Abstract
Purpose
This study aims to predict hematological toxicity induced by 223Ra therapy. We investigated the value of metabolically active bone tumor volume (MBTV) and total bone lesion activity (TLA) calculated on pretreatment fluorine-18-fluorocholine (18F-FCH) PET/CT in castrate-resistant prostate cancer (CRPC) patients with bone metastases treated with 223Ra radionuclide therapy.
Patients and methods
18F-FCH PET/CT imaging was performed in 15 patients with CRPC before treatment with 223Ra. Bone metastatic disease was quantified on the basis of the maximum standardized uptake value (SUV), total lesion activity (TLA=MBTV×SUVmean), or MBTV/height (MBTV/H) and TLA/H. 18F-FCH PET/CT bone tumor burden and activity were analyzed to identify which parameters could predict hematological toxicity [on hemoglobin (Hb), platelets (PLTs), and lymphocytes] while on 223Ra therapy. Pearson’s correlation was used to identify the correlations between age, prostate-specific antigen, and 18F-FCH PET parameters.
Results
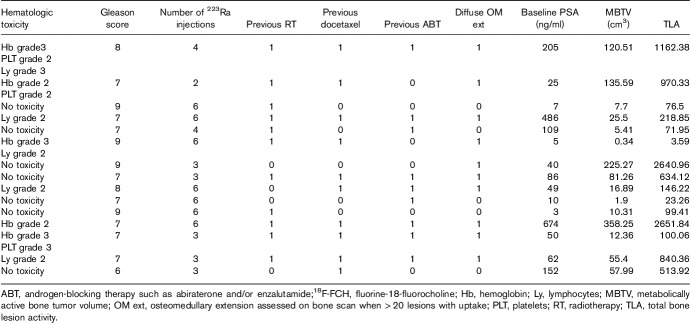
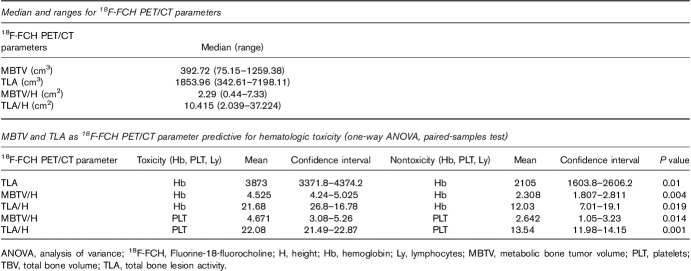
MBTV ranged from 75 to 1259 cm3 (median: 392 cm3). TLA ranged from 342 to 7198 cm3 (median: 1853 cm3). Patients benefited from two to six cycles of 223Ra (n=56 cycles in total). At the end of 223Ra therapy, five of the 15 (33%) patients presented grade 2/3 toxicity on Hb and lymphocytes, whereas three of the 15 (20%) patients presented grade 2/3 PLT toxicity.
Age was correlated negatively with both MBTV (r=−0.612, P=0.015) and TLA (r=−0.596, P=0.018). TLA, TLA/H, and MBTV/H predicted hematological toxicity on Hb, whereas TLA/H and MBTV/H predicted toxicity on PLTs at the end of 223Ra cycles. Receiver operating characteristic curve analysis allowed to define the cutoffs for MBTV (915 cm3) and TLA (4198 cm3) predictive for PLT toxicity, with an accuracy of 0.92 and 0.99.
Conclusion
Tumor bone burden calculation is feasible with 18F-FCH PET/CT with freely available open-source software. In this pilot study, baseline 18F-FCH PET/CT markers (TLA, MBTV) have shown abilities to predict Hb and PLT toxicity after 223Ra therapy and could be explored for patient selection and treatment optimization.
Keywords: fluorine-18-fluorocholine PET/CT, hematologic toxicity, 223Ra
Introduction
223Ra dichloride (223RaCl2) is the first α-particle emitter therapeutic agent approved by the European agency since 2013, with benefits in overall survival and delay in symptomatic skeletal events for patients with metastatic castrate-resistant prostate cancer (CRPC) 1. Recent post-hoc analyses of the phase III ALSYMPCA trial support the previously established safety profile and confirm the therapeutic efficacy of 223Ra 2–5.
The most common side effects are diarrhea, nausea, vomiting, and thrombocytopenia 2–4,6,7. In the ALSYMPCA trial, thrombocytopenia was the main hematologic toxicity induced by 223Ra rather than placebo, with grade 3/4 toxicity frequencies of 6% (3% grade 3 and 3% grade 4 toxicity) 3,8 and bone marrow suppression in less than 3% of patients, with no case of myelodysplasia or primary bone tumor 7.
