Abstract
Background
Human dental pulp is a highly vascular tissue that has an highly regenerative capacity due to its unique blood supply and the presence of progenitor or postnatal dental pulp stem cells. We aimed to isolate and compare the angiogenic characteristics of mouse endothelial cells prepared from dental pulp and periodontal ligament (PDL).
Methods
Pulp endothelial cells (EC) were isolated from 4-week-old wild type immorto mice. Mice were sacrificed and after mandible isolation, the molar and incisor teeth and the PDL of molar teeth were dissected. EC were prepared by collagenase digestion of tissues and affinity purification using magnetic beads coated with platelet/endothelial cell adhesion molecule-1 antibody. EC were isolated from dental pulp of incisors and molars, and PDL. Cells were characterized for expression of appropriate markers by fluorescence-activated cell sorting (FACS) analysis. Cell proliferation, migration, and capillary morphogenesis of EC were also evaluated. Ex vivo sprouting from various tissues was also assessed. Data were analyzed at the level of significance of P < 0.05.
Results
Incisor EC showed significantly more proliferation and migration compared with molar and PDL EC (P < 0.05). In addition, Molar and PDL EC formed a more extensive capillary network when plated on Matrigel. This is consistent with the normal proliferative and migratory characteristics of these cells compared to incisor EC (P < 0.05). PDL tissue showed significantly more sprouting area than molar and incisor pulp tissues (P < 0.05).
Conclusions
Mouse EC isolated from PDL, and incisor and molar pulp presented different angiogenic properties. The result of current study suggests that EC from different tooth tissues have unique characteristics related to their target tissue and function.
Keywords: Angiogenesis, Endothelial cells, Incisor, Molar, Regeneration
Introduction
Endothelial cells (EC) line the surface of whole vascular network starting from heart to the smallest capillaries, which control the passage of white blood cells and different substances into or out of the blood vessels. Besides this primary role as a permeable barrier for blood vessels, EC perform critical functions including regulation of hemostasis, the coagulation cascade, vascular tone, and immunity (Aird, 2007; Asahara et al., 1997; Saghiri et al., 2015a). Moreover, EC play a critical role in tissue regeneration through the process of angiogenesis. Angiogenesis is a multistep process involving the growth of new blood vessels from pre-existing capillaries, and is tightly regulated by the production of various angiogenic regulatory factors (Folkman, 1984; Risau, 1997). Some of these factors are released by the surrounding tissues in response to ischemia that activate nearby EC and initiate the process. This process includes degradation of extracellular matrix, proliferation and migration, and ultimately establishment of tubular structures (Faller, 1999).
Human dental pulp is a highly vascular tissue that has an highly regenerative capacity due to its unique blood supply and the presence of progenitor or postnatal dental pulp stem cells (DPSC) (Gronthos et al., 2000; Nakashima et al., 2009; Saghiri et al., 2015b). DPSC are recruited and differentiate into odontoblast-like cells to produce reparative dentin (Saghiri et al., 2015c). Vascularization plays a key role in determining whether injured pulp tissue survives or undergoes necrosis (Mantellini et al., 2006). In addition to participation in sprouting angiogenesis, it was demonstrated that EC may have a synergistic effect on DPSC. The co-culture of DPSC and EC enhanced osteo-/odontogenic properties of DPSC on one hand, and promoted the establishment and longevity of pre-existing blood vessel-like structures formed by EC on the other (Dissanayaka et al., 2012). Thus, the dental pulp EC and vasculature play a pivotal role in survival and regeneration of dental pulp after injuries.
