Abstract
Background:
Patients undergoing thoracotomy frequently experience acute pain and chronic post-thoracotomy pain (CPTP). There are few articles relating to the investigations on the effects of preoperative single-dose thoracic paravertebral block (PSTPVB) on acute pain and CPTP. We tested the hypothesis that adding PSTPVB to intravenous (IV) patient-controlled analgesia (PCA) would reduce acute pain scores and decrease the incidence and intensity of CPTP.
Methods:
Fifty-six patients undergoing elective thoracotomy were randomized to receive PSTPVB in addition to IV PCA (group T) or IV PCA alone (group C). A single 20-mL injection of 0.50% ropivacaine plus 10 mg dexamethasone in saline was administered preoperatively under ultrasound guidance; sufentanil was used for IV PCA. The acute pain intensity at rest and at coughing based on verbal rating scale, postoperative sufentanil consumption, and complications were evaluated at 6, 24, 48, and 72 hours after surgery. The incidence and intensity of CPTP were evaluated at 3 months after surgery.
Results:
Group T had significantly less acute pain compared with group C at all measurement times both at rest and at coughing (P < .05). The PCA cumulative sufentanil consumption, complications, and the incidence of CPTP between the 2 groups was not statistically significant (P > .05). The intensity of CPTP was significantly higher in group C than in group T (P < .05).
Conclusion:
This study indicated that adding PSTPVB to IV PCA improved acute postoperative pain and chronic pain in patients undergoing thoracotomy, but did not reduce the incidence of CPTP.
Keywords: acute pain, chronic pain, patient-controlled analgesia, thoracic paravertebral block, thoracotomy
1. Introduction
Acute pain after thoracotomy is considered 1 of the most intense types of postoperative pain.[1] Good analgesia after thoracotomy is particularly important not only for satisfaction of patients but also for prognosis of patients,[1,2] as poorly controlled acute pain may contribute to the impairment of respiratory function and the development of chronic post-thoracotomy pain (CPTP)[3–5] (pain that recurs or persists along a thoracotomy incision at least 2 months after the surgical procedure[6]).
Intravenous (IV) patient-controlled analgesia (PCA) using opioids may be used after thoracotomy, but the analgesic effect can be insufficient in some patients with severe pain. Thoracic epidural analgesia is effective in relieving acute postoperative pain after thoracotomy.[1] However, it carries the risks of nerve injury, dural puncture, epidural hematoma, and abscess.[7] A single-injection thoracic paravertebral block when used in conjunction with general anesthesia reduces acute postoperative pain in patients undergoing breast cancer surgery,[8] nephrectomy,[2] and thoracoscopic surgery.[9]
However, to date, there are few articles relating to the investigations on the effects of preoperative single-dose thoracic paravertebral block (PSTPVB) on acute pain and chronic pain after thoracotomy. In this randomized, controlled, double-blinded study, we tested the hypothesis that adding PSTPVB to IV PCA would reduce pain scores at rest and at coughing during the first 72 hours after thoracotomy, and decrease the incidence and intensity of CPTP compared with IV PCA alone in patients undergoing thoracotomy.
2. Methods
This randomized, controlled, double-blinded trial was approved by the institution's Ethics Examination Committee of Human Research (The document No.: 2016-P2-019-02, Beijing Friendship Hospital, Capital Medical University, Beijing, China), and written informed consent from the prospective patient cases, 56 American Society of Anesthesiologists physical status 1 to 3 patients scheduled for thoracotomy for lung or esophagus diseases were enrolled in the study. Exclusion criteria during the preoperative period were psychological disorders, contraindication to the use of PSTPVB or nonsteroidal anti-inflammatory drugs, cardiovascular surgery, gastroesophageal surgery, coagulopathy, pre-existing pain syndromes, pregnant patients, and severe hepatic, renal, or cardiovascular disorders. The intraoperative exclusion criterion was a decision of inoperability made by surgeon. During the postoperative period, exclusion criteria were patients who were re-operated and who reported infections. After hospital discharge, exclusion criterion was patients who died.