Because of the treatment duration of 24 weeks (6 months) with respect to an overall survival of 15 months 3, the risk of severe hematological toxicity needs to be limited. Severe hematological toxicities necessitated treatment interruption as only 387 (63%) of the 541 patients enrolled in the ALSYMPCA trial received all six 223Ra injections 3.
To date, there are no established metabolic or imaging markers to predict or to minimize the risk of grades 2–4 hematologic toxicity in patients eligible for 6-monthly injections of 223Ra.
Fluorine-18-fluorocholine PET-CT (18F-FCH PET/CT) has been used successfully to detect metastatic and recurrent prostate cancer 9,10. Measurements of metastatic global [visceral, lymph node (LN), and bone] tumor burden in prostate cancer on 18F-FCH PET/CT over the course of different treatments (abiraterone, enzalutamide, 223Ra) were found to be predictive of PSA progression being a potential surrogate marker of treatment outcome 11,12. Although alkaline phosphatase (ALP) emerged as the leading biomarker for 223Ra treatment response 2,3,5, previous studies suggested that 18F-FCH PET/CT could be a good imaging marker for prostate cancer cell proliferation 13,14. Several 18F-FCH PET/CT-derived metabolic parameters were previously shown to correlate with disease progression and overall survival 11,12,15 in CRPC patients undergoing various systemic therapies (chemotherapy, antiandrogen therapy, spiuleucel T, 223Ra). Segmentation algorithms were developed to delineate the metabolic volume corresponding to prostate cancer lesions 11,15,16 to correlate tumor burden to survival. In this pilot study, our aim is to assess the relevance of 18F-FCH PET-derived imaging parameters to predict hematological toxicity induced by 223Ra therapy with implications in treatment optimization and patient selection.
Patients and methods
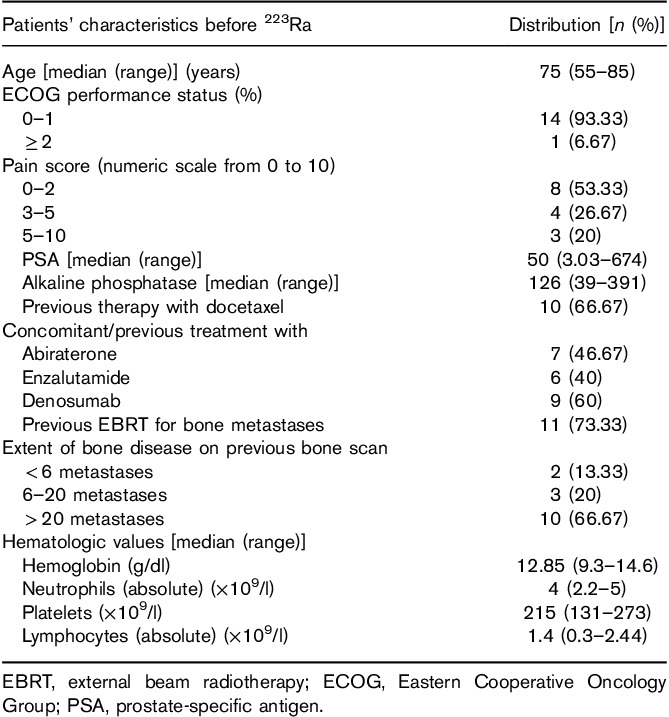
We prospectively analyzed 18F-FCH PET/CT from 15 patients, with symptomatic bone metastatic castration-resistant prostate cancer and no evidence of visceral metastases on 18F-FCH PET/CT, treated with 223Ra therapy. All patients had documented disease progression (on the basis of PSA kinetics and radiological criteria) before the initiation of 223Ra therapy. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Each patient received at least two injections of 55 kBq/kg 223Ra (Xofigo; Bayer HealthCare Pharmaceuticals Inc., Bayer santé, Lyon, France), administered at 4-week intervals for a maximum of six intravenous injections, at the Division of Nuclear Medicine, Department of Radiology, University Institute of Cancer from Toulouse-Oncopole (IUCT-Oncopole), between September 2014 and February 2017. The indication for 223Ra therapy was validated for each patient by the IUCT-Oncopole Prostate Cancer Tumor Board. 18F-FCH PET/CT before 223Ra therapy is a standard practice in our institution to rule out lymph node or visceral metastases. All patients treated and having performed PET/CT exams in our Department signed consent statements as requested by the ethical standards of the IUCT-Oncopole.
