Abstract
Objective
This study tested the mechanism of the oxidative stress (OS)-induced senescence pathway at the feto-maternal interface cells.
Methods
Primary amnion mesenchymal cells (AMCs), chorion and decidual cells isolated from the placental membranes of women at normal term (not in labor) were exposed to OS-inducing cigarette smoke extract (CSE) for 48 hours. Reactive oxygen species (ROS) was measured using 2′7′-dichlorodihydrofluorescein. Western blot analysis determined phosphorylated (P) p38MAPK and p53 expression. Senescence-associated β-Galactosidase (SA-β-Gal) and matrix metallopeptidase 9 (MMP9) histochemistry were used to measure senescence and inflammation respectively. Cotreatment of cells with the antioxidant, N-acetyl cysteine (NAC), or the p38MAPK inhibitor, SB203580 (SB), verified the activation specificity.
Results
CSE increased ROS production from AMCs, chorion cells, and decidual cells (P < 0.05) compared to controls. Western blot analysis determined that CSE induced p38MAPK activation (P < 0.05) and cotreatment with NAC inhibited ROS production and p38MAPK activation (P < 0.05) in all cell types. CSE did not increase p53 phosphorylation in any of the cells; however, AMCs showed constitutive P-p53 expression. CSE increased senescence in AMCs and chorion cells compared to controls (P = 0.01 and P = 0.003, respectively); however, senescence was not observed in decidual cells. Senescence was significantly reduced following cotreatment with SB and NAC (AMCs; P = 0.01 and chorion; P = 0.009). CSE increased MMP9 in all cells that was reduced by NAC.
Conclusion
OS induced p38MAPK activation and inflammation in all cell types that was associated with senescence in fetal cells but not in maternal cells.
Keywords: amnion mesenchymal cells, chorion, deciduas, oxidative stress, p38MAPK, senescence
Introduction
The maintenance of human pregnancy and subsequent labor and delivery of the fetus are effected by a variety of maternal and fetal endocrine and paracrine signaling pathways. Inflammatory mediators manifested by feto-maternal tissues throughout gestation are one of the effectors of labor and delivery. These inflammatory processes are intertwined with changes in the redox balance in the intrauterine tissues where oxygen requirements vary during each trimester [1–5]. Changes in the intrauterine oxidative environment are designed to support the fetus and fetal tissue growth, as reactive oxygen radicals are key signaling components of cell growth and tissue development [6]. Localized inflammation resulting from reactive oxygen species (ROS)-mediated signaling reactions allow tissue remodeling and growth. During pregnancy, the ROS-inflammatory axis works in tandem in a well-balanced system maintained by endogenous antioxidants and anti-inflammatory mediators [7]. Thus changes in ROS levels and inflammatory processes are well tolerated by feto-maternal tissues to maintain pregnancy [8]. An imbalance in this process is often associated with pregnancy complications and results in preterm birth [4].
At term, the transition from non-labor to labor status by feto-maternal uterine tissues results from the progressive imbalance of various homeostatic systems required to maintain pregnancy. An imbalance in both redox factors and inflammation is evident during labor and delivery compared to not in labor tissues (TNIL) at term [9, 10]. These events can be considered as natural and physiological events that overturn immune tolerance to ensure fetal delivery concurs with the completion of fetal growth and maturation. Mediators that switch mechanisms from a balanced to an imbalanced state have also been well reported [11–13]. Specifically, activation of immune cells and their chemotaxis to strengthen the inflammatory load in various gestational tissues [14]. Activation of immune mediators is not a random event but is mediated by various factors that determine the gestational length and the timing of delivery. These include both endocrine and paracrine factors and fetal signals of organ maturation [15]. These events are all linked to the competence of the fetus to exist as an individual outside of the uterine environment [16–21].
The search for upstream events that could cause immune intolerance during pregnancy and inflammatory overload at term to promote labor led to the examination of the mechanisms of placental membrane aging. Placental membranes, which are distinct from the placenta and perform unique functions to protect the fetus as well as provide structural integrity to the uterine cavity, were investigated [22]. Placental membrane growth and development coincided with fetal growth, and membrane longevity was equal to gestational length. Progressive aging of the placental membranes was aided by telomere attrition and activation of the oxidative stress (OS) signaling molecule, p38 mitogen-activated protein kinase (MAPK) [13]. p38MAPK-mediated senescence (mechanism of aging) is an inflammatory process [23, 24]. In vitro studies using amnion epithelial cells (AECs) from placental membranes and animal models of pregnancy showed that OS can accelerate senescence and the development of a senescence-associated secretory phenotype (SASP; sterile inflammation) [25, 26]. Similar to OS-mediated amnion senescence, decidual senescence has also been associated with preterm parturition. In this model, p53, which is an antitumor protein and proapoptotic activator, was linked as the senescence mediator [27]. Although the precise mechanisms of p53-mediated senescence have not been reported at term parturition in humans, it is likely that senescence and sterile inflammation occur at the feto-maternal interface and are not restricted to amnion cells alone.
The feto-maternal interface plays a major role in generating the necessary inflammation to transition quiescent maternal uterine tissues to an active labor state [28]. The development of senescence in response to intrauterine OS at term, and sterile inflammatory mediators generated in response to senescence, can be considered as paracrine signals that are capable of transitioning and activating maternal tissues. So far studies have been restricted to amnion and chorion cells [25, 29]. The objective of this study was to investigate the development of OS-induced senescence in various cell types at the feto-maternal interface, including amnion mesenchymal cells (AMCs), chorion cells, and decidual cells. Using similar experimental conditions to those reported for AECs, this study reports OS-mediated senescence and the SASP-generating effects produced by the distinct cell types.