2.1. Preoperative visit
At the preoperative visit, eligible patients were informed about the study and their written informed consents were received. All patients were instructed in how to evaluate their own pain by using a 10-cm verbal rating scale (VRS) (0 = no pain, 10 = maximum pain imaginable) and how to use a PCA device. Additionally, before the hospital discharge, they were also informed at the telephone interview about pain at 3 months after surgery.
2.2. Randomization and blinding method
Patients were stratified by disease sites (lung or esophagus), and randomized to receive PSTPVB in addition to IV PCA (group T) or IV PCA alone (group C) by drawing sequentially numbered, sealed envelopes each with a computer-generated allocation number for the analgesic technique. PSTPVB was performed in group T. In group C, the skin was penetrated 1 cm with a needle at the same site as in the group T, but no drug was injected. One investigator, who was not involved with the intraoperative and postoperative data collection, performed PSTPVB and sham procedures. Another investigator blinded to the group allocation recorded perioperative data and postoperative assessments. The telephone interview at 3 months after surgery was also conducted by the same investigator.
2.3. Intraoperative management
After arrival in the operating room, an intravenous infusion of lactated Ringer solution was then commenced on the hand or forearm contralateral to the side of surgery, and baseline electrocardiogram, Bispectral Index (BIS) monitor, noninvasive arterial blood pressure, SaO2, and heart rate were recorded.
In group T, before the induction of general anesthesia, PSTPVB was performed according to the method of Shibata and Nishiwaki.[10] The superior aspect of spinous process of the proposed thoracic level of skin incision was identified by bony landmarks with the patient in the lateral position. The thoracic paravertebral space was scanned by ultrasonography (Vivid S6; GE Medical Systems Israel Ltd, 4 Etgar Street, Tirat Carmel, Israel) with a linear 12L probe. A 22-G needle (Stimuplex; B. Braun AG, Melsungen, Germany) was positioned in the paravertebral space and a 20-mL bolus of a solution of 0.5% ropivacaine (Naropin; Astra-Zeneca, SE-151 85 Sodertalje, Sweden) plus 10 mg dexamethasone in saline was administered under real-time ultrasound monitoring at least 30 minutes before skin incision. In group C, a sham procedure was performed.
After placement of an arterial catheter, a standardized general anesthetic induction was conducted with intravenous midazolam 0.05 mg/kg, sufentanil (0.3 μg/kg), propofol (1.5–2.5 mg/kg), and cis-atracurium (0.2 mg/kg). Endobronchial intubation was performed with a double-lumen tube. For the maintenance of anesthesia, a continuous infusion of propofol and remifentanil was used with incremental doses of cis-atracurium and sufentanil. Target BIS values were set between 40 and 60. Before closing the incision, 40 mg parecoxib sodium was given intravenously. All patients were extubated at the end of surgery before transferring to the postanesthesia care unit. Patients left the operating room with a PCA pump (CPE-101, Royal Fornia Medical Equipment Ltd, Tangjia Bay, Zhuhai, China). The pump was programmed as follows: bolus dose 2 μg sufentanil, 5-minute lockout period; no background infusion. Afterward, patients were transferred to the intensive care unit of the thoracic department for close monitoring.
2.4. Postoperative ward and follow-up
When patients returned to the surgical ward, parecoxib sodium (40 mg/12 h) was administered intravenously or pethidine (50 mg/8 h) was injected intramuscularly as rescue analgesia if VRS score at rest was greater than 4, or if the patient demanded additional analgesia.
During the first 72 hours after surgery, patients were questioned about their pain at rest and at coughing by using VRS. Pain was evaluated at 6, 24, 48, and 72 hours after surgery by an investigator who was blinded to the group allocation. The side effects and unwanted effects were also recorded. Postoperative rehabilitation and drainage duration, postoperative morbidity, and mortality were evaluated and compared between the groups.
At 3 months after surgery, an investigator, who was blinded to the group allocation, interviewed patients by telephone, using a standard questionnaire. In the first 2 questions, patients were asked whether they were having pain at the moment or had ever experienced it from the last interview. They were asked to evaluate their pain with the same VRS as in the acute period. Then they were asked whether the pain (if any) was interfered with their daily life.