Symptomatic disease was defined as the regular use of nonopioid or opioid analgesic medication or treatment with external beam radiation therapy within the previous 12 weeks for cancer-related bone pain. Patient follow-up generally consisted of a monthly assessment of pain (numeric scale 0–10), opiate medication consumption, and laboratory assessments including hematologic status [hemoglobin (Hb), platelet (PLT) and lymphocyte counts], ALP, and PSA before every cycle (details on previous treatments and biology pre-223Ra are shown in Table 1).
Table 1.
Patient characteristics before 223Ra treatment (N=15)
18F-FCH PET/CT imaging
Patients refrained from eating and drinking for 3–4 h before undergoing PET/CT. Imaging was performed using GE Healtcare Discovery IQ PET/CT (GE Healthcare, 2014). The CT scanning parameters were as follows: 120 kV, maximum 190 mA/slice, rotation time of 0.5 s, slice thickness and interval of 2.5 mm, and pitch of 1.375. Patients were injected with 1.9 MBq/kg 18F-FCH and a computed tomography (CT) scan and sequential emission PET were obtained from the skull to the mid-thighs using 2 min acquisition per bed position 45 min after 18F-FCH injection. Images were reconstructed using the vendor-supplied maximum-likelihood expectation maximization algorithm, VPHD-S, with CT-driven attenuation correction 17.
Bone segmentation and PET quantifications
Image processing was performed using the Beth Israel PET/CT viewer plugin for Fiji (Fiji Is Just ImageJ) 18. This software is developed as a free and open-source PET/CT viewer for research purpose (http://petctviewer.org) 19,20. On the basis of Fiji/ImageJ, advanced image processing is allowed because of ImageJ services and plugins.
To calculate the metabolic bone tumor volume (MBTV), bone tumor volume was assessed on CT images, which were presegmented using Weka Trainable 21 segmentation on the basis of a machine learning algorithm. The previous dataset of 10 learning patients was used to feed the machine learning algorithm by manual delineation of bone tissue using a patient’s CT with no cancer disease. The bone classifier generator was applied to the CT series of the 18F-FCH PET/CT. All bone segmentations were controlled visually by a physician. The CT series were modified according to the bone segmentation; all nonsegmented voxels were assigned to a −1000 HU density.
To calculate MBTV, the PET/CT viewer handled image fusion and selected voxels with a double PET and CT value condition.
Bone metastatic disease was quantified on the basis of the standardized uptake value (SUV) representing the measured voxel activity divided by the injected radioactivity normalized by body weight. Bone lesions were defined as skeletal structures with a cutoff of SUV of at least 3 15,16 to select significant tumor uptake on the whole skeleton. MBTV for the whole skeleton was computed using the bone-segmented CT transposed to the PET volume; only voxels with an SUV of at least 3 and corresponding to a CT value more than −1000 (i.e selected during bone segmentation) were included in the final MBTV value (Fig. 1).
Fig. 1.
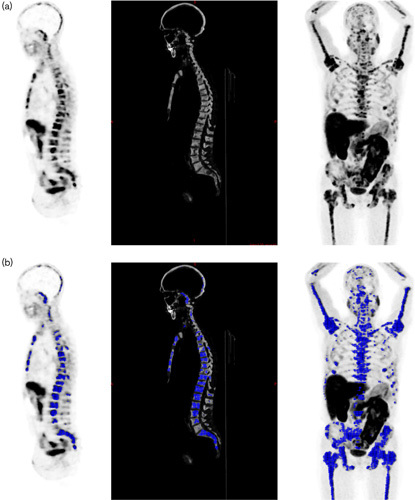
Quantification of tumoral bone burden in a prostate cancer patient with disseminated bone metastases: (a) bone volume measurement on whole-body computed tomography (CT) extrapolated on PET [sagittal view PET, right; sagittal view bone CT, center; maximum intensity projection (MIP), left]; (b) metabolic bone tumor volume measurement on PET with standardized uptake value>3, selected regions upon bone segmentation in blue: sagittal view PET on the right, sagittal view bone CT in the center, MIP on the left side.
The activity distribution within the volume of interest [total lesion activity (TLA)] was also computed as the product of SUVmean of the whole skeleton volume of interest and MBTV. MBTV and TLA were also normalized for patient height in m (MBTV/H, TLA/H).
Biological parameters
Hematologic toxicity was assessed following the Common Terminology Criteria for Adverse Events (CTCAE v.4.03). Evaluation was performed at baseline and before each dose of 223Ra. If the total leukocyte or PLT counts did not return to the normal range within six weeks of the last 223Ra administration, despite proper standard clinical management, we carried out new imaging and biology studies, and clinical and disease status were reassessed to carefully decide the premature end of treatment.
