Abstract
Objective
Aortic valve-in-valve implantation for prosthetic valve dysfunction is a good alternative to reoperative valve replacement. There are some limitations to this approach including the risk of coronary occlusion, patient prosthesis mismatch, and valve malposition. The incidence of coronary occlusion is higher in aortic valve-in-valve than de novo aortic stenosis cases. Multiple factors can contribute to this complication, and the type of bioprosthesis has been implicated.
Methods
We examined our experience of 80 aortic valve-in-valve cases with internally and externally mounted leaflet valves.
Results
Procedural success was achieved in 95% of cases with an overall 30-day mortality of 1.3%. Clinical and procedural outcomes were similar in the both cohorts.
Conclusions
Our data suggest that aortic valve-in-valve implantation can be safely performed in carefully selected patient with internally and externally mounted leaflet bioprosthesis.
Key Words: Aortic valve-in-valve, Transcatheter aortic valve replacement, Structural valve dysfunction, Bioprosthetic valves
With increasing number of bioprosthesis being implanted, structural valve dysfunction (SVD) requiring reoperative valve surgery is expected. Aortic valve-in-valve (AVIV) implantation using catheter-based prosthesis is becoming an acceptable therapeutic option for patients.1,2 However, coronary obstruction is a potential serious complication and is more common in AVIV cases than in transcatheter aortic valve replacement for native valve.2 Externally mounted leaflet valve (EMV) possesses certain design features that may theoretically increase the risk of coronary obstruction and multiple cases of this complication have been reported.3