The periodontium is defined as the tooth supporting structures including root cementum, periodontal ligament (PDL), alveolar bone, and the dentogingival junction. PDL is the soft and specialized connective tissue in periodontium, which contains well-defined and oriented collagen fiber bundles connecting the root cementum to the alveolar bone. PDL has several cells such as fibroblasts, cementoblasts, odontoclasts, osteoblasts, osteoclasts, undifferentiated mesenchymal cells, epithelial cell rests of Malassez, macrophages, and monocytes (Nanci and Bosshardt, 2006). In addition to these components, PDL has a complex and well-distributed vascular network derived from the alveolar bone and gingiva, which is oriented in two layers including the outer or peripheral and the inner layers. The outer layer consists of larger vessels, which play a great role in shock absorbing system, while the inner layer contains the capillary vessels that supply PDL structures with oxygen and nutrients (Masset et al., 2006). Besides the crucial functions of vascular networks, the EC of PDL vessels were shown to play an important role in proliferation of PDL and gingival fibroblasts (Jin and Yuan-zheng, 2013). It was also demonstrated that EC from PDL have more permeability compared to EC from other tissues (Maruyama and Sato, 2016). Thus, PDL EC cooperate with other cells in maintaining function and regeneration of PDL.
Based on the importance of EC in dental pulp and PDL regeneration, and the information gap regarding the properties of these cells, the present study aimed to evaluate and compare the characteristics of EC prepared from mice PDL, dental pulp of incisors, and molar teeth. We determined whether EC from the different tooth tissues had different properties, which may contribute to their angiogenic potential during regenerative and reparative procedures. These studies will advance our knowledge regarding the regulatory tissue specific function of these EC. This knowledge could aid in the development of new and effective therapies for regenerative and reparative dentistry.
Material and Methods
Experimental animals
All the experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology statement for the use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and use Committee of the University of Wisconsin School of Medicine and Public health. Immorto mice expressing a temperature-sensitive simian virus (SV) 40 large T antigen were obtained from Charles Rivers Laboratories (Wilmington, MA) and backcrossed to a C57BL/6J background and maintained as previously described (Lawler et al., 1998; Su et al., 2003). The isolated DNA from tail biopsies was used for screening of various transgenes as previously described (Scheef et al., 2009; Su et al., 2003).
Isolation and culture of EC
EC were isolated form one litter (6 or 7 pups) of 4-week-old mice. Briefly, first molar and incisor teeth were harvested. The molar and incisor tooth were broken at the cement enamel junction (CEJ), and the pulp tissue were collected under a dissecting microscope. Periodontal ligament (PDL) tissues isolated from mandibular first molars of mice extracted from same pups PDL tissues were separated from the root surface using a scalpel and were minced into the smallest size possible. All tissues were cut up using a razor blade and then digested in 5 mL of Collagenase type I (Worthington, Lakewood, NJ; 1 mg/mL in serum-free Dulbecco’s modified Eagle’s medium; DMEM) and incubated at 37°C for 40 min. (Supplementary Movie) The digested tissues were washed with DMEM containing 10% fetal bovine serum (FBS), and centrifuged. The pellet was re-suspended in 10 mL of DMEM with 10% FBS and passed through a nylon mesh with a pore size of 70 μm. The cells were pelleted and re-suspended in 1 mL of DMEM with 10% FBS and mixed with magnetic beads coated with PECAM-1 antibody prepared as previously described (Su et al., 2003; Zerfaoui et al., 2008). The mixture allowed to rock at 4°C for 1 h. Following incubation, the beads were collected using a magnet and washed six times with 1 mL of DMEM with 10% FBS. The beads were then re-suspended in 0.5 mL of EC growth medium as previously described (Su et al., 2003). The EC growth medium contained DMEM with 10% FBS, 2 mM L-glutamine, 2 mM sodium pyruvate, 20 mM HEPES, 1% nonessential amino acids, 100 μg/ml streptomycin, 100 U/ml penicillin, freshly added heparin at 55 U/ml (Sigma, St. Louis, MO), endothelial growth supplement 100 μg/ml (Sigma), and murine recombinant interferon-γ (R&D, Minneapolis, MN) at 44 units/ml.
Cells were maintained at 33°C with 5% CO2. Cells were progressively passed to larger plates, maintained, and propagated in 1% gelatin-coated 60 mm dishes. Three different isolations of EC were used in these studies and all cells were used prior to passage 15.