In patients with grade 3–4 toxicity, the next 223Ra treatment was postponed until hematological status returned to normal or to grade 1 hematological toxicity. Patients with grade 3–4 toxicities on Hb or Plt had red blood cell or platelets transfusions.
Statistics
Continuous variables are given as median and range and categorical variables as percentages. For pain evaluation, we used a standard numeric pain score (each patient was scored using a 10-point scale). Bone metastatic disease and 18F-FCH PET/CT bone tumor burden and activity were analyzed using comparisons of means analysis of variance to identify which parameters were predictive of hematological toxicity (on Hb, PLTs, and lymphocytes) at the end of radionuclidic bone therapy. Pearson’s correlation coefficient was used to identify the correlations between age, PSA, and 18F-FCH PET parameters.
Results
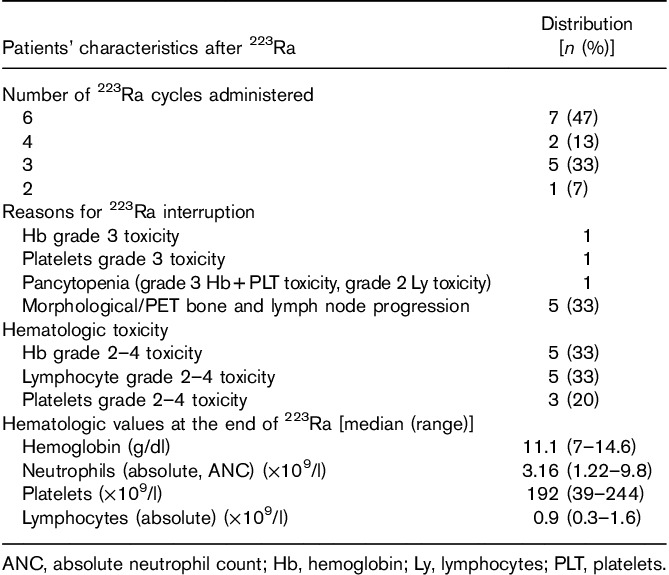
The distribution of patient characteristics assessed during the study is shown in Table 1. The majority of patients [10 (67%)] were treated with 223Ra after docetaxel chemotherapy. Abiraterone or enzalutamide was administered before 223Ra in seven (47%) and six (40%) of the patients respectively, whereas two patients received 223Ra and abiraterone concomitantly. Nine (60%) patients were treated concomitantly with a RANK ligand agonist as a bone-targeting agent. The median number of 223Ra doses administered was four (range: 2–6), for a total of cycles administered of 56. Only seven (47%) of 15 patients completed the planned six injections. Early treatment discontinuation occurred in eight patients because of disease progression [bone and LN progression in five (33%) patients] and because of hematologic toxicity [grade 2–4 anemia or thrombocytopenia in three (20%) patients] (Table 2).
Table 2.
Patients’ hematological characteristics at the end/interruption of 223Ra treatment (N=15)
Hematologic toxicity rate
At the end of the therapy, five of the 15 (33%) of patients presented persistent grade 2–4 toxicity on Hb and lymphocytes, whereas three (20%) of 15 patients presented grade 2–4 PLT toxicity (Tables 2 and 3). There were no grade 3–4 toxicities on absolute neutrophil counts.
Table 3.
Patients’ clinical history and 18F-FCH PET characteristics related to hematologic toxicity on 223Ra treatment
Severe hematologic toxicity during treatment (grade 3–4) necessitating transfusions occurred in three (20%) of 15 patients: one patient had pancytopenia (grade 3 Hb and lymphocyte toxicity and grade 2 PLTs toxicity); one patient had combined grade 3 Hb toxicity and grade 2 lymphocyte toxicity; and one patient had grade 3 Hb and PLTs toxicity because of 223Ra therapy (Table 3). All patients with severe hematologic toxicity presented diffuse osteomedullary disease.
MBTV and TLA
Among the 18F-FCH PET parameters, MBTV ranged from 75 to 1259 cm3 (median: 393 cm3) and TLA ranged from 343 to 7198 cm3 (median : 1854 cm3) (Table 4).
Table 4.
18F-FCH PET/CT parameters characterizing metabolic bone tumor burden before 223Ra treatment
Age was correlated negatively with both MBTV (r=−0.612, P=0.015) and TLA (r=−0.596, P=0.018). Age, Gleason score, previous hormonal therapy, radiotherapy, and docetaxel therapy were associated with TLA (P<0.001). TLA and TLA/H, and MBTV/H predicted hematological toxicity on Hb, whereas TLA/H and MBTV/H predicted PLTs toxicity at the end of 223Ra cycles (Table 4). Receiver opearting characteristic curve analysis allowed to define cutoffs for MBTV (915 cm3) and TLA (4198 cm3) that would predict PLT toxicity with an accuracy of 0.92 and 0.99, respectively (Fig. 2). In terms of toxicities on Hb or lymphocytes, receiver operating characteristic curves did not allow to define cutoffs.