Currently, there are only two commercially available aortic bioprosthesis with externally mounted leaflet for surgical aortic valve replacement, namely, the Mitroflow (Sorin Group, Milan, Italy) (Fig. 1A) and the Trifecta aortic valve (St. Jude Medical, St. Paul, MN USA) (Fig. 1B). The design features that are unique to these bioprosthesis include their bovine pericardial leaflets being mounted externally onto the valve stent and have a slightly taller stent profile. Those features contribute to their excellent hemodynamics with low transvalvular gradient.4,5 However, with their wider and taller stand, they may be more prone to coronary compromise at the time of AVIV. We first reported this lethal complication in an a case of AVIV with a SAPIEN (Edwards Lifesciences, Irvine, CA USA) into a failed Mitroflow valve in 2011 (Fig. 2).3 There is a common belief that AVIV should not be performed in EMV. We here reported our current results of AVIV into failed EMV, Sorin Mitroflow, and St Jude Medical Trifecta valves.
FIGURE 1.
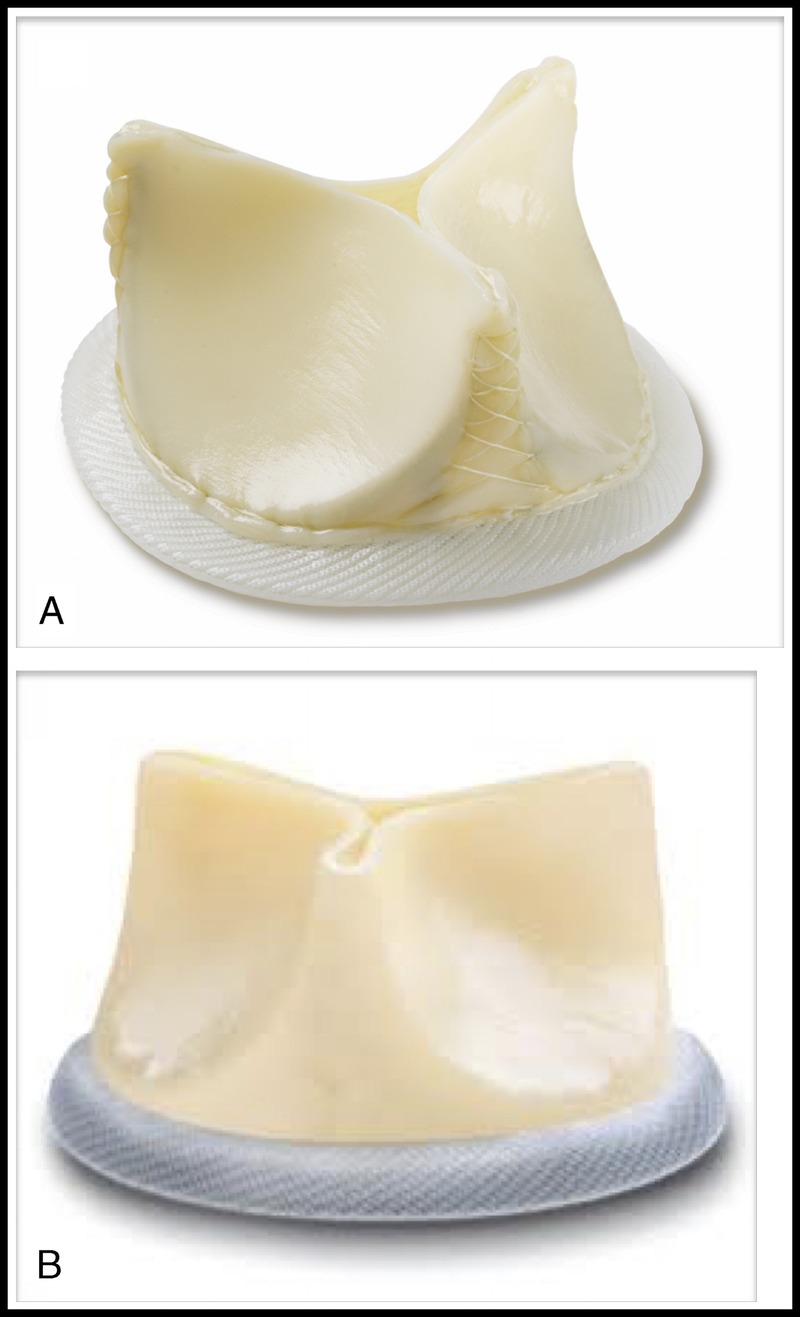
Externally mounted leaflet Prosthesis. A, Sorin Mitroflow pericardial valve. B, St. Jude Medical Trifecta pericardial valve.
FIGURE 2.
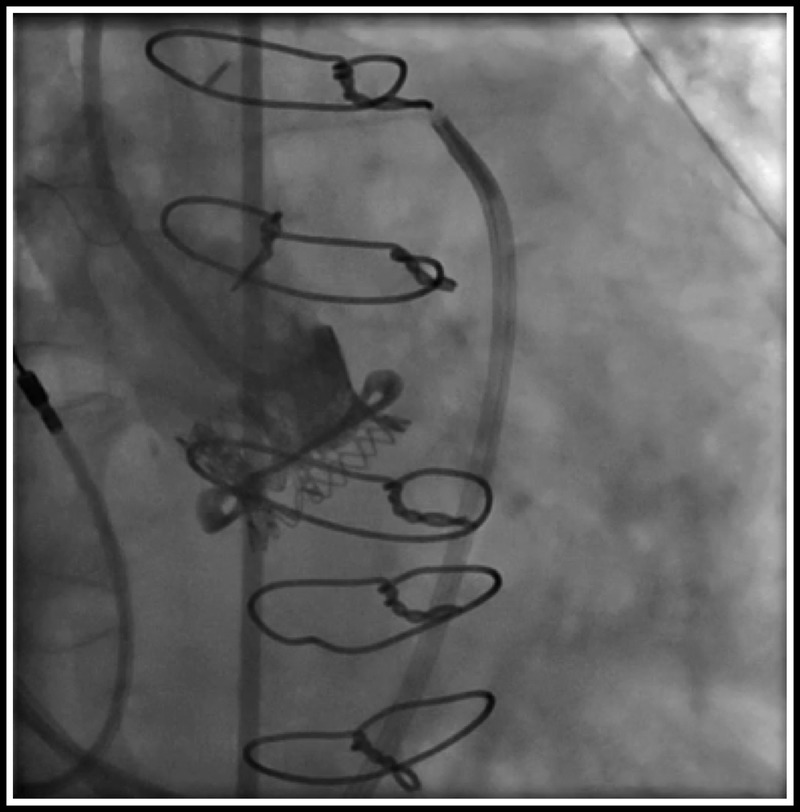
Total aortic occlusion after AVIV with a 23-mm SAPIEN valve into a failed Mitroflow bioprosthesis.
