be legalized for recreational use | ||
| Age group (years): 50–59 vs. 70–79 | 1.3 | 1.1 to 1.5b |
| Education: ≤high school vs. diploma/degree | NA | |
| Sex: men vs. women | 1.4 | 1.1 to 1.6b |
| Lifetime use: yes vs. no | 4.1 | 3.4 to 4.9b |
The odds ratio is the likelihood that, compared with the second cohort, the first cohort will “agree” or “strongly agree” with the statement as opposed to taking an “unsure/don’t know” or “disagree/strongly disagree” position. For example, compared with patients 70–79 years of age, those 50–59 years of age are 0.7 times as likely to either agree or strongly agree with the statement “Cannabis is harmful to the body”.
p < 0.001.
p < 0.01.
OR = odds ratio; CI = confidence interval; NA = not applicable.
Comfort Level Discussing Cannabis with Oncologists
When asked about their comfort level in telling oncologists about current cannabis use, only 96 respondents (5%) indicated that they would not feel comfortable telling their oncologists about their prior or current cannabis use. Another 548 respondents (27%) were unsure or did not complete the question. Of the 1094 respondents who had never used cannabis, 193 (18%) indicated that they had contemplated using cannabis as part of their cancer treatment. Of those 193, 168 (87%) felt comfortable discussing the issue with oncologists unprompted, 15 (8%) would feel comfortable discussing it if the oncologists brought it up, and only 1 (1%) felt uncomfortable discussing cannabis with oncologists [9 (5%) were unsure or didn’t respond].
DISCUSSION
Overall, 18% of respondents reported cannabis use within the 6 months before being surveyed, and 13% of all respondents reported use for cancer-related symptoms. The present work represents the first comprehensive and contemporary study describing the prevalence of cannabis use among Canadian patients with cancer. The study was performed at a provincial level and included thousands of responses.
Some limitations of the study are that patients might have been approached to complete the survey more than once if they attended a cancer centre multiple times during the study interval. Hence, multiple (up to a maximum of 5) responses could have been collected from the same individual. The instructions to the clerical staff—and the introductory statement circulated with the survey—were designed to avoid multiple survey completions, but to ensure confidentiality for patients who chose to participate, no patient tracking or personal identifiers were used. Furthermore, the survey was conducted in centres 1 and 2 two months before it was conducted in centres 3 and 4, potentially leading to an unknown confounder affecting the data collected.
By design, the survey was anonymous, which limited our ability to compare the characteristics of respondents with nonrespondents. It is reassuring that the demographics of the patients with a scheduled appointment were similar to those of the respondents to the survey (one exception being the ≥80 age category). Also, patients with skin, gynecologic, and hematologic malignancies appeared less likely to participate. That observation could be related to clinic flow in those tumour groups. Another limitation is that, by surveying patients in an outpatient cancer centre setting, we could not capture patients with early disease treated primarily with surgical modalities, patients with access difficulties, and patients with more advanced disease who have transitioned to care in the community. Finally, the survey was not able to identify whether any of the 119 lifetime users who had ever held authorizations or prescriptions for cannabis were reporting prior or current use of pharmaceutical cannabinoids such as nabilone.
Notwithstanding the foregoing limitations, the study showed a trend on logistic regression toward patients with any lifetime cannabis use being younger (p = 0.07). Upon further characterization, younger patients were less likely to believe that cannabis was harmful, that it interferes with other medications, or that cannabis should be used only under a doctor’s supervision. Although the study did not assess the self-reported efficacy of cannabis, a large proportion of the respondents who had used cannabis within the preceding 6 months (70%) reported at least 1 cancer-related symptom as a reason for their use. Another large proportion of respondents (55%) agreed or strongly agreed with the statement “cannabis helps treat symptoms related to cancer like nausea and pain.”
Other studies have reviewed attitudes toward cannabis in general. One prominent contribution was a survey of American Society of Clinical Oncology members by Doblin and Kleiman in 1991 (before 5-HT3 receptor therapy for nausea), which showed that 44% of Society members had recommended illegal cannabis use to their patients, and 54% felt that cannabis should be available by prescription4. In a more recent study by Ware et al.12, a questionnaire administered to 209 non-cancer patients found that cannabis users tended to be younger and more likely to use tobacco concurrently. In the Ware et al. cohort, 35% of patients had previously used cannabis, and of those, 15% used it specifically for pain relief. Those data are corroborated by data from the California Behavior Risk Factor Surveillance System, which showed that 5% of telephone respondents reported medical marijuana use and that respondents who used tended to be younger and to have conditions such as chronic pain7. A survey of a mixed cohort of patients from the United Kingdom reported on the opinions of 2969 respondents about cannabis use between 1998 and 20029. The investigators found that 25% of patients with chronic pain had used medicinal cannabis. Younger age was again associated with use. In the Netherlands, Gorter et al.3 found that, of patients who received prescriptions for cannabis for a variety of reasons, 64.1% (44% using for >5 months) reported a good or excellent effect on their symptoms.
