Abstract
Anastomosing hemangioma (ah) is a rare subtype of primary vascular tumour that can, clinically and radiologically, present similarly to malignant renal tumours such as renal cell carcinoma (rcc) and angiosarcoma. Rarely seen in the genitourinary system, the ah we report here occurred in a 40-year-old male patient diagnosed initially with rcc based on imaging and successfully treated by laparoscopic left radical nephrectomy, with adrenal sparing and perihilar lymph node dissection. The pathologic diagnosis of ah can be challenging on small biopsy specimens; we therefore opine that it is appropriate to excise these lesions to facilitate diagnosis and definitively exclude common renal cancers. However, in this review, we describe some radiologic and pathologic distinctions between ah and malignant tumours.
Keywords: Anastomosing hemangioma, renal cell carcinoma, radiology, pathology, renal lesions
INTRODUCTION
Vascular tumours of the kidney are rare1,2 and range from benign (hemangioma) to malignant (angiosarcoma). A recent review noted that the world literature contains approximately 200 reports of renal hemangioma and 40 of renal angiosarcoma3.
In 2009, Montgomery and Epstein4 described a series of vascular tumours—dubbed “anastomosing hemangioma” (ah)—with a complex architecture characterized by anastomosing vessels, rare hobnailing, and benign behaviour. Anastomosing hemangiomas have been reported to occur most commonly in the kidney, but also in other sites such as the testes, thigh, abdominal wall, ovaries, adrenal gland, liver, and gastrointestinal tract3.
CASE DESCRIPTION
Our 40-year-old male patient underwent magnetic resonance imaging (mri) to investigate right leg pain 9 months after a motor vehicle accident. An incidental 4.6 cm T2 hyperintense mass was seen in the left kidney. The mass was only partially imaged on mri, but on T2-weighted images, it had the appearance of a large parapelvic cyst.
The patient subsequently underwent dedicated renal-protocol computed tomography (ct) imaging and renal ultrasonography. He denied left-sided flank pain and had no constitutional symptoms. He reported some irritative voiding symptoms, and his wife suspected he was having hematuria. Although there was family history of metastatic lung cancer, no family history of renal cell carcinoma (rcc) or a genetic syndrome predisposing to rcc was present.
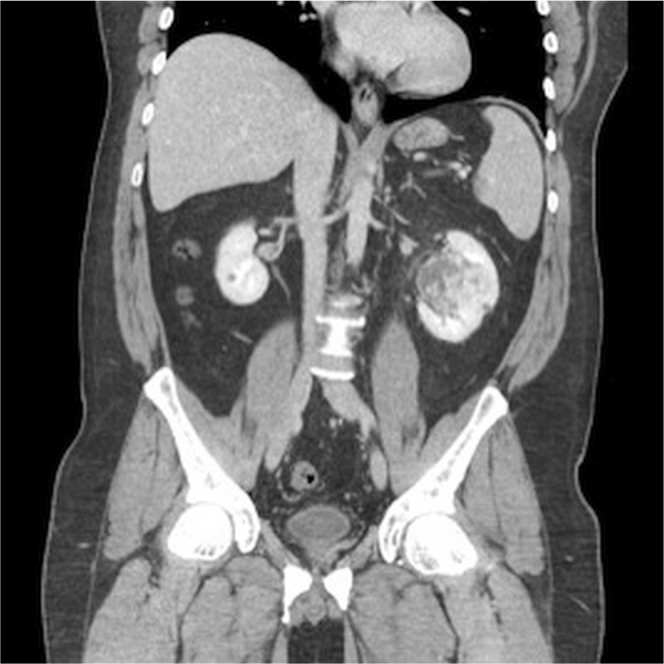
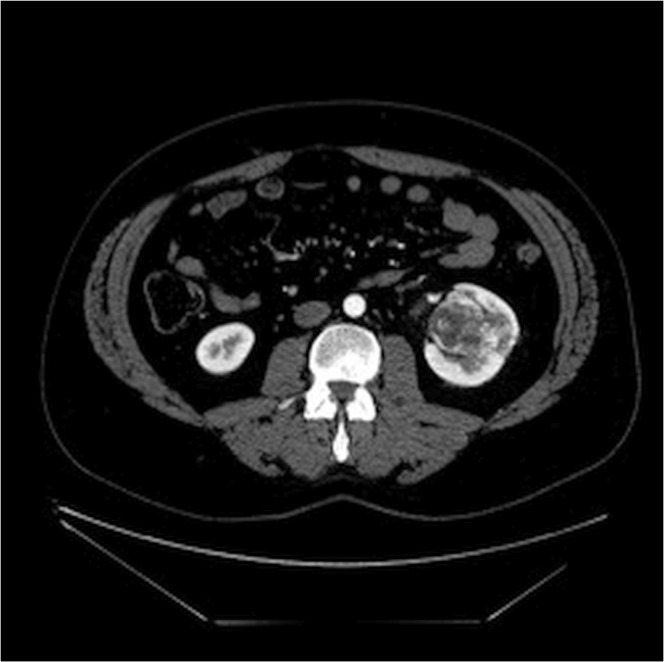