Fig. 2.
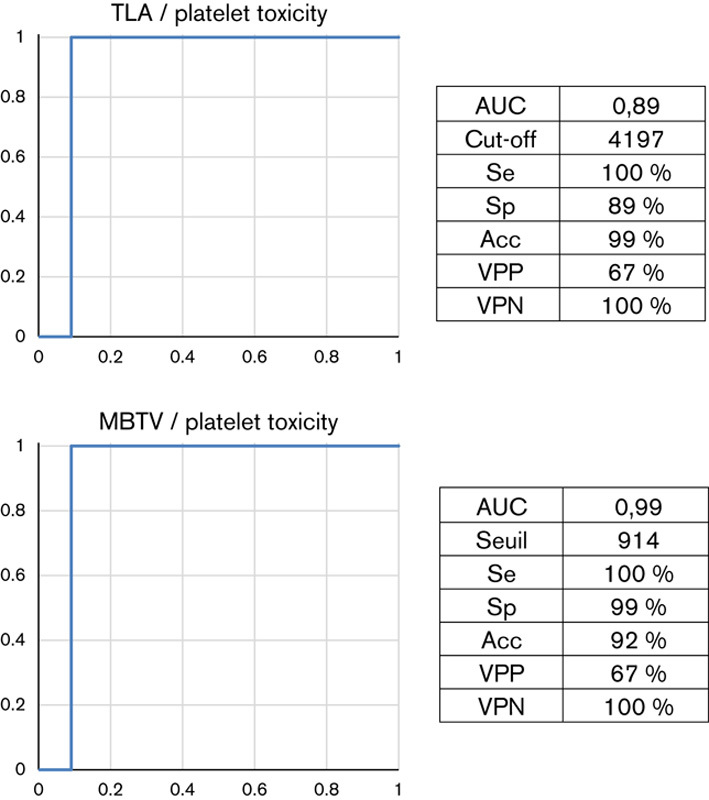
Receiver operating characteristic curves related to pretherapeutic metabolic 18F-FCH PET parameters (TLA and MBTV) and platelet toxicity at the end of 223Ra treatment. Acc, accuracy; AUC, area under the curve; 18F-FCH, fluorine-18-fluorocholine; MBTV, metabolically active bone tumor volume; Se, sensitivity; Sp, specificity; TLA, total bone lesion activity; VPN, negative predictive value; VPP, positive predictive value.
Discussion
223Ra therapy was shown to have a favorable therapeutic effect, good tolerance, and low toxicity, being the only bone-targeted drug associated with a survival benefit 2,3. Compared with β-emitters, the α-emitter 223Ra appears to have less toxic effects on the bone marrow, given its short-range emissions 22–24. It has been calculated that after six intravenous injections with 55 kBq/kg of 223Ra, the absorbed α dose to bone cells is about 16 Gy and the corresponding absorbed dose to the bone marrow is ~1.5 Gy 23, suggesting a very low risk of medullary toxicity. Indeed, 223Ra myelotoxicity is rare 1,25, with no cases of myelodysplastic syndrome, acute myelogenous leukemia, or aplastic anemia described in 2–3-year follow-up studies 4,6,26. Despite a better toxicity profile than β emitters, both anemia and thrombocytopenia have been reported with 223Ra in the ALSYMPCA trial, severe toxicities leading to early treatment interruption. Identification of predictive factors for hematologic toxicity may enable the selection of patients with a better benefit/risk balance for 223Ra therapy.
This study was designed to explore a segmentation methodology to assess bone tumoral infiltration using 18F-FCH PET/CT before 223Ra therapy and predict hematologic toxicity such as anemia and thrombocytopenia. In our study, we observed grade 3 hematologic toxicity in 20% of patients, with a high proportion of patients with grade 3 anemia or grade 3 thrombocytopenia in patients having received a previous treatment with docetaxel (30 and 10%), more frequently than in the ALSYMPCA trial (12 vs. 10% of patients with grade 3 anemia and 4 vs. 2% with grade 3 thrombocytopenia among patients treated previously with docetaxel vs. no previous use of docetaxel) 5.
Although anemia can be associated with abiraterone therapy, the two patients who received abiraterone concomitantly with 223Ra did not develop any hematological toxicity up to 6 months after the sixth and last injection of 223Ra.