In a comparable study from Israel in 2011, 279 of 17,000 patients with cancer (1.6%) had received a permit for cannabis use. Of those 279, 69 were surveyed (25% response rate)13. Respondents reported improvements in pain, appetite, well-being, and nausea with cannabis use. In our survey, 6% of respondents overall held an authorization for cannabis. Although respondents were not directly asked if cannabis had helped with the foregoing symptoms, more than half the current users endorsed cannabis use for such symptoms. Notably, in both jurisdictions, medical use was allowed with authorization, but recreational use was illegal.
Finally, in Washington State, where cannabis use is fully legalized, Pergam et al.6 administered a survey about cannabis use to patients visiting a Seattle cancer centre. Of the respondents to that survey, 66% had used cannabis previously, and 21% and 24% had used cannabis within the preceding week and year respectively. Those rates are higher than the rates of 13% and 18% for 1 week and 6 months respectively found in the present study. That difference might be explained by the finding in the Washington State survey that legalization influenced decision-making with respect to current use. The authors noted use for cancer-related symptoms in 75% of respondents, which is similar to the 70% found in the present study.
When considering route of administration, our study found that 81% of lifetime users had used dried leaves, and 41% had used oils or edibles. Those rates are lower than the rates in a multinational Internet-based survey of medicinal cannabis users conducted by Hazekamp et al.10, who found that 95% and 69% had tried inhaled and oral administration respectively. Interestingly, when planning future clinical trials, patients might prefer oral administration of cannabis, as was shown by Luckett et al.14 in a 2016 study.
CONCLUSIONS
This multicentre study provides the most comprehensive insight to date into cannabis use in the cancer-patient population. Of patients with cancer who responded to the survey, 1 in 5 had used cannabis within the preceding 6 months, and 1 in 8 were using cannabis for at least 1 cancer-related symptom.
Acknowledgments
This study was supported by unrestricted funding from Alberta Health Services and departmental funding from the Department of Oncology at the University of Calgary.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.United States, State of Colorado . Colorado Constitution of 1876. Denver, CO: State of Colorado; 2012. Art. XVIII, Sect. 16. Personal use and regulation of marijuana. [Google Scholar]
- 2.United States, State of Washington . Initiative Measure No. 502 concerns marijuana. Olympia, WA: State of Washington; 2011. [Google Scholar]
- 3.Gorter RW, Butorac M, Cobian EP, van der Sluis W. Medical use of cannabis in the Netherlands. Neurology. 2005;64:917–19. doi: 10.1212/01.WNL.0000152845.09088.28. [DOI] [PubMed] [Google Scholar]
- 4.Doblin RE, Kleiman MA. Marijuana as antiemetic medicine: a survey of oncologists’ experiences and attitudes. J Clin Oncol. 1991;9:1314–19. doi: 10.1200/JCO.1991.9.7.1314. [DOI] [PubMed] [Google Scholar]
- 5.Bridgeman MB, Abazia DT. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. P T. 2017;42:180–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Pergam SA, Woodfield MC, Lee CM, et al. Cannabis use among patients at a comprehensive cancer center in a state with legalized medicinal and recreational use. Cancer. 2017;123:4488–97. doi: 10.1002/cncr.30879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan-Ibarra S, Induni M, Ewing D. Prevalence of medical marijuana use in California, 2012. Drug Alcohol Rev. 2015;34:141–6. doi: 10.1111/dar.12207. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Sela G, Vorobeichik M, Drawsheh S, Omer A, Goldberg V, Muller E. The medical necessity for medicinal cannabis: prospective, observational study evaluating the treatment in cancer patients on supportive or palliative care. Evid Based Complement Alternat Med. 2013;2013:510392. doi: 10.1155/2013/510392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ware MA, Adams H, Guy GW. The medicinal use of cannabis in the UK: results of a nationwide survey. Int J Clin Pract. 2005;59:291–5. doi: 10.1111/j.1742-1241.2004.00271.x. [DOI] [PubMed] [Google Scholar]
- 10.Hazekamp A, Ware MA, Muller-Vahl KR, Abrams D, Grotenhermen F. The medicinal use of cannabis and cannabinoids—an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45:199–210. doi: 10.1080/02791072.2013.805976. [DOI] [PubMed] [Google Scholar]
- 11.Gierisch JM, Reiter PL, Rimer BK, Brewer NT. Standard definitions of adherence for infrequent yet repeated health behaviors. Am J Health Behav. 2016;34:669–79. doi: 10.5993/ajhb.34.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware MA, Doyle CR, Woods R, Lynch ME, Clark AJ. Cannabis use for chronic non-cancer pain: results of a prospective survey. Pain. 2003;102:211–16. doi: 10.1016/s0304-3959(02)00400-1. [DOI] [PubMed] [Google Scholar]
- 13.Waissengrin B, Urban D, Leshem Y, Garty M, Wolf I. Patterns of use of medical cannabis among Israeli cancer patients: a single institution experience. J Pain Symptom Manage. 2015;49:223–30. doi: 10.1016/j.jpainsymman.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Luckett T, Phillips J, Lintzeris N, et al. Clinical trials of medicinal cannabis for appetite-related symptoms from advanced cancer: a survey of preferences, attitudes and beliefs among patients willing to consider participation. Intern Med J. 2016;46:1269–75. doi: 10.1111/imj.13224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.