Material and Methods
Placental for this study were obtained from subjects who delivered at John Sealy Hospital, The University of Texas Medical Branch (UTMB), Galveston, TX, USA. The UTMB Institutional Review Board approved this study (protocol number 11–251) and waived the need to obtain informed written consent from subjects as only discarded placental samples were to be used.
AMC culture
Primary AMCs (n = 4) were isolated from the placental membranes of women experiencing normal parturient at term (e.g., not in labor) and undergoing a repeat elective cesarean section. Reflected amnion (~10 g) was peeled from the chorion layer and rinsed three or four times in sterile Hanks’ Balanced Salt Solution (HBSS) (Cat# 21-021-CV, Corning) to remove blood debris. The sample was then incubated with 0.05% trypsin/EDTA (Cat# 25-053-CI, Corning) for 1 hour at 37°C (water bath) to disperse the cells and remove the epithelial cell layer. The membrane pieces were then washed three to four times using cold HBSS to inactivate the enzyme. The washed membrane was transferred into a second digestion buffer containing Minimum Essential Medium Eagle (Cat# 10-010-CV, Corning), 1 mg/mL collagenase type IV, and 25 μg/mL DNase I and incubated in a rotator at 37°C for 1 hour. The digested membrane solution was neutralized using complete Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 media (Cat# 10-092-CV, Corning), filtered using a 70 μm cell strainer, and centrifuged at 3000 rpm for 10 minutes. The cell pellet was resuspended in complete DMEM/F12 media supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Cat# 35-010-CV, Corning), 10 ng/ml Epithelial growth factor (Cat# E4127, Sigma), 100 U/ml penicillin G, and 100 mg/ml streptomycin (Cat# 30-001-CI, Corning). The resuspended cells were subsequently seeded at a density of 50,000 cells per well in 6-well plates to yield cultures with 95%–99% purity. Cell viability was tested using the trypan blue exclusion method. The mesenchymal nature of the primary cell cultures was verified by immunocytochemistry, and all cultures had 95% vimentin-positive cells.
Chorion and decidual cell culture
Separation of the chorion and decidual involved blunt dissection with forceps and a scalpel. Each layer was minced by cross-cutting with scalpel blades. Tissues were processed in a digestion buffer containing 0.125% trypsin (Cat# 85450c, Sigma), 0.2% collagenase (Cat# C0130, Sigma), and 0.02% DNase I (Cat# DN25, Sigma) and incubated at 37°C for 60–90 minutes. Samples were subsequently neutralized with complete media (1:1 mixture of Ham’s F12/DMEM, supplemented with 5% heat-inactivated FBS, 10 ng/ml EGF, 100 U/ml penicillin G, and 100 mg/ml streptomycin) (Cat# 30-001-CI, Corning). After filtration, the cell solution was centrifuged at 3000 rpm for 10 minutes. A cell-separation gradient was prepared using an Optiprep column (Axis-Shield), with steps ranging from 4% to 40% of 4 mL each (4%, 6%, 8%, 10%, 20%, 30%, and 40%). Processed chorion or decidual cells were added to the top of the gradient and centrifuged (3000 × g) at room temperature for 35 minutes. Cell densities of 1.049–1.062 g/mL represented the chorion layer, while cell densities of 1.027–1.038 g/mL represented the decidua layer. Harvested cells were washed with DMEM, centrifuged, resuspended in DMEM, and plated at densities of 80,000 (decidua) and 100,000 cells (chorion) per well in 6-well plates to yield cultures with 95%–99% purity. Cell viability was tested using the trypan blue exclusion method, and the nature of the primary cell culture was verified by immunocytochemistry using anti-human cytokeratin-18 and vimentin antibodies. All cultures contained 95% vimentin-positive cells (decidua) and 90% cytokeratin-18-positive cells (chorion).
Feto-maternal interface tissue explant culture and cigarette smoke extract (CSE) treatment
The in vitro organ explant culture method for human placental membranes and the stimulation of membranes with CSE were performed as previously reported [30]. In short, 6-mm biopsies of placental membranes were collected from TNIL cesarean deliveries and placed in an organ explant system for 24 hours. CSE was prepared by bubbling smoke drawn from a single lit commercial cigarette (unfiltered Camel; R.J. Reynolds Tobacco Co., Winston Salem, NC) through 50 mL of tissue culture medium (Ham’s F12/DMEM mixture with antimicrobial agents) as detailed in our prior reports [25]. Placental membranes (n=6) were stimulated with CSE (1:50 dilution), CSE + N-acetyl cysteine (NAC), or TNIL control medium for 48 hours. The explants were then removed and placed in 500 μL of formalin (10%) in 1.5-mL Eppendorf tubes for fixation before analysis.