Fluorescence activated cell sorting (FACS) analysis
EC form 60-mm culture plates were rinsed with PBS containing 0.04% EDTA and incubated with 1.5 mL of Cell Dissociation Solution (Sigma, St. Louis, MO). Cells were then washed, collected from plates with DMEM containing 10% FBS, centrifuged, and blocked in 0.5 mL of Tris-buffered saline (TBS; 25 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.6) with 1% goat serum for 20 min on ice. Cells were then pelleted and incubated in 0.5 mL TBS with 1% BSA containing a specific primary antibody on ice for 30 min. The following antibodies were used: anti-VE-cadherin (Enzo Life Sciences; Farmingdale, NY), anti-PECAM-1 (BD Biosciences; San Jose, CA), and B4-lectin (Vector Laboratories; Burlingame, CA) at dilutions recommended by the supplier. Cells were then rinsed twice with TBS containing 1% BSA and incubated with appropriate FITC-conjugated secondary antibody (Pierce, Rockford, IL) prepared in TBS containing 1% BSA for 30 min on ice. Following incubation, cells were washed twice with TBS containing 1% BSA, re-suspended in 0.5 mL of TBS with 1% BSA and analyzed by a FACScan caliber flow cytometer (Becton Dickinson, Franklin Lakes, NJ). These experiments were repeated twice using two different isolations of EC with similar results.
Cell proliferation assay
Cell proliferation was assessed by counting the number of cells over a two weeks period. Cells (1×104) were plated in multiple sets of gelatin-coated 60-mm tissue culture plates, fed every other day for the duration of experiment. The number of cells was determined by counting every other day, on days not fed, in triplicates.
Scratch wound assay
Cells (1 × 106) were plated in 60-mm tissue culture dishes and allowed to reach confluence (1–2 days). Plates were wounded using a 1-mL micropipette tip, washed with growth medium twice to remove detached cells, and fed with growth medium containing 1 μmol/L 5-fluorouracil (Sigma) to block cell proliferation. Wound closure was monitored by phase microscopy at different time points (0, 24, 48 h) and images were captured in digital format. The migrated distance as percentage of total distance was determined for quantitative assessment of data as described previously (DiMaio and Sheibani, 2008).
Transwell migration assay
Cell migration was also determined using a transwell migration assay. Costar transwell inserts (8-μm pore size, 6.5-mm membrane, Lowell, MA) were coated with serum free DMEM containing fibronectin (2 μg/mL) on the bottom side at 4°C overnight. After washing with PBS, inserts were blocked in PBS containing 1% BSA for 1 h at room temperature and rinsed with PBS. Cells were trypsinized and re-suspended in serum-free medium, and 1 × 105 cells/0.1 mL was added to the top of inserts. The inserts were placed in a 24-well plate containing 0.5 mL of serum-free medium and incubated for 4 h at 33°C. Following incubation, cells were fixed with 2% paraformaldehyde for 10 min at room temperature stained with hematoxylin and eosin (H& E), and the inserts were mounted on a slide with the cell facing down. The number of cells migrated through the membrane was determined by counting 10 high-power fields (×200). Each experiment was done in triplicates and repeated with two different isolation of EC.
Capillary morphogenesis (Tubulogenesis) assay
Tissue culture plates (35 mm) were coated with 0.5 mL Matrigel (10 mg/mL, BD Biosciences) and incubated at 37°C for at least 30 min in order to allow the gel harden. The EC were removed by trypsin-EDTA, washed with DMEM containing 10% FBS. Cells (1.5×105 cells/mL) were re-suspended in serum free medium (2 mL) and applied to the Matrigel-coated plates. The plates were incubated at 37°C and photographed after 14 h using a Nikon microscope in a digital format. For quantitative assessment of the data, EC branches were skeletonized and the structures were convert to pixels similar to a previous study (Saghiri et al., 2013), the mean number of pixels was calculated for each image (×100).
Tissue sprouting assay
Tissues were dissected from euthanized 4-week-old molar, incisors, and PDL of molars. Freshly isolated tissues were transferred to cold serum-free DMEM and washed three times. Adipose tissues were carefully removed with microdissecting forceps and iridectomy scissors, and pulp pieces (0.5 ± 0.2 mm long) were sectioned. The pulp pieces were embedded on 0.3 ml of Matrigel gel-coated wells of a 12-well tissue culture plate (Fisher) and 0.5 ml of DMEM containing 1% serum, 100 of μg streptomycin, and 100 U/ml penicillin was applied for 2 days at 37°C. The medium was then replaced with EC growth medium, and the sprouting response of cultures was photographed after 4 days and quantified similar to a past study with ImageJ (Saghiri et al., 2013). For quantitative assessment of sprouting, the areas of sprouting per millimeter of tissue edge were assessed with the Image J software (National Institutes of Health).