This high toxicity rate led to frequent treatment interruption as less than half of the patients completed the six planned 223Ra administrations, and three (20%) patients specifically interrupted treatment because of hematological toxicity.
We found a significant association of the 18F-FCH PET parameter TLA with the occurrence of both anemia and thrombocytopenia during 223Ra treatment. We also identified a high predictive value for thrombocytopenia, which is more frequently associated with 223Ra therapy 3,6,7, whereas Hb and lymphocyte toxicity may be related more frequently to long-term consequences of previous radiotherapy and chemotherapy as reported previously 27,28.
In our pilot study, age, Gleason score, diffuse osteomedullary extension on bone scan, previous hormonal therapy, radiotherapy, and docetaxel therapy were associated with some 18F-FCH PET metabolic parameters, such as high TLA values in Kruskall–Wallis multiple comparisons, confirming that patients with advanced disease and multiple previous treatments presented more extensive bone disease.
Moreover, hematological toxicity such as grade 3 anemia observed in two patients was multifactorial as both had extensive progressive bone disease, previous chemotherapy with docetaxel and cabazitaxel, radiotherapy, and one of them had received treatment with 153Sm 3 years before 223Ra therapy. Both patients developed Hb toxicity as early as the end of the first cycle and had further documented medullary or lepto-meningeal extension.
Previous scoring systems related to quantification methods for bone tissue infiltration on planar bone scintigraphy using various automated systems rendering a bone scan index (BSI) as a percentage of the tumor burden on the total skeletal mass on the basis of identifying and quantifing hotspots as lesions on the basis of databases (e.g. EXINI bone 29,30, BONENAVI 31) enabled the prediction of overall survival after hormonal treatment 32 or docetaxel therapy 33 with decreasing BSI percentages. However, at present, there is only one study assessing BSI on initial bone scintigraphy assessment to predict hematologic toxicity before 223Ra 34.
Bone tumoral burden was also assessed on 18F-fluoride PET/CT using a volumetric semiautomatic quantification of whole-body skeletal tumor burden and was identified as an independent predictor of overall survival in 42 patients with CRPC treated with 223Ra 35. In a more recent publication, the same authors used their method of quantification on 18F-FCH PET/CT to assess bone marrow failure in 41 patients with metastatic prostate cancer after 223Ra therapy and identified that the assessment of total lesion burden was the single independent factor of bone marrow failure 36.
Compared with bone-targeting radiopharmaceuticals, 18F-FCH may provide a quantification of bone metastatic infiltration, enabling a direct estimation of tumoral cells mass, whereas bone scintigraphy and 18F-flouride approaches rely on indirect mechanisms of bone turnover.
To estimate the bone infiltration, we performed a double segmentation on 18F-FCH PET/CT: CT was first segmented to delineate bone tissue using a machine learning algorithm and then voxels were selected on the skeleton segmented PET with a cutoff of SUV of more than 3 as described previously by Kwee et al. 15 to isolate the bone tumoral infiltration from nonmetastatic bone. This double segmentation (bone tissue segmentation and SUV threshold) requires around 15 min of calculation time per patient and does not require any manual intervention. The full software package is freely available on http://petctviewer.org and is available for any operating system (Windows, Mac OSX, GNU/Linux).
This approach enables a direct quantification on 18F-FCH PET of the bone mass containing proliferating tumoral cells 13,14, using MBTV and TLA parameters, also normalized by height, which we found to be associated with the occurrence of hematologic toxicity. This may enable identification of patients for whom 223Ra therapy will be limited because of a high toxicity rate. Normalization by height of bone metabolic parameters might be interesting as 223Ra activity is prescribed as a function of the weight, but tumoral burden and volume is also dependent on a patient’s height.
As shown in our study, baseline 18F-FCH PET/CT appears to be suitable to predict hematological toxicity using quantification of bone tumoral infiltration, but can also provide additional information on disease staging before 223Ra therapy, enabling better patient selection, as patients with visceral extension are excluded. Moreover, recently published studies have shown interesting results related to the concordance between 18F-FCH PET/CT imaging, PSA, and ALP progression 11,12,15, suggesting a potential role for 18F-FCH PET/CT monitoring during treatment with an interesting economic approach, as it might help to avoid useless 223Ra treatment in patients with important bone tumor burden and hematologic toxicity predisposition aggravated by previous therapies.
Our pilot study showed the feasibility of bone tumoral burden quantification using an automatic method on the basis of a user-friendly open-source software. However, as our pilot study is limited by a low number of patients, further multicentric prospective studies with appropriate statistical evaluations of the number of patients to include would be needed to validate the prognostic power and the cutoff value of 18F-FCH PET MBTV and TLA parameters as tools for patient selection before 223Ra therapy.