Immunofluorescence staining
The purity of primary AMCs, chorion cells, and decidual cells was confirmed using immunocytochemistry staining for cytokeratin-18 (a biomarker of chorion cells) and vimentin (a biomarker of decidual and AMCs). After CSE treatment, cells were also stained for phosphorylated (P)-p38MAPK, P-p53, and α-smooth muscle actin. Cells were also stained using a DAPI nuclear stain. Cultures on glass chamber slides were fixed with cold methanol for 5 to10 minutes at −200°C. Cells were then washed twice in Tris-buffered saline (TBS) (Cat# 46-012-CM, Corning) and blocked with 3% bovine serum albumin TBS-Tween 20 for 1 hour at room temperature. After blocking, the cells were incubated overnight with primary antibodies at 40°C in humidified chambers. Primary antibodies for cytokeratin-18 (ab668, Abcam, Cambridge, MA), vimentin (ab92547, Abcam), P-p38MAPK (#9211, Cell Signaling, Danvers, MA), P-p53 (ab223868, Abcam), and α-smooth muscle actin (#14.9760, Thermo Fisher Scientific, Waltham, MA) were used at dilutions of 1:1000,1:750,1:400,1:400, and 1:500, respectively. A secondary antibody with no primary antibody was used as the negative control. Goat anti-mouse secondary antibody Alexa Fluor 594 (Life Technologies, Carlsbad, CA) and donkey anti-rabbit secondary antibody Alexa Fluor 488 (Abcam, Cambridge, MA) were used at dilutions of 1:1000 for 1 hour at room temperature. Cells were counterstained with DAPI for 1 minute. Slides were mounted using MoWiol 4-88 mounting medium and visualized using a Zeiss LSM 510 confocal microscope.
Cell stimulation with CSE
AMCs, chorion cells, and decidual cells were stimulated with 1:10 dilutions of CSE in culture media (as described above) for 3 hours at 37°C or 1:50 dilutions of CSE for 48 hours. To determine the specificity of the OS induction, antioxidant NAC (15 mM; Sigma, Cat#A7250) was used to inhibit ROS production, p38MAPK activation, senescence, and inflammation. SB203580 (SB; 13mM, Sigma #S8307), which is a p38MAPK functional inhibitor, was also used to prevent p38MAPK activation, senescence, and inflammation.
Measurement of ROS
AMCs, chorion cells, and decidual cells were grown to 70% confluence, loaded with 50 mM of 2′7′-dichlorodihydrofluorescein (DCF) diacetate (#C400, Invitrogen, Carlsbad, CA), and incubated at 37°C for 30 minutes. Cells were subsequently exposed to CSE or a combination of CSE and NAC (15 mM) for up to 1 hour. Fluorometric measurements were taken before the treatment and every 10 minutes for the first hour to determine changes in the ROS levels. DCF fluorescence was recorded in an FLx800 microplate reader at 528 nm after excitation at 485 nm. Results are expressed as arbitrary units, calculated using the mean slope of a linear regression of all points within the calculation zone.
Western blot analysis
AMCs, chorion cells, and decidual cells were homogenized in RIPA buffer containing protease inhibitors using a bullet blender (Next Advance, Averill Park, NY). Protein quantification was performed using the Pierce BCA protein assay kit (Thermo Fisher Scientific). Western blot analyses were performed using 20 μg of protein from each sample using standard protocols[26]. Antibodies used included p38MAPK (#9212, Cell Signaling), P-p38MAPK (#9211, Cell Signaling), P-p53 (ab223868, Abcam), and total p53 (AF1355, R&D Systems, Minneapolis, MN) at dilutions of 1:1000, 1:400, 1:400, and 1:250, respectively. To avoid interassay variability between blots, samples from the same experiments were run on the same gel for a given marker. All blots were reprobed with an antibody to β-actin (Sigma, St. Louis, MO). Detected bands were analyzed with densitometry using Image J software (National Institutes of Health, rsbweb.nih.gov/ij), and results were normalized to β-actin expression on the same blots.
Senescence-associated β-Galactosidase (SA-β-Gal) assay
Expression of the SA-β-Gal biomarker is independent of DNA synthesis and distinguishes senescent cells from quiescent cells [31]. Senescent cells were identified using a histochemical staining kit (ab65351), with blue cells visualized by light microscopy at 48 hours after treatment with CSE and/or NAC + SB. Four regions of interest were captured per condition using bright-field microscopy and a Nikon Eclipse TS100 (10x) (Melville, NY). Image modifications (brightness, contrast, and smoothing) were applied to all image sets using Light House, and Image J (National Institutes of Health, rsbweb.nih.gov/ij) was used to measure the staining intensity. Intensity was measured by converting images into greyscale, applying uniform thresholding, selecting regions randomly, and measuring the intensity of SA-β-Gal-stained cells in that region. An average of four regions were collected per replicate, and the number of cells with positive staining (blue colored cells) was analyzed.
Initial experiments to determine SA-β-Gal was not convincing in decidual cells and therefore additional experiments were conducted using flow cytometry. Flow cytometry using specific antibodies and reagents confirmed decidual SA-β-Gal activity[32]. Briefly, cells were incubated for one hour in complete DMEM growth medium supplemented with 100 nM bafilomycin A1 (baf A1) for one hour at 37°C. Without changing media, 5-Dodecanoylaminofluorescein di-b-D-galactopyranoside (C12FDG) was added (final concentration of 6 μM) and incubated at 37°C for one hour. Cells were harvested by trypsinization and centrifugation at 3000 g for 10 min at 4°C. The cell pellet was resuspended in 500 μL Coulter DNA Prep Stain (Beckman Coulter, Indianapolis, IN), which contains propidium iodide (PI) to indicate viable and non-viable cells, and run immediately on the CytoFlex flow cytometer (Beckman Coulter). Unstained, control AECs were used as negative controls for gating. Data were analyzed using the Cytexpert software (Beckman Coulter) and cells positive for C12FDG and negative for PI (viable) were considered for analysis.
Immunohistochemistry for matrix metallopeptidase 9 (MMP9)
To measure the CSE-induced SASP, biopsies of cultured TNIL placental membranes were immunostained for MMP9. Membranes from TNIL patients treated with CSE or CSE + NAC for 48 hours for were used for MMP9 localization. Formalin fixed, paraffin embedded explants sectioned at 5 μm thickness were immunostained using specific primary antibody to MMP9 (Cat#D603H, Cell Signaling).