Statistical differences between control and treated samples were evaluated using Graphpad Prism software (La Jolla, CA) according to the Tukey Multiple Comparison test with a P value <0.05 considered significant.
Results
FACS analysis
EC were isolated from pulp and PDL tissue of molars and incisors of mice as described in methods. To demonstrate that these cells are EC we examined the expression of various EC markers including PECAM-1/CD31, VE-cadherin, and B4-lectin by FACS analysis. All EC expressed significant amounts of these markers. We observed a modest increase in the level of PECAM-1 expressed in the incisor EC compared with EC from the PDL and molar teeth (Fig. 1). The purity of cultures was confirmed by immunofluorescence staining and was greater than 98% (not shown). These cells also exhibited a similar morphology as shown in the phase micrograph (Fig. 1).
Figure 1.
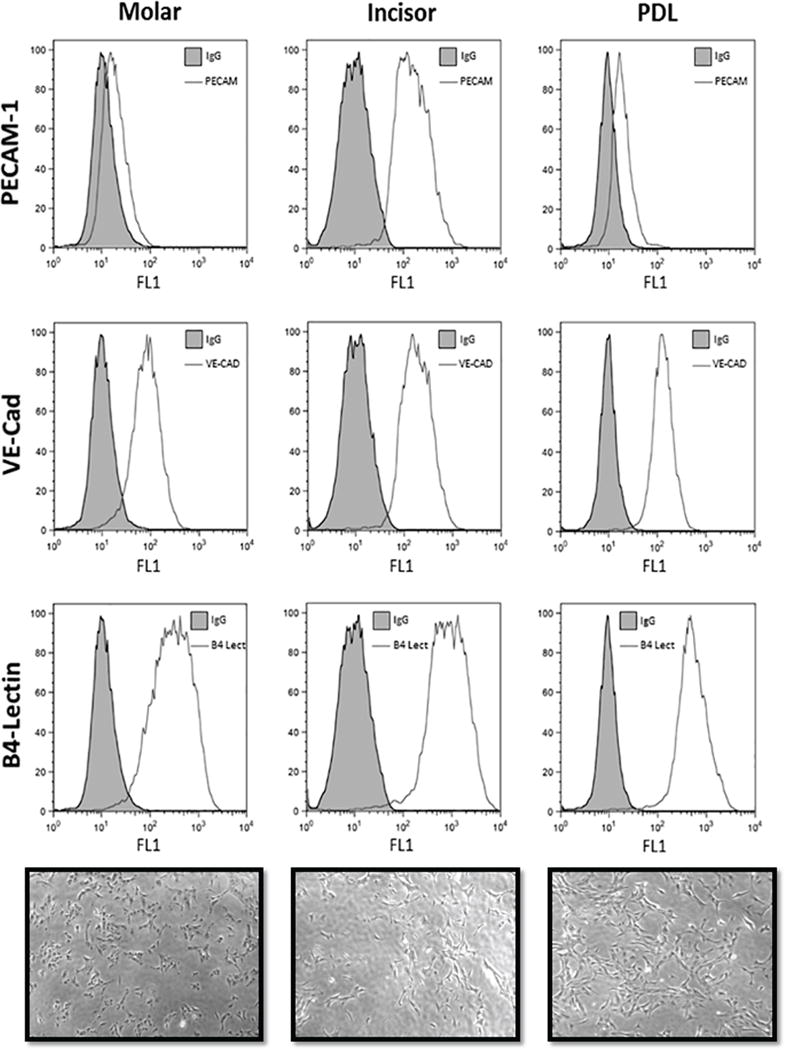
The graph of FACS analysis of the experimental groups demonstrating the PECAM-1, VE-Cad, B4-Lectin expression in ECs isolated from PDL, incisor and molar teeth. An increased PECAM-1 expression was noticed in the EC isolated from incisors in compared with EC isolated from molar or PDL.