METHODS
A retrospective review of our institutional database of all AVIV cases from March 2009 to February 2015 was performed. All patients with symptomatic aortic prosthetic SVD were assessed by a multidisciplinary Heart Team and were deemed too high risk for conventional aortic valve re-replacement. Transcatheter AVIV implantations were performed retrograde via the femoral artery or transapically thru the left ventricular apex. Implant procedures were performed in the hybrid operating room with the guidance of fluoroscopy and/or transesophageal echocardiography.
Follow-up and Data Collection
All patients were followed up by the transcatheter valve clinic including telephone interviews and office visits. Data were prospectively collected and entered into the institutional transcatheter valve database. The mean follow-up period was 3.4 years with longest follow-up of 6.7 years. Procedural success and complications were reported according to VARC-2 (Valve Academic Research Consortium) definitions.6
Statistical Analysis
Continuous variables are described as mean ± SD or median with interquartile range. Categorical variables are described by frequencies and percentages and paired Student t test was employed to compare continuous variables. All analysis was performed using the SPSS Version 17.0 software (IBM, Chicago, IL USA).
RESULTS
From March 2009 to February 2015, a total of 80 patients underwent successful AVIV for prosthetic SVD in our institution. Overall, 19 patients (24%) had the Mitroflow and the Trifecta valves as the original prosthesis, 17 and 2, respectively. The remaining cohort had internally mounted leaflet valves (IMVs), except one patient with a previous Freestyle Porcine prosthesis (Medtronic, Minneapolis, MN USA). Figure 3 demonstrated the proportion and type of failed bioprosthesis in our cohort. The demographics of this study cohort are listed in Table 1. Patients' baseline characteristics are not dissimilar in both groups with age, sex, major comorbidities, and calculated The Society of Thoracic Surgeons (STS) Risk Score. However, there were more patients in the IMV cohort with chronic renal insufficiency. The mean ± SD age of prosthesis was 12.3 ± 5.2 years after aortic valve replacement (AVR) at the time of AVIV implantation. The primary mode of failure was stenosis in 30%, regurgitation in 42%, and mixed in 28% of cases. The manufacturers' labeled size ranged from 19 mm to 29 mm with median of 23 mm. A variety of transcatheter valve was implanted into the failed prosthesis, including SAPIEN, SAPIEN XT, SAPIEN 3 (Edwards Lifesciences, Irvine, CA USA), CoreValve (Medtronic, Minneapolis, MN USA), PORTICO (St. Jude Medical, Minneapolis, MN USA), and JenaClip (JenaValve Technology GmbH, Munich, Germany) (Fig. 4). Procedural access was performed transfemorally in 73% of all cases, 77% in IMV and 58% in EMV cohort, respectively. The remaining patients underwent AVIV via the left ventricular apex. Overall procedural success was 95% with the need for a second transcatheter valve in two cases, both occurred in IMV group. No access site, major vascular complication, and stroke occurred in any patients. A patient developed acute kidney injury in the IMV cohort with normalization of renal function at discharge, not requiring perioperative dialysis.
FIGURE 3.
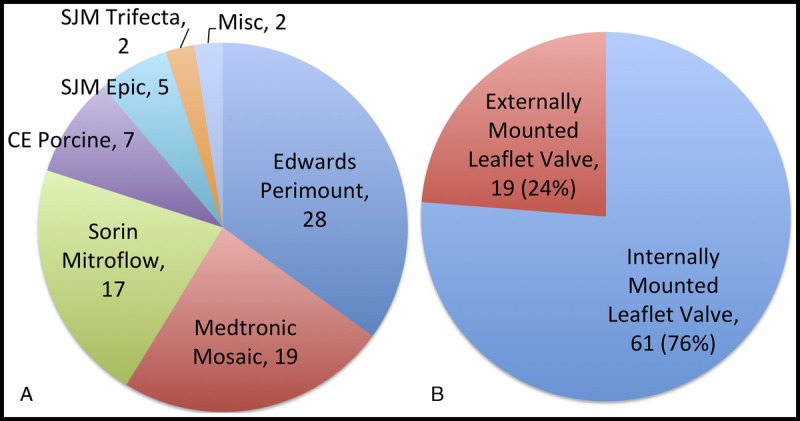
A, Type of failed aortic bioprosthesis that underwent AVIV implant. B, The number of IMVs and EMVs.