Statistical analysis
For the quantitative ROS data, a two-way repeated measures ANOVA was used to detect statistical significance considering the treatment and time factors as the variables. A one-way ANOVA was used to analyze the western blotting data and SA-β-Gal intensity staining. Tukey’s multiple comparison tests were performed to correct for pairwise treatment effects for both two-way and one-way ANOVAs. All data were analyzed using Graph Pad Prism 6.
Results
To provide adequate power to detect statistically significant differences between the various treatment groups, six cultures of each cell type were used for all experiments (determined based on our pilot experiments). Controls used in this study were untreated cells r tissues in an organ explant system grown or maintained under normal cell culture conditions. The water-soluble portion of CSE was used as a laboratory reagent to induce OS and not necessarily as a risk factor for pregnancy. Previous reports showed that CSE produces OS that mimics term labor and delivery and the OS observed in major subsets of preterm birth compared to proxies for infections (e.g., lipopolysaccharides ) and inflammation (e.g., tumor necrosis factor-α) [32].
Characteristics of placental membrane cells
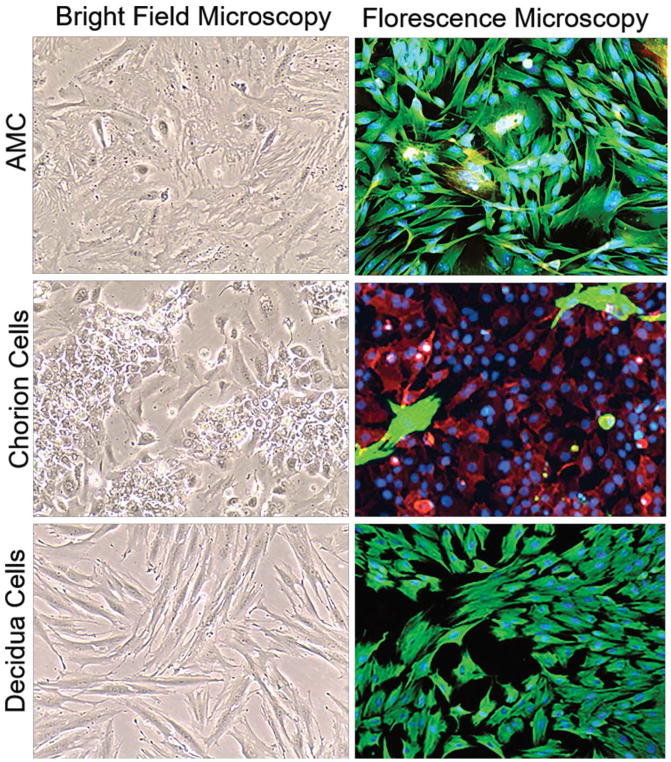
The feto-maternal interface is comprised of multiple layers including fetal-derived AMCs, chorion cells, and maternal decidual cells. AMCs exhibit an elongated morphology and express vimentin (green) in vitro (Fig. 1). In vivo, chorion trophoblasts form multiple layers of tightly-packed CK-18+ cells which mark the feto-maternal interface. In vitro, these cells exhibit a cobblestone morphology and express CK-18, while decidual cells have an elongated morphology and express vimentin (Fig. 1).
Figure 1. Characterization of placental membrane cells.
Bright-field and fluorescence microscopy showing the morphology of cultured primary amnion mesenchymal cells (AMCs), chorion cells, and decidual cells. Fluorescence microscopy showing vimentin (green) and cytokeratin-18 (CK-18; red) localization in AMCs, chorion cells, and decidual cells in vitro. Images were taken at 10X magnification.
CSE induces ROS in fetal- and maternal-derived cells
CSE treatment increased ROS levels in all cell types within 2 minutes of exposure. ROS production in AMCs and chorion cells was significantly higher within 10 minutes of CSE treatment compared to untreated controls (P < 0.05 and P < 0.001, respectively, for all time points) (Fig. 2A and B); however, NAC cotreatment reduced ROS levels to below control levels (P < 0.05 for all time points) (Fig. 2A and B). ROS production in decidual cells was also significantly higher following CSE treatment when compared to the untreated controls (P < 0.001 for all time points), and cotreatment with NAC reduced ROS levels below control levels (P < 0.001 for all time points). NAC treatment alone produced ROS levels that were similar to those observed in the controls of all cell types (Supplemental Fig. 1A–C). ROS levels in fetal-derived cells, AMCs, and chorion cells were lower than in maternal decidual cells, thus suggesting a different OS response in feto-maternal cells.
Figure 2. ROS production in fetal membrane cells.
A) Cigarette smoke extract (CSE) treatment of amnion mesenchymal cells (AMCs) significantly increased ROS production at 10 minutes (P < 0.05) and 20 minutes (P < 0.05) compared to control (untreated) AMCs. Cotreatment with N-acetyl cysteine (NAC) and CSE significantly reduced ROS production in AMCs (P < 0.05 for all time points).
B) CSE treatment of chorion cells significantly increased ROS production (P < 0.001) at all time points compared to control chorion cells. Cotreatment with NAC and CSE significantly prevented ROS production in chorion cells (P < 0.05 at all-time points).
C) CSE treatment of decidual cells significantly increased ROS production (P < 0.001) at all time points compared to control decidual cells. Cotreatment with NAC and CSE significantly reduced ROS production in decidual cells (P < 0.001 at all-time points).