2.5. Outcomes
The primary outcome measure was the acute postoperative pain score at 24 hours at rest after surgery. The secondary outcome measures were the acute postoperative pain scores at 6, 24, 48, and 72 hours after surgery both at rest and at coughing, postoperative sufentanil consumption, postoperative complications, and the incidence and intensity of CPTP at 3 months.
2.6. Sample size estimation
Because there were no comparable data, we conducted a pilot study on 10 patients who had undergone thoracotomy with a mean pain score of 2.56 (1.66) and 4.46 (1.60) in group T and group C at 24 hours at rest after surgery. A sample size of 21 per group was obtained by PASS 11.0 (NCSS Statistical Software, Kaysville, UT) with a power of 90% at a level of α = 0.01; thus, 28 patients per group were needed considering a 30% dropout rate.
2.7. Statistical analysis
A statistical analysis was performed using SPSS Version 22.0 (SPSS Inc., Chicago, IL). Data are reported as mean and SD if the data were normally distributed. Data are presented as median and interquartile range (IQR) if the data were not normally distributed. Qualitative data are reported as percentages. For comparison between 2 groups, Student t test or Mann–Whitney U test was used, depending on whether the data were distributed normally or not. Chi-square test (Fisher exact test, 2-tailed) was used to compare categorical variables. P < .05 was considered significant.
3. Results
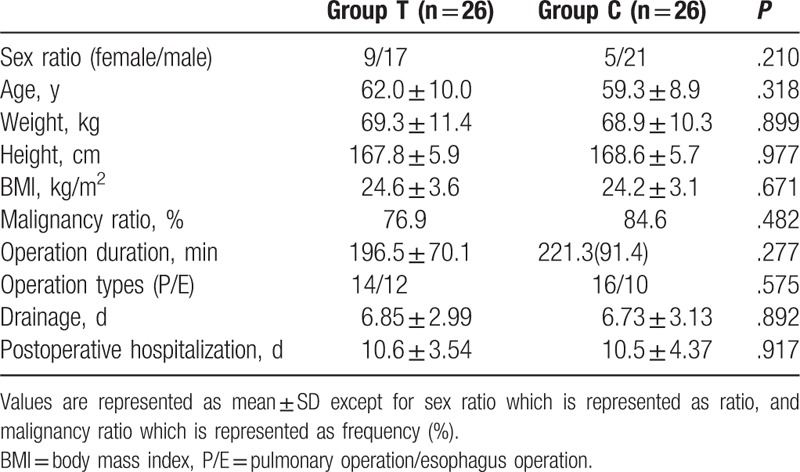
In all, 80 patients were screened and 56 were enrolled into the study. In total, 4 patients were subsequently excluded from the analysis because of wound infection (n = 1), reoperation (n = 1), inoperability (n = 1), or death (n = 1). Thus, in all, 52 patients completed the study, 26 in group T and 26 in group C (Fig. 1). Patient characteristics are presented in Table 1. There was no statistical difference in terms of sex ratio, age, height, weight, body mass index (BMI), malignancy ratio, surgery duration time, postoperative hospitalization time, and chest tube extubation between the 2 groups (Table 1).
Figure 1.