TABLE 1.
Patient Demographics
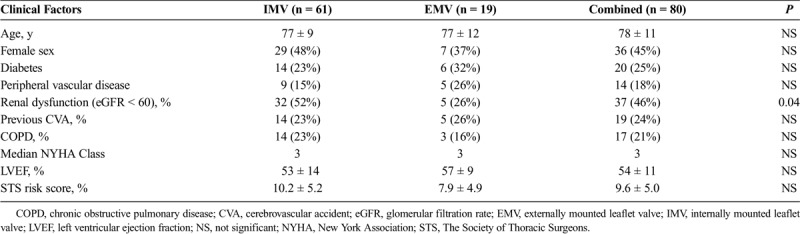
FIGURE 4.
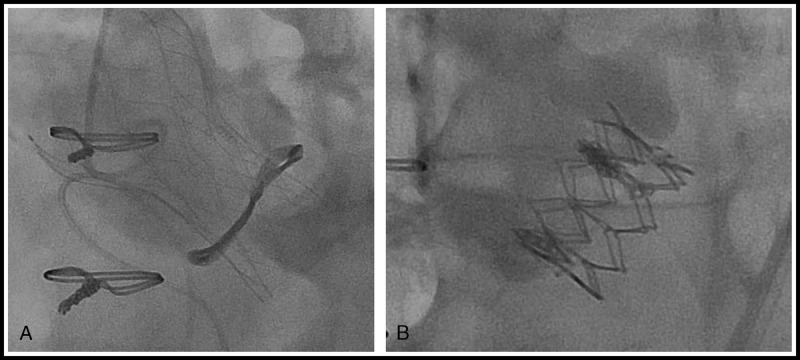
Aortic valve-in-valve with (A) SJM PORTICO transcatheter valve into a failed Sorin Mitroflow prosthesis and (B) Edwards SAPIEN XT transcatheter valve into a failed Trifecta prosthesis.
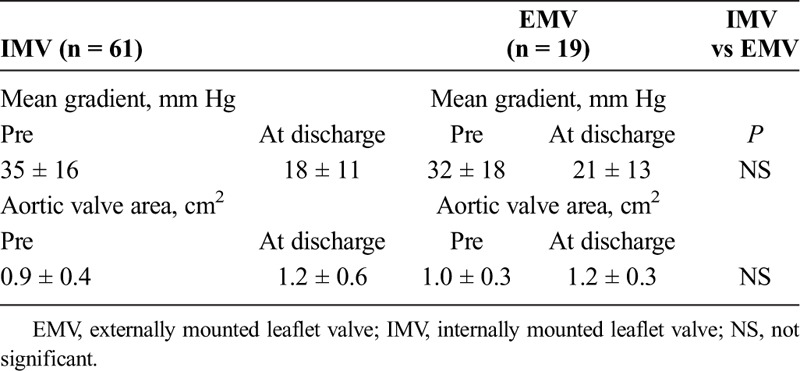
In total, there were two cases of coronary occlusion in our series. The previously reported case with a failed Mitroflow valve3 developed total aortic occlusion after transapical AVIV with a 23-mm SAPIEN valve. Emergent femoral-femoral cardiopulmonary (CPB) was initiated and an open reoperative AVR was carried out. Unfortunately, this patient developed multisystem failure, died 2 days postoperatively, and remained the only 30-day mortality in our entire series. Another patient with a failed 25-mm Perimount pericardial valve (Edwards Lifesciences, Irvine, CA USA), an IMV with high-risk features of coronary occlusion underwent transfemoral AVIV with a 26-mm SAPIEN XT valve. A coronary guide and a 4 × 16-mm drug-eluting coronary stent was preloaded and stationed in the mid LAD for potential rescue left main stenting. In addition, the femoral vessels were wired for CPB in case of hemodynamic collapse. Partial subtotal left main occlusion after AVIV occurred leading to cardiogenic shock, patient was placed and stabilized on femoral-femoral CPB efficaciously. Left main stenting with the preloaded stent was carried out with good results, and patient was weaned from CPB and had an unremarkable recovery (Fig. 5). Overall clinical and procedural outcomes are excellent and not significant different from both groups (Table 2). All patients had significant reduction in transvalvular gradient and improvement in valve area post AVIV in both groups (Table 3).