An asterisk above the blue line represents a significant difference between control and CSE-treated cells, while an asterisk below the last line represents a significant difference between CSE and CSE and NAC cotreated cells.
OS-induced p38MAPK activation in both fetal and maternal-derived cells
ROS induced the activation of p38MAPK in AMCs, chorion cells, and decidual cells in culture (Fig. 3) similar to that we reported previously in AECs. Western blot and immunofluorescence analysis revealed that CSE treatment induced the phosphorylation of p38MAPK within 6 hours. In AMCs, CSE significantly increased P-p38MAPK compared to controls (P = 0.0005), while CSE + NAC reduced P-p38MAPK (P = 0.005) (Fig. 3A). These results were further verified by immunofluorescence staining, which documented increased nuclear localization of P-p38MAPK after CSE treatment. Nuclear translocation of P-p38MAPK was inhibited by the p38MAPK inhibitor SB (Fig. 3B). In chorion cells, CSE significantly increased P-p38MAPK compared to controls (P = 0.01), while CSE + NAC reduced P-p38MAPK (P = 0.007) (Fig. 3C). Nuclear localization of P-p38MAPK increased in chorion cells after CSE treatment, while treatment with CSE + SB inhibited its translocation (Fig. 3D). In decidual cells, CSE significantly increased P-p38MAPK (P < 0.0001) compared to controls, while CSE + NAC reduced P-p38MAPK (P < 0.0001) (Fig. 3E). Nuclear localization of P-p38MAPK in decidual cells was similar to that seen in AMCs, with relative fluorescence increasing following CSE treatment and translocation reducing following CSE + SB treatment (Fig. 3F). NAC treatment alone did not stimulate p38MAPK activation in any cell type (Supplemental Fig. 1D). SB reduced P-p38MAPK translocation to the nucleus in all cell types.
Figure 3. Cigarette smoke extract (CSE) induces p38MAPK activation in placental membrane cells.
A–F) CSE treatment of amnion mesenchymal cells (AMCs), chorion cells, and decidual cells for up to 6 hours induced phosphorylation (P) of p38MAPK, as determined by western blots and immunofluorescence.
A) CSE treatment induced higher levels of P-p38MAPK in AMCs than in control cells (P = 0.0005), while CSE + N-acetyl cysteine (NAC) reduced P-p38MAPK levels (P = 0.005). The treatment did not alter the total p38MAPK level.
B) Nuclear translocation of P-p38MAPK increased following CSE treatment but was inhibited by the p38MAPK inhibitor, SB203580 (SB).
C) CSE treatment induced higher levels of P-p38MAPK in chorion cells than in control cells (P = 0.009), while CSE + NAC reduced P-p38MAPK levels (P = 0.006). The treatment did not alter the total p38MAPK level.
D) Nuclear localization of P-p38MAPK increased in chorion cells after CSE treatment, while CSE + SB treatment prevented this translocation.
E) CSE treatment induced higher levels of P-p38MAPK in decidual cells compared to controls (P < 0.0001), while cotreatment with CSE + NAC reduced P-p38MAPK activation (P < 0.0001). The treatment did not alter the total p38MAPK level.
F) Nuclear localization of P-p38MAPK in decidual cells was similar to that in AMCs. The relative fluorescence increased with CSE, while CSE + SB treatment reduced translocation.
Bar graphs represent densitometry, and the y-axis represents relative densitometry units. Confocal microscopy images were captured at 63X magnification. Red staining indicates α-smooth muscle actin (α-SMA), green staining represents P-p38MAPK, and blue represents the DAPI nuclear stain.
OS did not induce p53 activation in placental membrane cells
Although OS treatment stimulated p38MAPK activation, it did not activate p53, which is another molecule that responds to cellular stress. CSE treatment of AMCs for 3 hours did not result in a statistically significant change in P-p53 levels compared to the controls (Fig. 4A). This is likely because P-p53 is a constitutive component of AMCs. Immunofluorescence results confirmed the nuclear localization and constitutive expression of P-p53 regardless of the treatment (Fig. 4A; white arrows). NAC treatment alone did not stimulate P-p53 in AMCs (Supplemental. Fig. 1E). Chorion and decidual cells did not show P-p53 expression in control cells or in cells following CSE treatment.
Figure 4. Cigarette smoke extract (CSE) does not induce p53 activation in AMCs.
Amnion mesenchymal cells (AMCs) constitutively expressed P-p53 and total p53 regardless of the treatment. Immunofluorescent staining documented nuclear localization of P-p53 after CSE treatment (white arrows). Confocal microscopy images were captured at 63X magnification. Red staining represents α-smooth muscle actin (α-SMA), green staining represents P-p53, and blue staining represents the DAPI nuclear stain.