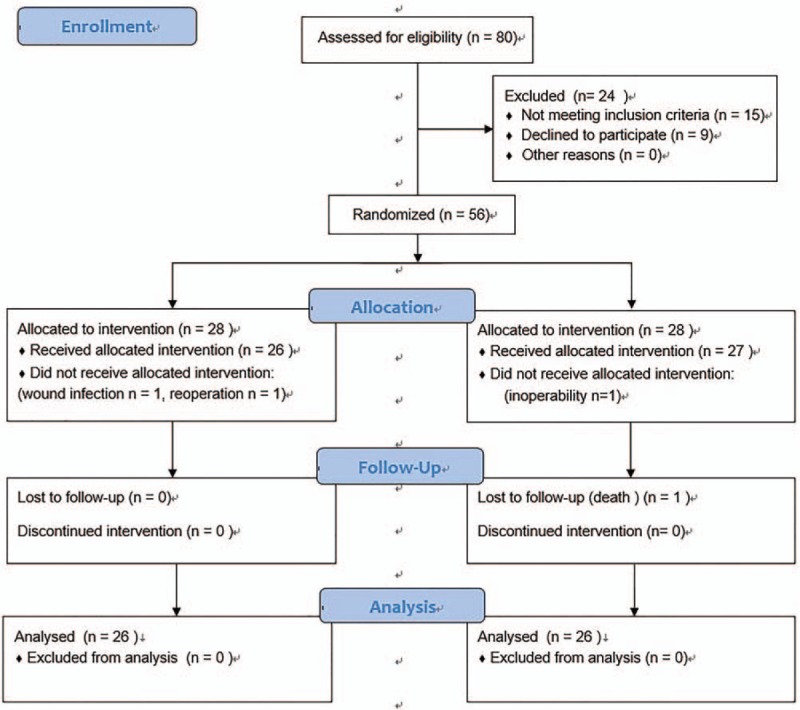
Consolidated standards of reporting trials (CONSORT) flow diagram.
Table 1.
Patient characteristics.
3.1. Primary outcome
The pain score at 24 hours at rest after surgery was significantly lower in group T than in group C (P < .05) (Fig. 2).
Figure 2.
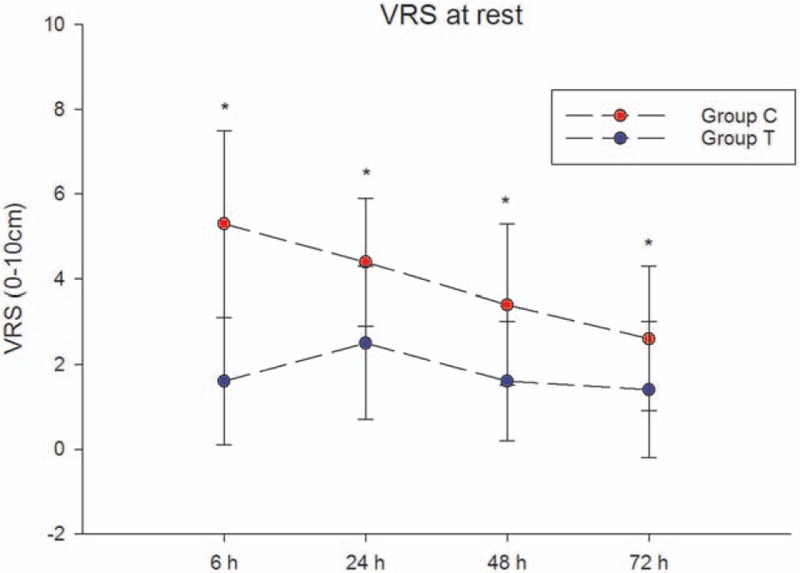
Acute pain scores (VRS) during 72 hours after surgery at rest. Data are presented as mean ± SD. The pain scores were significantly lower in group T than in group C at all time points at rest (P < .05). VRS = verbal rating scale.
3.2. Secondary outcomes
The acute pain scores during the first 72 hours after surgery at rest and at coughing are shown in Fig. 2 and Fig. 3, respectively. The pain scores were significantly lower in group T than in group C at all time points at rest and at coughing (P < .05). The number of patients with VRS scores ≤3 is shown in Fig. 4.
Figure 3.