Proliferation assay
All three EC types demonstrated an increasing cell number with time (P=0.0001) (Figure 2). In addition, all incisor EC demonstrated a statistically different mean rate of cell proliferation compared with other EC types, with incisor EC, molar EC, and PDL EC having mean rates of 62.18, 40.86, and 40.60, respectively (P=0.0001). However, there was no significant difference between PDL EC and molar EC proliferation (Figure 2).
Figure 2.
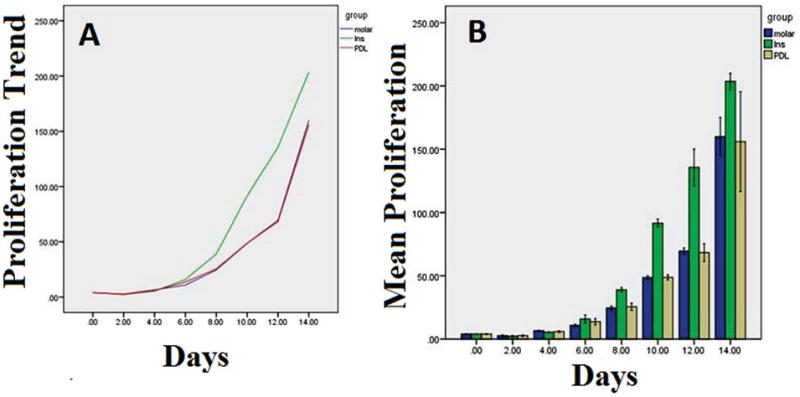
A: The trend of the proliferation rate of the ECs isolated from PDL, incisor (Ins) and molar teeth. B: The box plot of the means and standard deviations of the proliferation rate of the ECs isolated from PDL, incisor (Ins) and molar teeth.
Transwell migration assay
The scratch wound assays were used to assess the migration of EC over 48 hours of culture. Mean % wound closure of PDL and incisor EC was 45.05 and 61.19, respectively. The 95% confidence interval (CI) regarding to the mean difference was −26.30 to −5.98. The CI demonstrates that the difference between incisor and PDL EC in mean % wound closure was statistically significant (P<0.01). Similarly, the mean % wound closure for incisor EC was statistically higher than molar EC (mean=40.24) (P<0.001). The 95% CI related to mean difference between incisor and molar EC were (−31.10 to −10.79), not containing zero. Thus, in spite of a modest difference between mean of molar and PDL EC migration, the difference was not significant (P>0.05). The related 95% CI was −5.36 to 14.96 (Fig. 3A). Similar results were observed using a transwell migration assay. The mean number of migrating incisor and molar EC was 21.8 and 8.8, respectively. The 95% confidence interval (CI) regarding the mean difference was 4.92 to 21.07, indicating that the difference between migrating incisor and molar EC was statistically significant (P<0.01). Similarly, the mean number of migrating incisor EC was statistically higher than migrating PDL EC (mean=9.4) (P<0.01), with the 95% confidence interval related to mean difference between incisor and PDL categories being 4.33 to 20.47. It should be noted that in spite of a modest difference between mean of molar and PDL EC, there was no significant difference (P> 0.05). The related 95% confidence interval was −8.67 to 7.47 (Fig. 3B).
Figure 3.