OS induces senescence and senescence-associated inflammation in fetal- and maternal-derived cells
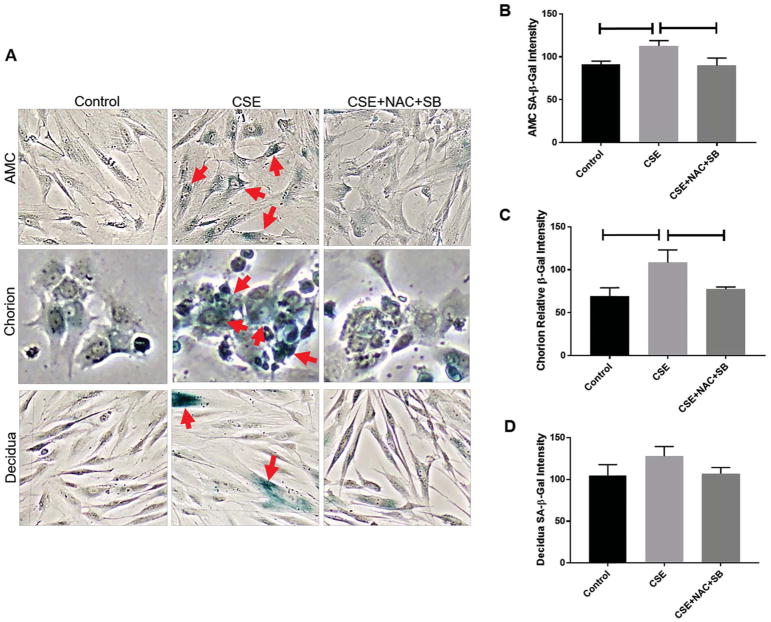
Fetal- and maternal-derived cells were stained for SA-β-Gal (blue staining) to determine senescence in response to OS-induced p38MAPK activation (Fig. 5A). In AMCs, the mean relative intensity unit (RIU) of SA-β-Gal was higher following 48-hour CSE exposure compared to untreated controls (110.4 ± 6.22 RIU vs. 90.43 ± 3.94 RIU, P = 0.02), while CSE + NAC + SB significantly inhibited senescence (89.59 ± 8.86 RIU, P = 0.01) (Fig. 5B). In chorion cells, CSE treatment increased senescence compared to control cells (99.84 ± 14.73 RIU vs. 62.32 ± 32 RIU, P = 0.003), while CSE + NAC + SB significantly inhibited senescence (23.25 ± 26.42 RIU, P = 0.009) compared to CSE alone (Fig. 5C). In decidual cells, CSE treatment did not induce senescence, as indicated by no significant difference in SA-β-Gal between CSE-treated and control cells (129.4 ± 11.62 RIU vs. 108.3 ± 12.95 RIU). Similarly, CSE + NAC + SB did not change senescence (110.3 ± 7.27 RIU) compared to CSE alone (Fig. 5D). Additionally, flow cytometry for SA-β-Gal confirmed the lack of decidual senescence (Supplemental Fig. 2). NAC treatment alone did not stimulate senescence in any cell type (Supplemental Fig. 1F).
Figure 5. Cigarette smoke extract (CSE) induces senescence in placental membrane cells.
A) Cigarette smoke extract (CSE) treatment (48 hours) induced senescence-associated-β-Galactosidase (SA-β-Gal) staining (blue stain; red arrows) in amnion mesenchymal cells (AMCs) and chorion cells but had little effect in decidual cells.
B) CSE treatment significantly induced SA-β-Gal (P = 0.02) in AMCs compared to control cells, while CSE + N-acetyl cysteine (NAC) significantly reduced SA-β-Gal staining (P = 0.01).
C) CSE treatment significantly induced SA-β-Gal (P = 0.003) in chorion cells compared to control cells, while CSE + NAC significantly inhibited SA-β-Gal staining (P = 0.009) compared to CSE alone.
D) SA-β-Gal staining was not significantly different between CSE-treated and control decidual cells or after cotreatment with NAC. All bright-field images were captured after 48 hours of treatment and at 20X magnification. Data are documented in relative intensity units (RIU).
SASP is a well-reported phenomenon of sterile inflammation. MMP9 was used as a surrogate for OS-mediated SASP. MMP9 expression increased in AMCs, chorion cells, and decidual cells when tissues were stimulated with CSE for 48 hours, while CSE + NAC decreased the expression of MMP9 compared to CSE treatment alone (Fig. 6A–C).
Figure 6. Cigarette smoke extract (CSE) induces senescence-associated inflammation in placental membrane cells.
A) Term not in labor (TNIL) explants that were cultured for 48 hours (controls) expressed no MMP9 in any placental membrane layers (i.e., amnion epithelial cells (AECs), amnion mesenchymal cells (AMCs), chorion cells, or decidual cells).
B) CSE treatment of TNIL explants for 48 hours induced the MMP9 expression in all cell layers.
C) Cotreatment with CSE + NAC reduced MMP9 expression.
Discussion
Senescence of feto-maternal gestational tissues is a physiologic process associated with human pregnancy and parturition. Senescence is accelerated by increased OS at term. We tested the extent of senescence at term feto-maternal interface cells. The principal findings of this study were that OS induced ROS production, activation of p38MAPK, and activation of senescence and senescence-associated inflammation (MMP9) in AMCs and chorion cells; however, ROS production and p38MAPK activation did not cause decidual cell senescence but still led to increased inflammatory MMP9 levels irrespectively. Like AECs, other cells in the placental membrane also respond to OS and cause senescence and inflammation through the p38MAPK-mediated pathway. This report was based on primary cell culture models and hence communication between different cells did not exist; however, it has already been shown that term- and preterm-labor membranes show both OS markers and senescence thus supporting the findings of this report [30, 33].
The feto-maternal interface is comprised of fetal amniochorionic membranes and decidua formed from differentiated maternal endometrial stromal fibroblasts. This report showed that fetal cell responses were similar to those reported in AECs. A notable difference was that AMCs showed constitutive expression of active p53, which is a proapoptotic and prosenescence marker associated with decidual tissue senescence in mouse models [27]. The p53 pathway is one of two major tumor suppressor pathways that regulate cellular senescence, and p53 has been considered as the “guardian of the genome” during pregnancy. It is likely that p53 performs specific functions related to AMC turnover and does not necessarily function to impact senescence. In conclusion, placental membrane cells (AECs, AMCs, and chorion cells) react similarly to CSE-induced OS and produce senescence and inflammation.