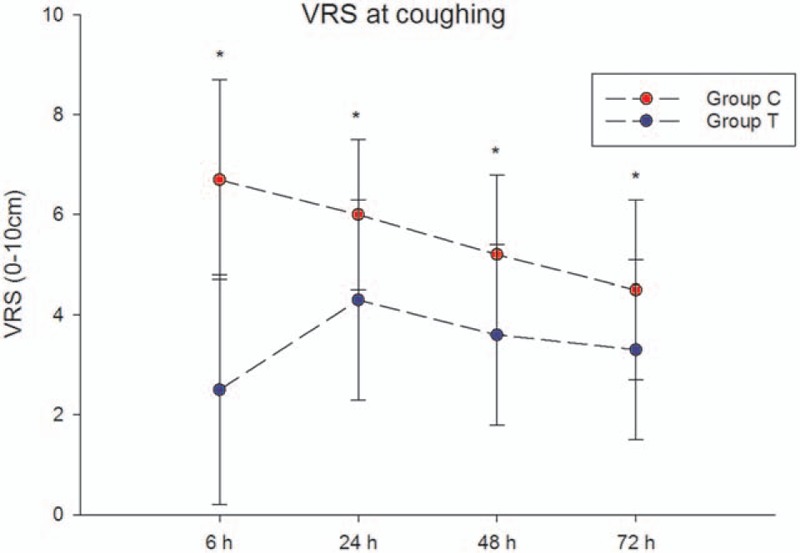
Acute pain scores (VRS) during 72 hours after surgery at coughing. Data are presented as mean ± SD. The pain scores were significantly lower in group T than in group C at all time points at coughing (P < .05). VRS = verbal rating scale.
Figure 4.
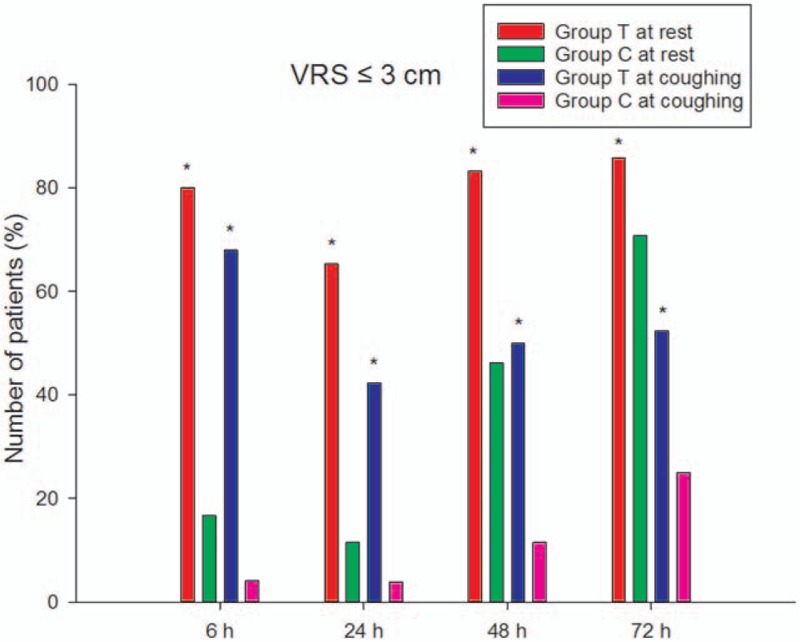
Number of patients with VRS scores ≤3 cm (ie, sufficient postoperative analgesia) during 72 hours after surgery at rest and at coughing. VRS = verbal rating scale.
The PCA cumulative sufentanil consumption and postoperative adverse effects are shown in Fig. 5 and Table 2, respectively. The PCA cumulative sufentanil consumption between the groups was not statistically significant at all time points. There was no statistical difference in terms of nausea/vomiting, drowsiness, atrial arrhythmia, and urinary retention among the 2 groups.
Figure 5.