FIGURE 5.
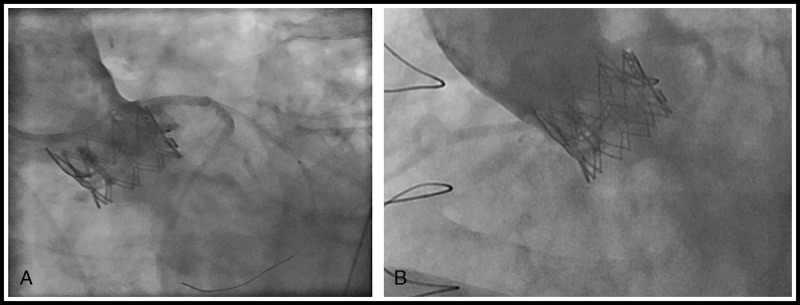
Coronary complication post-SAPIEN XT into Perimount with (A) subtotal left main obstruction and (B) patent left main poststenting.
TABLE 2.
Clinical Outcomes After AVIV Implantation
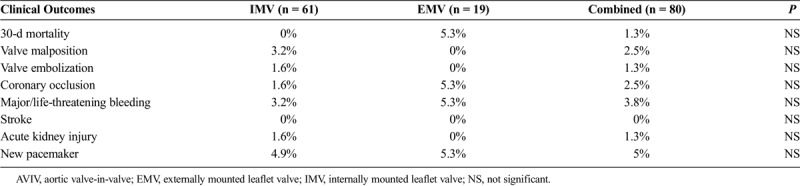
TABLE 3.
Echocradiographic Data Pre and Post Aortic Valve-in-Valve Implantation
DISCUSSIONS
Since our first experience with AVIV in 2007, AVIV has established itself as a viable alternative to surgical re-replacement. Our overall 30-day and in hospital mortality of 1.3% in a high-risk cohort is excellent and is noninferior to surgically redo AVR with an STS risk predicated mortality of 9.7%.
Coronary complication can occur in both types of bioprosthesis. Patient native anatomy including the height of the coronaries, the dimension of the sinuses, sinotubular junction, the ascending aorta, and the position of the failed prosthesis has to be evaluated carefully to avoid such complication. Coronary occlusion occurs in patients with more than one risk factor. In our facility, this assessment includes aortic root angiography in an optimal projection that is perpendicular to both the surgical bioprosthesis and the coronary ostia, and left anterior oblique projection with cranial angulation is generally required. In addition, a projection having “1-2” postalignment is commonly used (Fig. 6). This technique is of little value when the bioprosthetic valve posts are radiolucent (i.e., Mitroflow). In addition, semiselective injection of contrast in coronary ostia may provide optimal assessment of the geometric relationship between the failed surgical valve and the coronary ostia with little contrast. This injection is usually performed in a projection that is both perpendicular to the surgical valve and to the coronary ostium. In these cases, adequate reflux of contrast allows for assessment of the relationship between the bioprosthetic valve and the ostium of the left main and identification cases at increased risk. In cases with coronary compromise is highly suspected, a balloon valvuloplasty should be considered with an aortogram (Fig. 7).
FIGURE 6.