The ct imaging revealed a heterogeneously enhancing solid left renal mass centred within the renal sinus and extending into the parenchyma in the interpolar region, measuring approximately 5.3 cm (Figures 1 and 2). The mass was well-defined and less dense than kidney parenchyma on the unenhanced images (21 HU). No fat or calcification was evident within the lesion. It demonstrated avid peripheral nodular enhancement in the corticomedullary phase (up to 260 HU). In images from the nephrographic phase, centripetal filling of the enhancing portion of the tumour was observed (132 HU). No invasion of the collecting system or renal veins was demonstrated on imaging.
FIGURE 1.
Coronal reconstruction of venous-phase nephrography images demonstrate avid nodular enhancement in the arterial phase, which fills centripetally in the nephrographic phase. The degree of enhancement in the enhanced phases is similar to the degree of vascular enhancement.
FIGURE 2.
Corticomedullary-phase computed tomography imaging.
The imaging was felt to be most representative of a centrally located conventional rcc, as discussed with radiology and staff urology. Biopsy was felt not to be necessary, given a greater than 90% likelihood of the tumour being rcc. There was no evidence of metastatic disease.
The patient subsequently underwent successful laparoscopic left radical nephrectomy, with adrenal sparing and perihilar lymph node dissection. Initial pathology reported angiosarcoma, but on review of the pathology, the presence of anastomosing channels and the lack of marked atypia, pleomorphism, and mitosis revealed ah. Five resected periaortic lymph nodes were all negative for malignancy.
The patient recovered well postoperatively and had no complications at his 1-month clinical follow-up. At the time of the present report, the patient has undergone follow-up ct imaging of chest, abdomen, and pelvis, and abdominal ultrasonography, at 6-month intervals—all negative for disease recurrence.
RADIOLOGY REVIEW
With only 64 cases of renal ah described in the literature5, there is a paucity of information about its imaging appearance. Here, we present a unique case of multimodality imaging of this unusual tumour.
O’Neill et al.6 described the ct appearance of a 6 cm renal ah as a well-circumscribed mass at 22 HU before contrast and avid heterogeneous enhancement up to 147 HU. They described discontinuous peripheral nodular enhancement in both adrenal and renal ahs.
Our case of renal ah shares ct imaging features with those described by O’Neill et al. We also identified avid peripheral nodular enhancement and marked arterial enhancement up to 260 HU (Figures 1 and 2). Centripetal filling in the enhancement was also seen in our example, but has not been described in prior reports. In fact, the behavior of the lesion is reminiscent of benign hepatic hemangioma. Unfortunately, our dynamic renal-mass protocol does not include an excretory phase to determine whether the hemangioma continued to fill centrally with time.