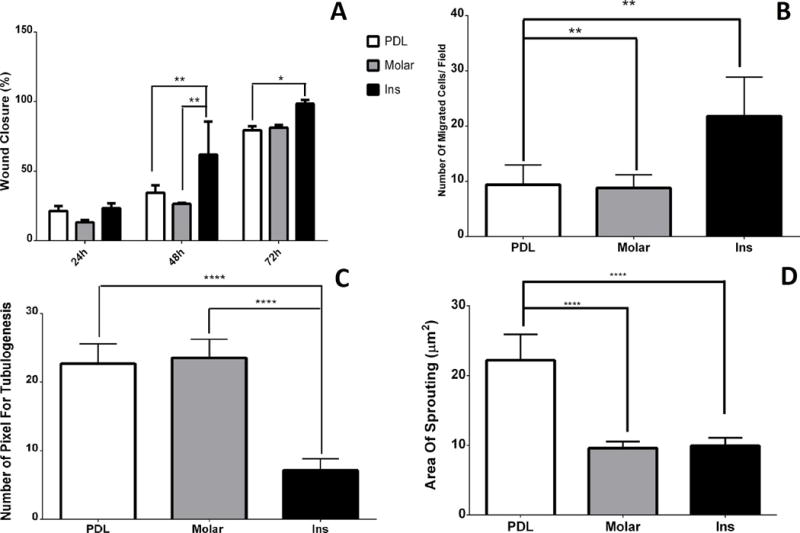
A) The box plots of the means and standard deviations of the percentage of wound closure assay of experimental groups during 24, 48, and 72 hours culture. (*): Significant difference was detected between values of incisor group with PDL group after 72 hours; (**): Significant differences were detected between values of incisor group with PDL and molar groups after 48 hours. B) The box plots of the means and standard deviations of the number of migrating ECs per field of experimental groups. (**): Significant differences were noticed between values incisor group with PDL and molar groups. C) The box plots of the means and standard deviations of the number of pixel of tubulogenesis of experimental groups. (****): Significant differences were indicated between tubulogenesis values of PDL and molar groups with incisor group. D) The box plots of the means and standard deviations of the area of sprouting (μm2) of experimental groups. (****): Significant differences were indicated between area of sprouting values of incisor and molar groups with PDL group.
Capillary morphogenesis (Tubulogenesis) assay
Capillary morphogenesis is a unique characteristic of EC where they organize into a capillary network when plated in Matrigel. The mean pixel density for incisor EC networks was 7.16 and statistically lower than PDL (mean=22.73) and Molar (mean=23.56) EC networks (for both P<0.0001). The 95% confidence intervals regarding to the former and latter comparisons were 11.39 to 19.74 and 16.40 to 20.57, respectively. However, there was no statistically significant difference between mean pixel densities for PDL and molar EC (P> 0.05). This is consistent with the 95% confidence interval (-5.006 to 3.34) (Fig. 3C).
Tissue sprouting assay
To assess whether the capillary morphogenesis of various EC in culture was consistent with their in vivo sprouting capacity, we next determined the degree of sprouting angiogenesis by excised PDL, incisor and molar tissue samples. We observed a significant difference in mean sprouting area for PDL tissue (mean = 22.2) compared with molar (mean = 9.58) and incisor (mean = 9.94) pulp tissues (for both P<0.0001). Though there was a modest difference between incisor and molar pulp tissues, this difference was not statistically significant (95% CI: −3.54 to 4.26; P>0.05). The 95% CI for comparing mean area of sprouting between PDL and molar was −16.52 to −8.72, and −16.16 to −8.36 for differences between incisor and PDL tissues (Fig. 3D).
Discussion
Mice incisors have continuous growth, while molars have limited growing patterns. Mice incisors represent an exceptional aspect of growth and development as the amelogenesis does not stop for the duration of a mouse’s life time. Hence, mice incisors have been widely used as a study model to evaluate hard and soft tissue development and regeneration in teeth (Goldberg et al., 2014). Here we aimed to evaluate and compare the angiogenic properties of EC prepared from incisor and molar teeth, and PDL. We successfully isolated and cultured these EC from mouse tissues, for the first time, and confirmed their phenotype using FACS analysis. The FACS analysis showed modest differences in expression of EC markers among different EC. The EC isolated from incisors showed increased PECAM-1 expression compared with EC of molar and PDL (Fig. 1). This difference was consistent with other results where ECs of incisor showed significantly different characteristics than ECs of molar and PDL.
The results of EC proliferation, transwell migration, and scratch wound assays showed significant differences only between incisor EC with molar and PDL EC. This difference can be explained by the fact that incisor teeth in mice have exceptional and continuous growth pattern (Goldberg et al., 2014). In mice, the incisor teeth, unlike the molar teeth, have a turnover of about 35–45 days, and the normal eruption rate is about 2.8 mm per week in lower incisors and 2 mm per week in upper incisors (Goldberg et al., 2014). Based on the fact that the proliferation, transwell migration, and scratch wound assays are mainly relevant to the production and regeneration of new EC, our results are consistent with the continuous growth pattern of incisor teeth. It seems that EC in mice incisor teeth, due to continuous renewal and growth of these teeth, have more turnover and proliferation rate compared to the ECs of molar and PDL, which showed slower growth pattern.