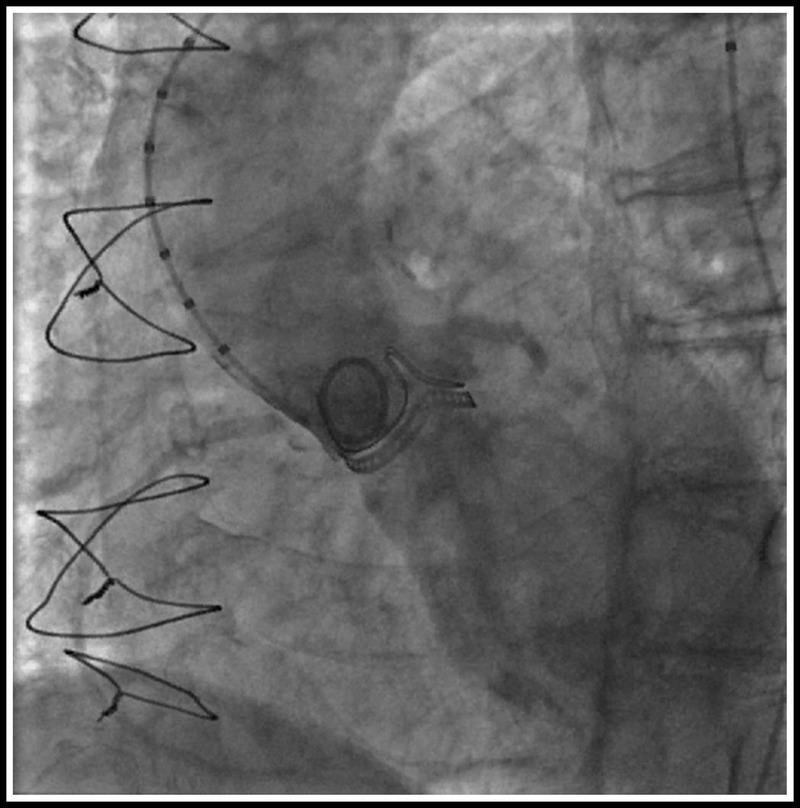
Aortic root angiography with “1-2” postalignment to left coronary ostia.
FIGURE 7.
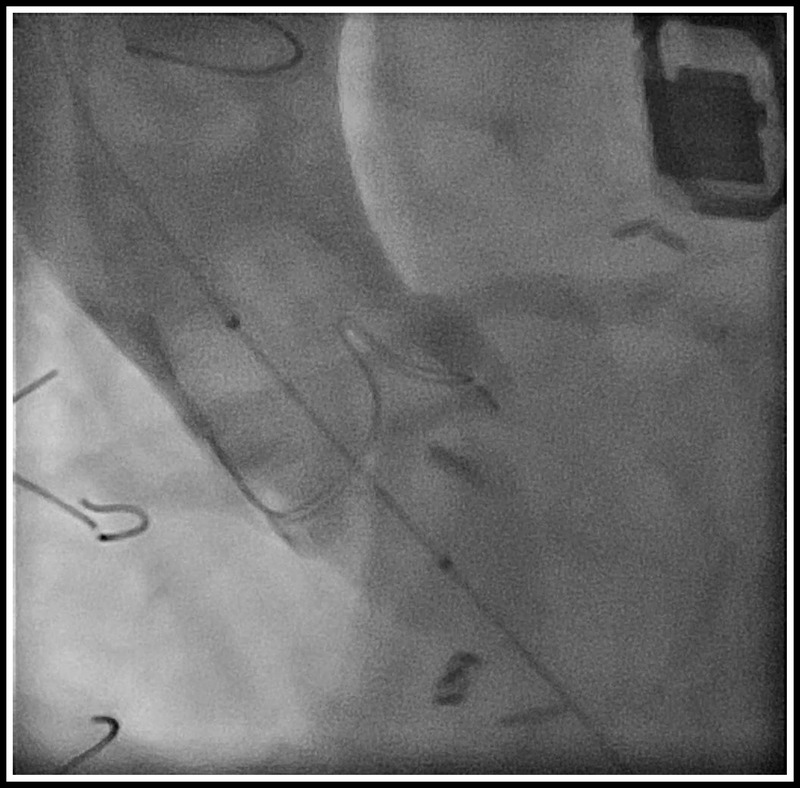
Aortic root angiography during balloon valvuloplasty with “1-2” postalignment to left coronary ostia with no risk of coronary occlusion.
Multidetector computed tomography was routinely used for assessing the risk of coronary occlusion in AVIV. The anticipated distance of the transcatheter heart valve (THV) to the coronary ostia was estimated [virtual THV-coronary distance (VTC)]. This is optimally performed by superimposing a virtual ring simulating the diameter of the anticipated, fully expanded THV centered along the geometrical center of the surgical prosthesis followed by a measurement from the ring toward the coronary ostium. This distance provides a marker of the capacity of the root to accommodate the THV while maintaining flow to the coronary arteries and also accounts for a possible eccentric position of the surgical prosthesis within the aortic root. Smaller VTC distances may confer an increased hazard for coronary occlusion. In general, the risk of coronary complication is considered high with a VTC distance of less than 3 mm, intermediate at 3 to 6 mm, and low if greater than 6 mm.7
However, a re-referral to cardiothoracic surgeon for reconsideration of conventional redo AVR should be considered. However, in cases where redo AVR is prohibitive, prewiring with preloaded stent in the coronary may be an option in some cases.