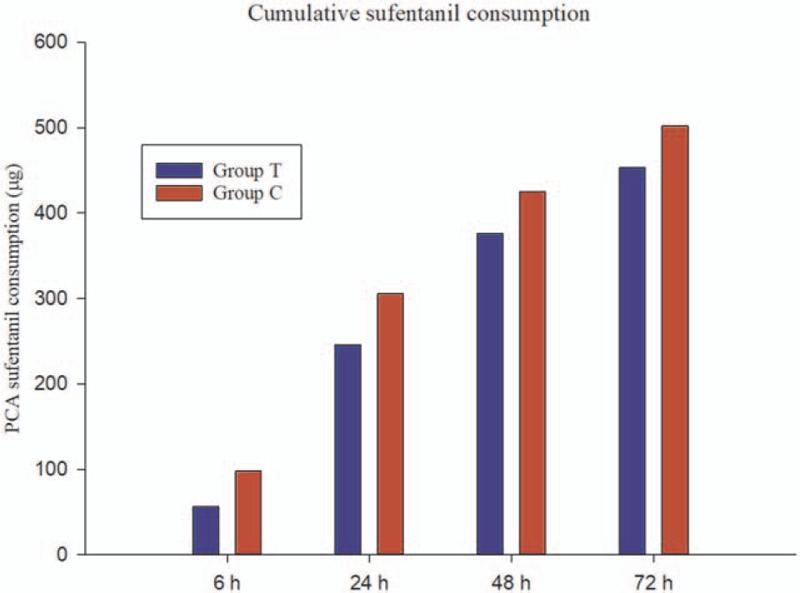
The cumulative sufentanil consumption by intravenous patient-controlled analgesia (μg). The difference between the groups was not statistically significant at all time points. PCA = patient-controlled analgesia.
Table 2.
Adverse effects.
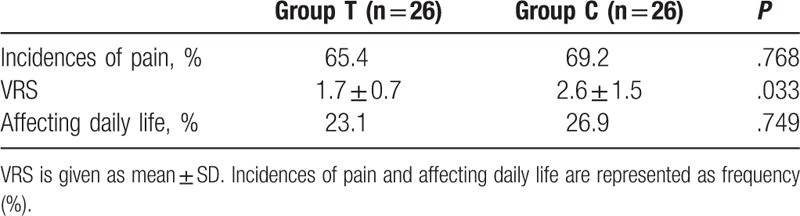
The results of the questionnaire concerning the long-term data are shown in Table 3. There was no statistically significant difference in the incidence of chronic pain between the 2 groups. The pain scores were significantly higher in group C than in group T. There was no statistically significant difference in the number of patients reported that their lives were affected by pain between the 2 groups.
Table 3.
Results of the questionnaire at the third month.
4. Discussion
We found that adding PSTPVB to IV PCA produced clinically significantly lower acute pain scores up to 72 hours after surgery, reduced chronic pain scores, but did not reduce the incidence of CPTP at 3 months compared with using IV PCA alone in patients undergoing thoracotomy. In my opinion, this is the first randomized, controlled, double-blind trial to evaluate the effects of PSTPVB on acute and chronic pain after thoracotomy.
The primary outcome measure in this article was the VRS score at 24 hours at rest after surgery. However, compared with group C, the scores both at rest and at coughing were still lower in group T after 72 hours; this phenomenon is consistent with the findings of other authors who successfully used PSTPVB with different surgical procedures.[8,9,11] The possible reasons for this phenomenon may be explained as follows:
-
1.
The relatively sparse vascularity of the paravertebral space[11]: The vascularity has an impact on local anesthetics uptake and removal. Reduced vascularity can slow the removal of drug and thus prolong the duration of action.
-
2.
The pre-emptive effect of PSTPVB[12]: PSTPVB which can produce not only a very dense afferent blockade of sensory information,[13] but also completely block transmission within the sympathetic chain due to the anatomy of the paravertebral space[12] may have reduced central sensitization, thus reducing postoperative pain.[9]
-
3.