The CSE-induced ROS production in decidua was substantially higher on the maternal side than on the fetal side. Interestingly, ROS did not result in the senescence of decidual cells compared to fetal cells, even with p38MAPK activation. Unlike fetal cells, maternal tissues are likely protected from senescence. Decidualized endometrium is a close component of the placental membrane, although it is functionally distinct from the membranes. Decidua likely act to amplify inflammatory signals propagated from the placental membranes to increase overall uterine inflammation [15]. Resident immune cells within the decidua and immune cell-like properties of decidual cells ensure the activation of inflammation [34]. Ongoing studies suggest that exosomes released from senescent placental membrane cells (amnion and chorion) can increase the inflammatory load in decidual cells. It is likely that a continued inflammatory surge from decidual cells is required to ensure fetal and placental delivery. As senescence may compromise such functional capabilities of decidua, senescence is likely to be muted even with p38MPAK activation. A p53-mediated senescence pathway was previously reported in decidua [27]. Our western blot analysis could not corroborate this data and we report that human decidual cells do not produce active p53. The presence of P-p53 in AMCs validated the experimental conditions and antibody specificity. One major role of decidua is to accommodate and support the developing embryo, and the roles of p53 in developing tissues are likely different from its roles in normal adult tissues. Decidual cells may support pregnancy by providing immune tolerance whereas it may promote parturition by amplifying inflammation. To note, decidua contain resident immune cells and often get infiltrated by immune cells at term. These cells do play a role in decidual homeostasis. We have not examined the changes in these cells in response to oxidative stress. It is likely that these cells may also play a role in determining decidual senescence that is not tested in our in vitro model.
Programmed aging of the placental membrane coincides with fetal growth and organ maturation [35]. Mechanistically, senescence involves telomere shortening and p38MAPK activation, which result in functional changes in intrauterine tissues. Senescence is accelerated at term when ROS levels are increased. These are likely to be programmed events that cause the inflammation required to promote labor-associated changes in uterine tissues. Elevated sterile inflammatory signals (i.e., SASP) secreted from both senescent placental membranes (e.g., AEC, AMCs, and chorion cells) and inflamed decidua are likely to contribute to the onset of labor at term. Premature senescence activation may contribute to spontaneous preterm birth and preterm premature rupture of the membranes.
In summary, cells in the feto-maternal interface show differential senescence in response to OS, with fetal cells responding more rigorously than maternal decidual cells. Fetal cells demonstrate a SASP response, whereas decidual cells show an innate immune response to OS.
Supplementary Material
A) N-acetyl cysteine (NAC) treatment alone significantly decreased ROS production at 30 minutes (P < 0.05) compared to control amnion mesenchymal cells (AMCs).
B) NAC treatment alone significantly decreased ROS production at 20 minutes (P < 0.05) compared to control chorion cells.
C) NAC treatment alone significantly decreased ROS production at all time points (P < 0.05) compared to control decidual cells.
D) NAC treatment did not induce or suppress P-p38MAPK in AMCs, chorion cells, or decidual cells.
E) NAC treatment did not induce P-p53 in AMCs.
F) Treatment with NAC + p38MAPK inhibitor SB203580 did not induce senescence in AMCs, chorion cells, or decidual cells.
A–B) Cigarette smoke extract (CSE) treatment or CSE+ N-acetyl cysteine (NAC)+SB203580 did not induced SA-β-Gal in decidual cells measured by flow cytometry.
Highlights.
Cigarette smoke extract induces ROS in fetal and maternal uterine cells
OS activation of p38MAPK leads to senescence in mesenchymal and chorion cells.
Mesenchymal and chorion cell senescence is associated with sterile inflammation
p38MAPK mediated inflammation in decidual cells is independent of senescence.
Fetal but not maternal cells are vulnerable to OS induced senescence and inflammation
Acknowledgments
Samantha Sheller-Miller and Lauren Richardson are predoctoral trainees in the Environmental Toxicology Training Program (T32ES007254), which is supported by the National Institute of Environmental Health Sciences of the National Institutes of Health of the United States and administered through the University of Texas Medical Branch in Galveston, Texas.