With respect to these outcomes, EC isolated from PDL and molar teeth showed a greater potential for sprouting and tubulogenesis than incisor EC. Tubulogenesis is a crucial process in angiogenesis and vasculogenesis, which begins with release of vascular endothelial growth factor-A (VEGF-A). VEGF-A signaling induces an increase in expression of Delta-like 4 (DLL4), which is a transmembrane protein and Notch ligand. The cells that express the higher levels of DLL4 become the tip cells, which express the sprouting phenotype. Meanwhile, the tip cells induce the up-regulation of Notch signaling in neighboring cells in order to inhibit the expression of VEGF receptors and these remain as the stalk cells positive for Jagged-1 (Eilken and Adams, 2010). The tip cell is a non-proliferative cell, which develops filopodia that enable these cells to undergo chemotaxis toward angiogenic factors. However, the stalk cells are highly proliferative cells that contribute to the elongation of sprout and finally lumenized to form the functional blood vessel (Tung et al., 2012). This description shows that the sprouting or tubulogenesis process although is related to proliferation and migration of EC, but it is controlled by different mechanisms in angiogenesis events. This fact can explain the results gained in this study as the EC isolated from PDL and molar teeth showed lower proliferative and migratory values, but in tubulogenesis they excel the incisor EC. The limitation of present study include the mechanism of action and signaling pathways that will shed light on differences of angiogenic phenotype between ECs isolated from PDL, incisor molar teeth. In addition, similar studies can be designed and performed in case of human dental pulp and PDL tissues. Understanding the mechanisms responsible for pro-proliferative and pro-migratory phenotype of incisors may provide new clues in terms of tooth regenerative strategies.
Conclusions
Based on outcomes of this study the following conclusions can be drawn:
ECs isolated from incisors present superior angiogenic properties of proliferation and migration. ECs isolated from mice PDL and molar teeth form extensive EC networks, which is consistent with their normal migratory and proliferative activity.
The angiogenic characteristics of dental pulp EC in mice are tissue specific and are regulated by local environmental clues, and are consistent with tissues need and function.
Supplementary Material
Acknowledgments
The authors express special thanks to Regenerative Group of Dr. H Afsar Lajevardi Research Cluster. NS is supported by an unrestricted award from Research to Prevent Blindness to the Department of Ophthalmology, Retina Research Foundation, P30 EY016665, P30 CA014520, EPA 83573701, and EY022883. CMS is supported by RRF Daniel M. Albert Chair. This publication is dedicated to the memory of Dr. H Afsar Lajevardi, a legendry Pediatrician (1953–2015).
Footnotes
Authors’ contributions:
MAS: designed and conducted experiment, analyzed/interpreted data, wrote and proofed/revised the manuscript. AA: designed experiment, analyzed/interpreted data, wrote and proofed/revised the manuscript. CMS: designed and conducted experiments, analyzed/interpreted data, wrote and proofed/revised the manuscript. NS: designed experiment, analyzed/interpreted data, wrote and proofed/revised the manuscript.
Competing financial interests: The authors declare no competing financial interests.
Supplementary Materials. The video of method used for isolation and culture of EC.