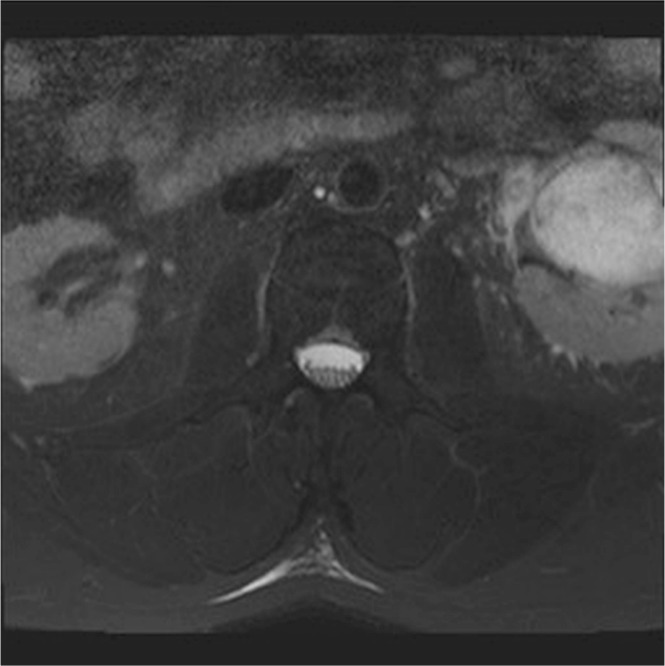
The mri appearance of a renal ah has not previously been described. In our case, the mass was incidentally detected on an mri of the spine and was imaged only with T2 weighting (Figure 3). The marked T2 hyperintensity of the lesion in our example mimicked a parapelvic cyst. That feature is also seen in mri images of hepatic hemangiomata.
FIGURE 3.
Axial T2 fat-saturated image from magnetic resonance imaging of the lumbar plexus demonstrates a mass centred in the left renal sinus, with marked T2 hyperintensity mimicking a parapelvic cyst.
PATHOLOGY REVIEW
The pathology report located and described the tumour at the mid-pole renal sinus extending into the parenchyma. No invasion of the renal vein or artery had occurred, but the tumour appeared to focally invade the sinus fat. A smooth cyst was identified in the capsule, but otherwise, it was tan brown and unremarkable. The medulla was well defined. The renal sinus was distorted by the mass, and the calyces had a pale tan, unremarkable appearance. Some distortion in the calyces and pelvis had occurred because of shifting from the tumour, but they were otherwise grossly unremarkable. Lobulation was appreciated in the perinephric fat.
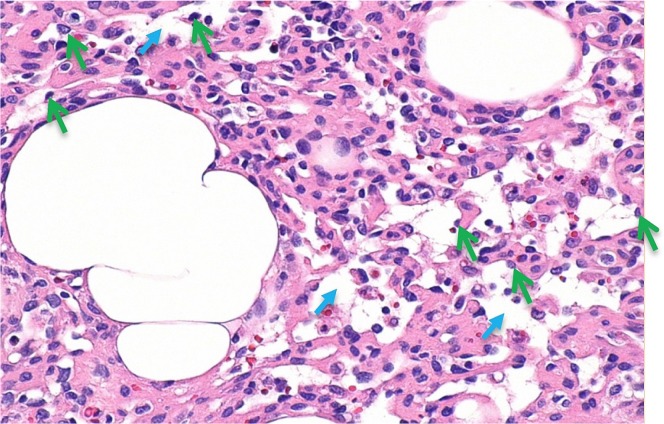
Interestingly, the tumour showed a trabecular arrangement of plump cells, with a prominent vascular channel-type pattern, moderate atypia, and pleomorphism without appreciable mitosis. On further review, a vascular neoplasm composed of complex anastomosing capillary vascular channels lined by hobnail-like endothelial cells was revealed (Figure 4).
FIGURE 4.
High-magnification view of anastomosing channels with occasional red blood cells. The green arrows mark hobnail endothelial cells. The smaller blue arrows mark anastomosing channels. Hematoxylin and eosin stain. 20× original magnification. Horizontal dimension of the image is approximately 0.42 mm.