Limitations
Our study is retrospective and the sample size is relatively small. It may be underpowered to detect any difference in mortality and other clinical outcomes.
CONCLUSIONS
Aortic valve-in-valve can be performed safely in both IMV and EMV bioprosthetic valves with excellent clinical outcomes (Table 2). Coronary occlusion occurred is more common in AVIV, 2.5% in our series, 7% in the Valve-in-Valve International Data registry, and native transcatheter aortic valve replacement (1%). Careful evaluation with aortogram and computed tomography imaging are crucial to avoid such complication. Aortic valve-in-valve is an acceptable alternative to redo AVR in carefully selected patients.
CLINICAL PERSPECTIVE
This case series from Dr. Cheung and his colleagues at St. Paul's Hospital in Vancouver examined their results with 80 aortic valve-in-valve cases with internally and externally mounted leaflet valves. They achieved procedural success in 95% of cases with an overall 30-day mortality of 1.3%. The preoperative Society of Thoracic Surgeons PROM of these patients was 9.6 ± 5%. Outcomes were similar in both cohorts of patients suggesting that aortic valve-in-valve implantation can be safely performed in selected patients with both internally and externally mounted leaflet prostheses.
This is an interesting retrospective study from a pioneering center in transcatheter valve replacement. They report superb results with aortic valve-in-valve replacement and provide excellent guidance on how to prevent complications particularly coronary occlusion in these patients. However, this was a relatively small study and was underpowered to detect any major differences in outcomes between internally versus externally mounted bioprostheses. A larger clinical registry will be useful in elucidating further details of proper patient selection. However, this report and others have clearly demonstrated that aortic valve-in-valve implantation remains an acceptable alternative to redo aortic valve replacement in this patient population.
Footnotes
Disclosures: Anson W. Cheung, MD, is a consultant for Abbott Cardiovascular (St. Jude Medical), St. Paul, MN USA; David A. Wood, MD, is a consultant for Abbott Cardiovascular (St. Jude Medical), St. Paul, MN USA, Edwards Lifesciences, Irvine, CA USA, and Medtronic, Inc, Minneapolis, MN USA, and John G. Webb, MD, is a consultant for Edwards Lifesciences, Irvine, CA USA. Jian Ye, MD, Danny Dvir, MD, and John G. Webb, MD, declare no conflicts of interest.
REFERENCES
- 1.Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121:1848–1857. [DOI] [PubMed] [Google Scholar]
- 2.Dvir D, Webb J, Brecker S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation. 2012;126:2335–2344. [DOI] [PubMed] [Google Scholar]
- 3.Gurvitch R, Cheung A, Bedogni F, Webb JG. Coronary obstruction following transcatheter aortic valve-in-valve implantation for failed surgical bioprostheses. Catheter Cardiovasc Interv. 2011;77:439–444. [DOI] [PubMed] [Google Scholar]
- 4.Bavaria JE, Desai ND, Cheung A, et al. The St Jude Medical Trifecta aortic pericardial valve: results from a global, multicenter, prospective clinical study. J Thorac Cardiovasc Surg. 2014;147:590–597. [DOI] [PubMed] [Google Scholar]
- 5.Bleiziffer S, Eichinger WB, Hettich IM, et al. Hemodynamic characterization of the Sorin Mitroflow pericardial bioprosthesis at rest and exercise. J Heart Valve Dis. 2009;18:95–100. [PubMed] [Google Scholar]
- 6.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33: 2403–2418. [DOI] [PubMed] [Google Scholar]
- 7.Dvir D, Leipsic J, Blanke P, et al. Coronary obstruction in transcatheter aortic valve-in-valve implantation: preprocedural evaluation, device selection, protection, and treatment. Circ Cardiovasc Interv. 2015;8: e002079. [DOI] [PubMed] [Google Scholar]