Dexamethasone as a local anesthetic adjuvant by inhibiting potassium channel-mediated discharge of nociceptive C-fibers, attenuating the release of inflammatory mediators, and reducing ectopic neuronal discharge can improve the duration of peripheral nerve block.[14,15] In a review article, 9 studies (801 patients) were included with 393 patients receiving dexamethasone (4–10 mg). Dexamethasone prolonged the analgesic duration of local anesthetics from 730 to 1306 minutes.[16]
There was no significant difference in the incidence of CPTP between the 2 groups at 3 months. The incidence of CPTP in this study is consistent with published data describing CPTP in 62% to 80% of patients at 3 months after thoracotomy.[17,18] There are few data evaluating CPTP in patients receiving PSTPVB. Several studies have shown that thoracic epidural analgesia that covers the whole perioperative period in addition to the postoperative phase is significantly better to reduce the incidence of CPTP than IV PCA.[19,20] This phenomenon may be explained by the excellent effect of the thoracic epidural analgesia: complete perioperative blockade of the nociceptive input to the central nervous system may have attenuated central sensitization, thereby leading to less CPTP.[21] Compared with thoracic epidural analgesia, the efficacy of PSTPVB will wane over time. In this study, PSTPVB did not reduce the PCA cumulative sufentanil consumption. This result is in accordance with several studies of paravertebral block in thoracoscopic surgery.[9,22,23] We also found that nearly half of patients in group T had VRS scores >3 cm at 24, 48, and 72 hours after surgery at coughing. All of this means that sufficient postoperative analgesia may not be provided by PSTPVB alone. High consumption of analgesics during the acute post-thoracotomy period was associated with a higher risk of CPTP.[5,17,19]
The 3-month telephone interview was performed according to the experience of Sentürk et al[20]; the investigator did not ask if the patients had pain at rest or at coughing, so the VRS score at 3 months was to be considered an average score. The average VRS score of CPTP in our study was mild (VRS ≤3 cm) and there was no significant difference in the ratio of affecting daily life between the 2 groups. However, the VRS score of CPTP was found to be significantly lower in the paravertebral block group in our study. The pathogenesis of CPTP is still not completely clear, but the central and peripheral mechanisms are considered to play a significant role.[24] The reason for the lower VRS score of CPTP in the paravertebral block group may be that PSTPVB partially reduces central sensitization.[25]
Our study has limitations. First, we did not test directly whether PSTPVB was done successfully by testing sensory blockade because of the blinded nature of the trial. But, considering that we used real-time ultrasound to guide the block and all blocks were performed by a single experienced anesthesiologist, we believe that the majority of the blocks were effective. Second, the number of patients we recruited was to assess the effect of PSTPVB on acute postoperative pain, and may be insufficient to show the beneficial effects of PSTPVB on CPTP. So, a further research is needed to assess the effects of PSTPVB on CPTP.
5. Conclusion
In conclusion, adding PSTPVB to IV PCA improved acute postoperative pain and chronic pain in patients undergoing thoracotomy, but did not reduce the incidence of CPTP.
Acknowledgment
The authors thank Na Zeng for help in statistical analyses.
Author contributions
XLL and YZ contributed equally and should be co-first authors to this work.
Conceptualization: Ye Zhang.
Data curation: Tian Dai, Lei Wan.
Investigation: Tian Dai.
Methodology: Guan-Nan Ding.
Writing – original draft: Xiu-Liang Li.
Writing – review & editing: Ye Zhang.
Footnotes
Abbreviations: CPTP = chronic post-thoracotomy pain, IV PCA = intravenous patient-controlled analgesia, PSTPVB = preoperative single-dose thoracic paravertebral block, VRS = verbal rating scale.
The authors have no conflicts of interest to disclose.