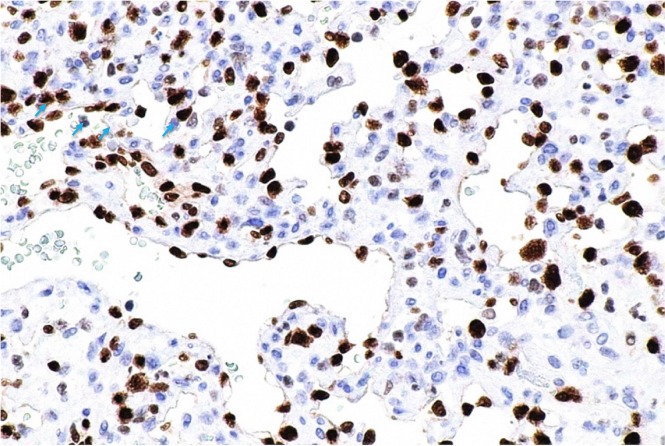
Immunohistochemistry was negative for cytokeratins (AE1/AE3, CAM5.2, CK7), epithelial membrane antigen, S100, human herpes virus 8, and hmb-45. The lesional cells were positive for erg, CD34, CD31, actin, and factor viii, supporting vascular differentiation (Figure 5). Podoplanin, calretinin, wt1, and desmin were negative. Staining for Ki-67 was seen in approximately 15% of the tumour cells.
FIGURE 5.
ETS-related gene (ERG) immunostain (brown) marks the nuclei of the lesional (endothelial) cells (blue arrows). 20× original magnification. Horizontal dimension of the image is approximately 0.42 mm.
Given the above findings, angiosarcoma was initially considered in the differential diagnosis. However, the presence of vascular neoplasm composed of complex anastomosing capillary vascular channels and a lack of marked cytologic atypia, pleomorphism, and mitotic activity were in keeping with the characteristics of ah described in current literature7,8. External consultation confirmed that diagnosis.
DISCUSSION
Although ah is uncommon, its recognition and differentiation from malignant entities such as rcc and angiosarcoma would be helpful in its management. Accurate diagnosis can allow for a conservative treatment approach or selective embolization8 rather than radical nephrectomy, which has been identified as the mainstay of treatment in a review of 64 cases of renal ah5.
The under-recognition of ah before nephrectomy occurs because of similarities to malignant tumours in its clinical and radiographic features; the diagnosis is made primarily on histology and immunochemistry7. More frequently seen in patients with end-stage renal disease and compromised renal function5, ah can often mimic the symptoms of rcc. Hematuria, hypertension, and flank pain are nonspecific features seen in ah, angiosarcoma, and rcc4. All of those entities can demonstrate echogenicity on ultrasonography and can appear as a well-demarcated, solid, heterogeneous avidly-enhancing lesion on contrast-enhanced ct9. Hypervascular conventional rcc is much more frequently encountered than ah in the kidney, and those lesions have a large overlap in imaging appearance, making a confident prospective imaging diagnosis of a renal vascular tumour unlikely.
However, certain features could lead the radiologist to consider the diagnosis. A solid renal mass with marked T2 hyperintensity, almost akin to a cyst, could be a clue. Centripetal filling of avid contrast enhancement from the periphery toward the centre on dynamic ct or mri could be another clue that might prompt percutaneous biopsy rather than nephrectomy. A renal mass that behaves similarly to a hepatic hemangioma on ct or mri could in fact be a vascular tumour, such as an ah, rather than the much more common rcc.
Histologically, ah can mimic angiosarcoma (hyaline globules, anastomosing vascular pattern, and hobnailing endothelial cells), but lacks features of malignancy such as mitotic activity, multilayering, and atypia of endothelial cells7,8. Given a lack of clinical or radiologic findings that are specific to ah, and the serious morbidity associated with a misdiagnosed rcc or angiosarcoma, nephrectomy appears to be the safest management option. A recent case report by Burton et al.10 also demonstrated the possibility of multifocal disease or recurrence of ah in a patient 14 years after partial nephrectomy for papillary renal carcinoma.