References
- Aird WC. Phenotypic heterogeneity of the endothelium I. Structure, function, and mechanisms. Circulation Research. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- DiMaio TA, Sheibani N. PECAM-1 isoform-specific functions in PECAM-1-deficient brain microvascular endothelial cells. Microvascular Research. 2008;75:188–201. doi: 10.1016/j.mvr.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayaka WL, Zhan X, Zhang C, Hargreaves KM, Jin L, Tong EH. Coculture of dental pulp stem cells with endothelial cells enhances osteo-/odontogenic and angiogenic potential in vitro. Journal of Endodontics. 2012;38:454–463. doi: 10.1016/j.joen.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Current Opinion in Cell Biology. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Faller DV. Endothelial cell responses to hypoxic stress. Clinical and Experimental Pharmacology and Physiology. 1999;26:74–84. doi: 10.1046/j.1440-1681.1999.02992.x. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis, Biology of endothelial cells. Springer; 1984. pp. 412–428. [Google Scholar]
- Goldberg M, Kellermann O, Dimitrova-Nakov S, Harichane Y, Baudry A. Comparative studies between mice molars and incisors are required to draw an overview of enamel structural complexity. Frontiers in Physiology. 2014;5:359. doi: 10.3389/fphys.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Yuan-zheng Y. The effect of vascular endothelial cells on the proliferation of periodontal ligament cells and gingival fibroblasts. Shanghai Journal of Stomatology. 2013;22 [PubMed] [Google Scholar]
- Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. Journal of Clinical Investigation. 1998;101:982. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantellini MG, Botero T, Yaman P, Dennison JB, Hanks CT, Nör JE. Adhesive resin and the hydrophilic monomer HEMA induce VEGF expression on dental pulp cells and macrophages. Dental Materials. 2006;22:434–440. doi: 10.1016/j.dental.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Sato S. Effect of high-glucose conditions on human periodontal ligament endothelial cells: in vitro analysis. Odontology. 2016:1–8. doi: 10.1007/s10266-016-0235-8. [DOI] [PubMed] [Google Scholar]
- Masset A, Staszyk C, Gasse H. The blood vessel system in the periodontal ligament of the equine cheek teeth–Part I: The spatial arrangement in layers. Annals of Anatomy-Anatomischer Anzeiger. 2006;188:529–533. doi: 10.1016/j.aanat.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Iohara K, Sugiyama M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine and Growth Factor Reviews. 2009;20:435–440. doi: 10.1016/j.cytogfr.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Nanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontology. 2006;2000(40):11–28. doi: 10.1111/j.1600-0757.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Saghiri MA, Asatourian A, Orangi J, Sorenson CM, Sheibani N. Functional role of inorganic trace elements in angiogenesis—Part I: N, Fe, Se, P, Au, and Ca. Critical Reviews in Oncology/Hematology. 2015a;96:129–142. doi: 10.1016/j.critrevonc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Saghiri MA, Asatourian A, Sheibani N. Angiogenesis in regenerative dentistry. Oral surgery, oral medicine, oral pathology and oral radiology. 2015b;119:122. doi: 10.1016/j.oooo.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghiri MA, Asatourian A, Sorenson CM, Sheibani N. Role of Angiogenesis in Endodontics: Contributions of Stem Cells and Proangiogenic and Antiangiogenic Factors to Dental Pulp Regeneration. Journal of Endodontics. 2015c doi: 10.1016/j.joen.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghiri MA, García-Godoy F, Asgar K, Lotfi M. The effect of Morinda Citrifolia juice as an endodontic irrigant on smear layer and microhardness of root canal dentin. Oral Science International. 2013;10:53–57. [Google Scholar]
- Scheef EA, Sorenson CM, Sheibani N. Attenuation of proliferation and migration of retinal pericytes in the absence of thrombospondin-1. American Journal of Physiology-Cell Physiology. 2009;296:C724–C734. doi: 10.1152/ajpcell.00409.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Molecular Vision. 2003;9:171–178. [PubMed] [Google Scholar]
- Tung JJ, Tattersall IW, Kitajewski J. Tips, stalks, tubes: notch-mediated cell fate determination and mechanisms of tubulogenesis during angiogenesis. Cold Spring Harbor Perspectives in Medicine. 2012;2:a006601. doi: 10.1101/cshperspect.a006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerfaoui M, Suzuki Y, Naura AS, Hans CP, Nichols C, Boulares AH. Nuclear translocation of p65 NF-κB is sufficient for VCAM-1, but not ICAM-1, expression in TNF-stimulated smooth muscle cells: Differential requirement for PARP-1 expression and interaction. Cellular Signalling. 2008;20:186–194. doi: 10.1016/j.cellsig.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