Funding: This study was supported by 1R01HD084532 grant (NIH/NICHD) to R Menon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dennery PA. Effects of oxidative stress on embryonic development. Birth Defects Res C Embryo Today. 2007;81(3):155–62. doi: 10.1002/bdrc.20098. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122(4):369–82. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 4.Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Frontiers in Immunology. 2014;5:567. doi: 10.3389/fimmu.2014.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai M, Barker G, Menon R, Lappas M. Increased oxidative stress in human fetal membranes overlying the cervix from term non-labouring and post labour deliveries. Placenta. 2012;33(8):604–10. doi: 10.1016/j.placenta.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11(4):173–86. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 7.Rinaldi SF, Hutchinson JL, Rossi AG, Norman JE. Anti-inflammatory mediators as physiological and pharmacological regulators of parturition. Expert Rev Clin Immunol. 2011;7(5):675–96. doi: 10.1586/eci.11.58. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T, Margolis L. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213(6):836.e1–836.e18. doi: 10.1016/j.ajog.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72(5):458–74. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biondi C, Pavan B, Lunghi L, Fiorini S, Vesce F. The role and modulation of the oxidative balance in pregnancy. Curr Pharm Des. 2005;11(16):2075–89. doi: 10.2174/1381612054065747. [DOI] [PubMed] [Google Scholar]
- 12.Saito S, Nakashima A, Myojo-Higuma S, Shiozaki A. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J Reprod Immunol. 2008;77(1):14–22. doi: 10.1016/j.jri.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Menon R, Yu J, Basanta-Henry P, Brou L, Berga SL, Fortunato SJ, Taylor RN. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One. 2012;7(2):e31136. doi: 10.1371/journal.pone.0031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feyaerts D, Benner M, van Cranenbroek B, van der Heijden OWH, Joosten I, van der Molen RG. Human uterine lymphocytes acquire a more experienced and tolerogenic phenotype during pregnancy. Sci Rep. 2017;7(1):2884. doi: 10.1038/s41598-017-03191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update. 2016;22(5):535–60. doi: 10.1093/humupd/dmw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casey ML, Winkel CA, Porter JC, MacDonald PC. Endocrine regulation of the initiation and maintenance of parturition. Clin Perinatol. 1983;10(3):709–21. [PubMed] [Google Scholar]
- 17.Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69(3):212–30. doi: 10.1111/aji.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009;23(7):947–54. doi: 10.1210/me.2009-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317–26. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20(2):154–67. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 21.Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regul Pept. 2002;108(2–3):159–64. doi: 10.1016/s0167-0115(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 22.Nunes V, Cross J, Speich JE, Morgan DR, Strauss JF, 3rd, Ramus RM. Fetal membrane imaging and the prediction of preterm birth: a systematic review, current issues, and future directions. BMC Pregnancy Childbirth. 2016;16(1):387. doi: 10.1186/s12884-016-1176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bracchitta G, Catalfo A, Martineau S, Sage E, De Guidi G, Girard PM. Investigation of the phototoxicity and cytotoxicity of naproxen, a non-steroidal anti-inflammatory drug, in human fibroblasts. Photochem Photobiol Sci. 2013;12(5):911–22. doi: 10.1039/c3pp25326k. [DOI] [PubMed] [Google Scholar]
- 24.Akasaka E, Takekoshi S, Horikoshi Y, Toriumi K, Ikoma N, Mabuchi T, Tamiya S, Matsuyama T, Ozawa A. Protein oxidative damage and heme oxygenase in sunlight-exposed human skin: roles of MAPK responses to oxidative stress. Tokai J Exp Clin Med. 2010;35(4):152–64. [PubMed] [Google Scholar]
- 25.Menon R, Boldogh I, Urrabaz-Garza R, Polettini J, Syed TA, Saade GR, Papaconstantinou J, Taylor RN. Senescence of primary amniotic cells via oxidative DNA damage. PLoS ONE [Electronic Resource] 2013;8(12):e83416. doi: 10.1371/journal.pone.0083416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere Fragment Induced Amnion Cell Senescence: A Contributor to Parturition? PLoS ONE [Electronic Resource] 2015;10(9):e0137188. doi: 10.1371/journal.pone.0137188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest. 2010;120(3):803–15. doi: 10.1172/JCI40051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon R, Mesiano S, Taylor RN. Programmed Fetal Membrane Senescence and Exosome-Mediated Signaling: A Mechanism Associated With Timing of Human Parturition. Front Endocrinol (Lausanne) 2017;8:196. doi: 10.3389/fendo.2017.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canzoneri BJ, Feng L, Grotegut CA, Bentley RC, Heine RP, Murtha AP. The chorion layer of fetal membranes is prematurely destroyed in women with preterm premature rupture of the membranes. Reprod Sci. 2013;20(10):1246–54. doi: 10.1177/1933719113483009. [DOI] [PubMed] [Google Scholar]
- 30.Menon R, Boldogh I, Hawkins HK, Woodson M, Polettini J, Syed TA, Fortunato SJ, Saade GR, Papaconstantinou J, Taylor RN. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. American Journal of Pathology. 2014;184(6):1740–51. doi: 10.1016/j.ajpath.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol. 2007;371:21–31. doi: 10.1007/978-1-59745-361-5_3. [DOI] [PubMed] [Google Scholar]
- 32.Dixon CL, Richardson L, Sheller-Miller S, Saade G, Menon R. A distinct mechanism of senescence activation in amnion epithelial cells by infection, inflammation, and oxidative stress. Am J Reprod Immunol. 2017 doi: 10.1111/aji.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behnia F, Taylor BD, Woodson M, Kacerovsky M, Hawkins H, Fortunato SJ, Saade GR, Menon R. Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol. 2015;213(3):359.e1–16. doi: 10.1016/j.ajog.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 34.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–7. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonney EM, Ramkamar Differential senescence in feto-maternal tissues during mouse pregnancy, placenta. 2017 doi: 10.1016/j.placenta.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) N-acetyl cysteine (NAC) treatment alone significantly decreased ROS production at 30 minutes (P < 0.05) compared to control amnion mesenchymal cells (AMCs).
B) NAC treatment alone significantly decreased ROS production at 20 minutes (P < 0.05) compared to control chorion cells.
C) NAC treatment alone significantly decreased ROS production at all time points (P < 0.05) compared to control decidual cells.
D) NAC treatment did not induce or suppress P-p38MAPK in AMCs, chorion cells, or decidual cells.
E) NAC treatment did not induce P-p53 in AMCs.
F) Treatment with NAC + p38MAPK inhibitor SB203580 did not induce senescence in AMCs, chorion cells, or decidual cells.
A–B) Cigarette smoke extract (CSE) treatment or CSE+ N-acetyl cysteine (NAC)+SB203580 did not induced SA-β-Gal in decidual cells measured by flow cytometry.