Conclusion
The predictive value of pretherapeutic 18F-FCH PET/CT for Hb and PLT toxicity after 223Ra therapy may stem from the capacity to assess whole-body cellular tumor burden and the extent of osteomedullary infiltration. We showed for the first time the feasibility of measuring TLA and MBTV on 18F-FCH PET/CT using a new automatic, rapid, segmentation software. Further studies are necessary to assess pretherapeutic 18F-FCH PET-based metabolic markers (TLA, MBTV) predicting PLT and Hb toxicity that occurs during 223Ra therapy, with potential implications in patient selection and therapy optimization.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.EMA. European Medical Agency: Product Information and EPAR Authorized Presentations EUR MED Agency 2013;1–86.
- 2.Parker C, Sartor O. Radium-223 in prostate cancer. N Engl J Med 2013; 369:1659–1660. [DOI] [PubMed] [Google Scholar]
- 3.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369:213–223. [DOI] [PubMed] [Google Scholar]
- 4.Parker CC, Pascoe S, Chodacki A, O’Sullivan JM, Germa JR, O’Bryan-Tear CG, et al. A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol 2013; 63:189–197. [DOI] [PubMed] [Google Scholar]
- 5.Hoskin P, Sartor O, O’Sullivan JM, Johannessen DC, Helle SI, Logue J, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol 2014; 15:1397–1406. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson S, Larsen RH, Fossa SD, Balteskard L, Borch KW, Westlin JE, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res 2005; 11:4451–4459. [DOI] [PubMed] [Google Scholar]
- 7.Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 2014; 15:738–746. [DOI] [PubMed] [Google Scholar]
- 8.Vogelzang NJ, Coleman RE, Michalski JM, Nilsson S, O’Sullivan JM, Parker C, et al. Hematologic safety of radium-223 dichloride: baseline prognostic factors associated with myelosuppression in the ALSYMPCA Trial. Clin Genitourin Cancer 2017; 15:42.e8–52.e8. [DOI] [PubMed] [Google Scholar]
- 9.Beheshti M, Rezaee A, Geinitz H, Loidl W, Pirich C, Langsteger W. Evaluation of prostate cancer bone metastases with 18F-NaF and 18F-fluorocholine PET/CT. J Nucl Med 2016; 57:55s–60s. [DOI] [PubMed] [Google Scholar]
- 10.Colombie M, Campion L, Bailly C, Rusu D, Rousseau T, Mathieu C, et al. Prognostic value of metabolic parameters and clinical impact of (1)(8)F-fluorocholine PET/CT in biochemical recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015; 42:1784–1793. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki KS, Kuang Y, Kwee SA. Changes in skeletal tumor activity on (18)F-choline PET/CT in patients receiving (223)Radium radionuclide therapy for metastatic prostate cancer. Nucl Med Mol Imaging 2015; 49:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J, Sato MM, Coel MN, Lee KH, Kwee SA. Prediction of PSA progression in castration-resistant prostate cancer based on treatment-associated change in tumor burden quantified by 18F-fluorocholine PET/CT. J Nucl Med 2016; 57:1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picchio M, Mapelli P, Panebianco V, Castellucci P, Incerti E, Briganti A, et al. Imaging biomarkers in prostate cancer: role of PET/CT and MRI. Eur J Nucl Med Mol Imaging 2015; 42:644–655. [DOI] [PubMed] [Google Scholar]
- 14.Oprea-Lager DE, van Kanten MP, van Moorselaar RJ, van den Eertwegh AJ, van de Ven PM, Bijnsdorp IV, et al. [18F]fluoromethylcholine as a chemotherapy response read-out in prostate cancer cells. Mol Imaging Biol 2015; 17:319–327. [DOI] [PubMed] [Google Scholar]
- 15.Kwee SA, Lim J, Watanabe A, Kromer-Baker K, Coel MN. Prognosis related to metastatic burden measured by (1)(8)F-fluorocholine PET/CT in castration-resistant prostate cancer. J Nucl Med 2014; 55:905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reske SN, Blumstein NM, Neumaier B, Gottfried HW, Finsterbusch F, Kocot D, et al. Imaging prostate cancer with 11C-choline PET/CT. J Nucl Med 2006; 47:1249–1254. [PubMed] [Google Scholar]
- 17.Reynes-Llompart G, Gamez-Cenzano C, Romero-Zayas I, Rodriguez-Bel L, Vercher-Conejero JL, Marti-Climent JM. Performance characteristics of the whole-body discovery IQ PET/CT system. J Nucl Med 2017. [DOI] [PubMed] [Google Scholar]