Current Canadian Urological Association guidelines on small renal masses, defined as masses less than 4 cm in greatest dimension, recommend management with partial nephrectomy—or radical nephrectomy for masses not amenable to the former approach. Alternative treatment with probe ablation is possible, usually for lesions less than 3 cm. Although core-needle biopsies are increasingly performed, they are not yet the standard of care in Canada for the histologic characterization of small renal masses. Consideration of active surveillance is limited to the elderly and infirm11. Interestingly, in the recent review by Perdiki et al.5 describing 64 cases of renal ah, most of which were smaller than 4 cm, almost all cases were treated with radical nephrectomy before diagnosis (only 2% underwent excision). In the present report, we describe a large renal mass suspicious for rcc, treated according to current best practice with radical nephrectomy, which was eventually revealed to be a benign ah. This case raises the question of whether it might be possible to spare patients an unnecessary surgery if a secure diagnosis of ah could be established with imaging or biopsy. Future research could investigate radiologic differentiation of these tumours and the increased role of core-needle biopsy.
SUMMARY
Anastomosing hemangiomas are rare benign vascular tumours of the kidney that can present similarly to malignant renal tumours such as rcc and angiosarcoma clinically and on imaging. Definitive diagnosis requires a pathology assessment, including histology and immunohistochemistry. Identifying ways to characterize ah before surgical treatment would be ideal; however, given the high probability of a solid renal mass being malignant and the rarity of benign tumours, the current management guideline of first-line surgical resection appears reasonable.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30:1525–40. doi: 10.1148/rg.306105517. [DOI] [PubMed] [Google Scholar]
- 2.Brown JG, Folpe AL, Rao P, et al. Primary vascular tumors and tumor-like lesions of the kidney: a clinicopathologic analysis of 25 cases. Am J Surg Pathol. 2010;34:942–9. doi: 10.1097/PAS.0b013e3181e4f32a. [DOI] [PubMed] [Google Scholar]
- 3.Omiyale AO. Anastomosing hemangioma of the kidney: a literature review of a rare morphological variant of hemangioma. Ann Transl Med. 2015;3:151. doi: 10.3978/j.issn.2305-5839.2015.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery E, Epstein JI. Anastomosing hemangioma of the genitourinary tract: a lesion mimicking angiosarcoma. Am J Surg Pathol. 2009;33:1364–9. doi: 10.1097/PAS.0b013e3181ad30a7. [DOI] [PubMed] [Google Scholar]
- 5.Perdiki M, Datseri G, Liapis G, et al. Anastomosing hemangioma: report of two renal cases and analysis of the literature. Diagn Pathol. 2017;12:14. doi: 10.1186/s13000-017-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neill AC, Craig JW, Silverman SG, Alencar RO. Anastomosing hemangiomas: location of occurrence, imaging features, and diagnosis with percutaneous biopsy. Abdom Radiol (NY) 2016;41:1325–32. doi: 10.1007/s00261-016-0690-2. [DOI] [PubMed] [Google Scholar]
- 7.Wetherell DR, Skene A, Manya K, Manecksha RP, Chan Y, Bolton DM. Anastomosing haemangioma of the kidney: a rare morphological variant of haemangioma characteristic of genitourinary tract location. Pathology. 2013;45:193–6. doi: 10.1097/PAT.0b013e32835c782b. [DOI] [PubMed] [Google Scholar]
- 8.Zhao M, Li C, Zheng J, Sun K. Anastomosing hemangioma of the kidney: a case report of a rare subtype of hemangioma mimicking angiosarcoma and review of the literature. Int J Clin Exp Pathol. 2013;6:757–65. [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HS, Koh BH, Kim JW, et al. Radiologic findings of renal hemangioma: report of three cases. Korean J Radiol. 2000;1:60–3. doi: 10.3348/kjr.2000.1.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton KR, Jakate K, Pace KT, Vlachou PA. A case of recurrent, multifocal anastomosing haemangiomas. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-220076. pii: bcr-2017-220076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jewett MA, Rendon R, Lacombe L, et al. on behalf of the Canadian Urological Association Canadian guidelines for the management of small renal masses (srm) Can Urol Assoc J. 2015;9:160–3. doi: 10.5489/cuaj.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]