References
- [1].Kavanagh BP, Katz J, Sandler AN. Pain control after thoracic surgery. A review of current techniques. Anesthesiology 1994;81:737–59. [DOI] [PubMed] [Google Scholar]
- [2].Baik JS, Oh AY, Cho CW, et al. Thoracic paravertebral block for nephrectomy: a randomized, controlled, observer-blinded study. Pain Med 2014;15:850–6. [DOI] [PubMed] [Google Scholar]
- [3].Sabanathan S, Mearns AJ, Bickford SP, et al. Efficacy of continuous extrapleural intercostal nerve block on post-thoracotomy pain and pulmonary mechanics. Br J Surg 1990;77:221–5. [DOI] [PubMed] [Google Scholar]
- [4].Sabanathan S. Has postoperative pain been eradicated? Ann R Coll Surg Engl 1995;77:202–9. [PMC free article] [PubMed] [Google Scholar]
- [5].Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg 2009;36:170–80. [DOI] [PubMed] [Google Scholar]
- [6].Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl 1986;3:S1–226. [PubMed] [Google Scholar]
- [7].Yeung JH, Gates S, Naidu BV, et al. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev 2016;2:D9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kairaluoma PM, Bachmann MS, Korpinen AK, et al. Single-injection paravertebral block before general anesthesia enhances analgesia after breast cancer surgery with and without associated lymph node biopsy. Anesth Analg 2004;99:1837–43. [DOI] [PubMed] [Google Scholar]
- [9].Vogt A, Stieger DS, Theurillat C, et al. Single-injection thoracic paravertebral block for postoperative pain treatment after thoracoscopic surgery. Br J Anaesth 2005;95:816–21. [DOI] [PubMed] [Google Scholar]
- [10].Shibata Y, Nishiwaki K. Ultrasound-guided intercostal approach to thoracic paravertebral block. Anesth Analg 2009;109:996–7. [DOI] [PubMed] [Google Scholar]
- [11].Copik M, Bialka S, Daszkiewicz A, et al. Thoracic paravertebral block for postoperative pain management after renal surgery: a randomised controlled trial. Eur J Anaesthesiol 2017;34:596–601. [DOI] [PubMed] [Google Scholar]
- [12].Lonnqvist PA. Pre-emptive analgesia with thoracic paravertebral blockade? Br J Anaesth 2005;95:727–8. [DOI] [PubMed] [Google Scholar]
- [13].Richardson J, Jones J, Atkinson R. The effect of thoracic paravertebral blockade on intercostal somatosensory evoked potentials. Anesth Analg 1998;87:373–6. [DOI] [PubMed] [Google Scholar]
- [14].Attardi B, Takimoto K, Gealy R, et al. Glucocorticoid induced up-regulation of a pituitary K+ channel mRNA in vitro and in vivo. Receptors Channels 1993;1:287–93. [PubMed] [Google Scholar]
- [15].Johansson A, Hao J, Sjolund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand 1990;34:335–8. [DOI] [PubMed] [Google Scholar]
- [16].Choi S, Rodseth R, Mccartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: a systematic review and meta-analysis of randomized trials. Br J Anaesth 2014;112:427–39. [DOI] [PubMed] [Google Scholar]
- [17].Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta Anaesthesiol Scand 1999;43:563–7. [DOI] [PubMed] [Google Scholar]
- [18].Ju H, Feng Y, Yang BX, et al. Comparison of epidural analgesia and intercostal nerve cryoanalgesia for post-thoracotomy pain control. Eur J Pain 2008;12:378–84. [DOI] [PubMed] [Google Scholar]
- [19].Tiippana E, Nilsson E, Kalso E. Post-thoracotomy pain after thoracic epidural analgesia: a prospective follow-up study. Acta Anaesthesiol Scand 2003;47:433–8. [DOI] [PubMed] [Google Scholar]
- [20].Sentürk M, Ozcan PE, Talu GK, et al. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth Analg 2002;94:11–5. [DOI] [PubMed] [Google Scholar]
- [21].Kissin I. Preemptive analgesia. Why its effect is not always obvious. Anesthesiology 1996;84:1015–9. [DOI] [PubMed] [Google Scholar]
- [22].Hill SE, Keller RA, Stafford-Smith M, et al. Efficacy of single-dose, multilevel paravertebral nerve blockade for analgesia after thoracoscopic procedures. Anesthesiology 2006;104:1047–53. [DOI] [PubMed] [Google Scholar]
- [23].Marret E, Bazelly B, Taylor G, et al. Paravertebral block with ropivacaine 0.5% versus systemic analgesia for pain relief after thoracotomy. Ann Thorac Surg 2005;79:2109–13. [DOI] [PubMed] [Google Scholar]
- [24].Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother 2009;9:723–44. [DOI] [PubMed] [Google Scholar]
- [25].Karmakar MK, Samy W, Li JW, et al. Thoracic paravertebral block and its effects on chronic pain and health-related quality of life after modified radical mastectomy. Reg Anesth Pain Med 2014;39:289–98. [DOI] [PubMed] [Google Scholar]