- 18.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012; 9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanoun S, Tal I, Berriolo-Riedinger A, Rossi C, Riedinger JM, Vrigneaud JM, et al. Influence of software tool and methodological aspects of total metabolic tumor volume calculation on baseline [18F]FDG PET to predict survival in Hodgkin lymphoma. PLoS One 2015; 10:e0140830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arganda-Carreras I, Kaynig V, Rueden C, Eliceiri KW, Schindelin J, Cardona A, et al. Trainable Weka segmentation: a machine learning tool for microscopy pixel classification. Bioinformatics 2017; 33:2424–2426. [DOI] [PubMed] [Google Scholar]
- 22.Bruland OS, Nilsson S, Fisher DR, Larsen RH. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res 2006; 12:6250s–6257s. [DOI] [PubMed] [Google Scholar]
- 23.Lassmann M, Nosske D. Dosimetry of 223Ra-chloride: dose to normal organs and tissues. Eur J Nucl Med Mol Imaging 2013; 40:207–212. [DOI] [PubMed] [Google Scholar]
- 24.Hobbs RF, Song H, Watchman CJ, Bolch WE, Aksnes AK, Ramdahl T, et al. A bone marrow toxicity model for (2)(2)(3)Ra alpha-emitter radiopharmaceutical therapy. Phys Med Biol 2012; 57:3207–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xofigo (Radium Ra-223 Dichloride) Injection [Prescribing Information]. EMA 2013 assessment study. Wayne, NJ: Bayer HealthCare Pharmaceuticals Inc; 2013. [Google Scholar]
- 26.Nilsson S. Radionuclide therapies in prostate cancer: integrating radium-223 in the treatment of patients with metastatic castration-resistant prostate cancer. Curr Oncol Rep 2016; 18:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saad F, Carles J, Gillessen S, Heidenreich A, Heinrich D, Gratt J, et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol 2016; 17:1306–1316. [DOI] [PubMed] [Google Scholar]
- 28.Gillessen S, Omlin A, Attard G, de Bono JS, Efstathiou E, Fizazi K, et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC). Ann Oncol 2015; 26:1589–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anand A, Morris MJ, Kaboteh R, Bath L, Sadik M, Gjertsson P, et al. Analytic validation of the automated bone scan index as an imaging biomarker to standardize quantitative changes in bone scans of patients with metastatic prostate cancer. J Nucl Med 2016; 57:41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadik M, Suurkula M, Hoglund P, Jarund A, Edenbrandt L. Improved classifications of planar whole-body bone scans using a computer-assisted diagnosis system: a multicenter, multiple-reader, multiple-case study. J Nucl Med 2009; 50:368–375. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima K, Nakajima Y, Horikoshi H, Ueno M, Wakabayashi H, Shiga T, et al. Enhanced diagnostic accuracy for quantitative bone scan using an artificial neural network system: a Japanese multi-center database project. EJNMMI Res 2013; 3:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsui Y, Shiina H, Yamamoto Y, Haramoto M, Arichi N, Yasumoto H, et al. Prediction of survival benefit using an automated bone scan index in patients with castration-resistant prostate cancer. BJU Int 2012; 110:E628–E634. [DOI] [PubMed] [Google Scholar]
- 33.Kaboteh R, Gjertsson P, Leek H, Lomsky M, Ohlsson M, Sjostrand K, et al. Progression of bone metastases in patients with prostate cancer – automated detection of new lesions and calculation of bone scan index. EJNMMI Res 2013; 3:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fosbol MO, Petersen PM, Kjaer A, Mortensen J. Radium-223 therapy of advanced metastatic castration-resistant prostate cancer: quantitative assessment of skeletal tumor burden for prognostication of clinical outcome and hematological toxicity. J Nucl Med 2017. [DOI] [PubMed] [Google Scholar]
- 35.Etchebehere EC, Araujo JC, Fox PS, Swanston NM, Macapinlac HA, Rohren EM. Prognostic factors in patients treated with 223Ra: the role of skeletal tumor burden on baseline 18F-fluoride PET/CT in predicting overall survival. J Nucl Med 2015; 56:1177–1184. [DOI] [PubMed] [Google Scholar]
- 36.Etchebehere EC, Araujo JC, Milton DR, Erwin WD, Wendt RE, Swanston NM, et al. Skeletal tumor burden on baseline 18F‐fluoride PET/CT predicts bone marrow failure after 223Ra therapy. Clin Nucl Med 2016; 41:268–273. [DOI] [PubMed] [Google Scholar]