Abstract
Rationale:
Hepatocellular carcinoma (HCC) is a highly invasive cancer associated with high mortality rates. Although sorafenib is currently recommended as standard treatment for advanced HCC, its treatment efficacy is limited. Effective treatments for patients with advanced HCC that progresses on or after sorafenib treatment or patients who are intolerant of sorafenib remain an unmet medical need.
Patient concerns:
We report an advanced HCC patient with many lung metastases who failed sorafenib treatment.
Diagnoses:
Sorafenib refractory HCC patient with a large number of lung metastases.
Interventions:
The apatinib alone was used as second line therapy.
Outcomes:
The patient achieved partial response (PR) soon after the treatment, which was maintained for approximately 1 year. During the entire process, the lung metastases continued to diminish. Finally, only a few lesions remained
Lessons:
Apatinib alone may be a good second-line therapy for advanced HCC patients who are refractory to sorafenib. However, further investigation in future prospective clinical studies is warranted.
Keywords: apatinib, hepatocellular carcinoma, second-line therapy, sorafenib refractory
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third most common cause of cancer-related death.[1] Patients often present with unresectable, recurrent, or metastatic HCC, for which chemotherapy has been shown to be ineffective.[2,3] Sorafenib is currently acknowledged as a standard therapy for advanced HCC, but its efficacy is very limited, especially in Asian populations, extending overall survival only 2.3 months.[3] Effective treatments for patients with advanced HCC that progresses on or after sorafenib treatment or patients who are intolerant of sorafenib remain an unmet medical need.[4]
Apatinib is a small-molecule tyrosine kinase inhibitor that selectively binds to vascular endothelial growth factor (VEGF) receptor 2, resulting in a decrease in VEGF-mediated endothelial cell migration, proliferation, and tumor microvascular density.[5,6] It has been recommended as a third-line treatment for metastatic gastric cancer patients.[6] However, there have been very few reports evaluating its efficacy and safety in patients with HCC. In addition, in past reports apatinib has always been combined with transcatheter arterial chemoembolization (TACE) or radiotherapy.[7–9] We report here a case of advanced sorafenib-refractory HCC with many lung metastases treated with apatinib alone as second-line therapy showing an excellent effect in our hospital. This report was approved by the West China Hospital institutional review board, and the patient provided written informed consent.
2. Case presentation
A 43-year-old man was brought to the outpatient department of our hospital with symptoms of cough and hemoptysis 2.5 years ago. HCC with lung metastases was first diagnosed at his local hospital in April 2015. He had a history of hepatitis B infection and liver cirrhosis for 13 years. The patient also had a history of hypertension and diabetes for 4 years. At the time of his visit, he was taking nifedipine 30 mg daily and irbesartan 150 mg twice daily for hypertension and entecavir 0.5 mg daily for hepatitis B. He also received insulin 14 IU twice daily for diabetes. A liver puncture biopsy confirmed the diagnosis of HCC. The patient then began taking sorafenib 400 mg twice a day. In addition, he underwent TACE twice (in April and June 2015). Computed tomography (CT) at our hospital (September 18, 2015) showed a large number of lung metastases (Figs. 1A, 2A, 3A, and 4A) and 3 liver masses with lipiodol deposition (Fig. 5A). His alpha fetoprotein (AFP) level was 183.50 ng/mL. The evaluation indicated progression disease (PD) because there were many new and enlarged lung metastases compared with the CT images from his local hospital in April 2015. Therefore, treatment with apatinib was suggested, and the patient began to take apatinib 750 mg once daily and stopped sorafenib on September 23, 2015. The patient received no other treatment. Three months later (December 23, 2015), a CT evaluation showed an excellent effect. The lung metastases had diminished remarkably, and a few metastases had disappeared (Figs. 1B, 2B, 3B, and 4B). The liver masses were almost the same (Fig. 5B). The evaluation indicated partial response (PR). His AFP level was decreased to 59.00 ng/mL. The patient continued to take apatinib. The CT evaluation on April 12 (Figs. 1C, 2C, 3C, and 4C) and November 18, 2016 (Figs. 1D, 2D, 3D, and 4D) showed that the lung metastases had continued to diminish and many lung metastases disappeared. The masses in the liver remained the same (Fig. 5C, D). Therefore, the tumor evaluation showed continued PR for approximately 1 year. The patient's AFP level was 70.09 ng/mL. However, this patient got a serious lung infection on January 10, 2017 and died on January 15, 2017.
Figure 1.
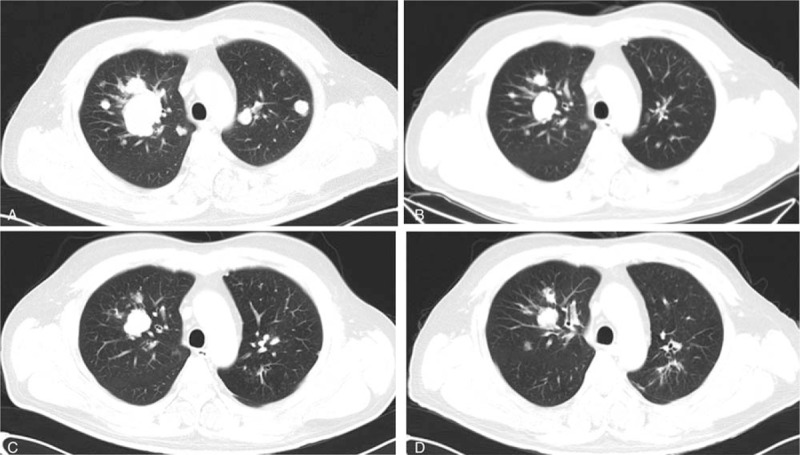
A, The lung metastases before the apatinib treatment (September 18, 2015). B, The lung metastases 3 months after the apatinib treatment (December 23, 2015). C, The lung metastases 7 months after the apatinib treatment (April 12, 2016). D, The lung metastases 14 months after the apatinib treatment (November 18, 2016).
Figure 2.
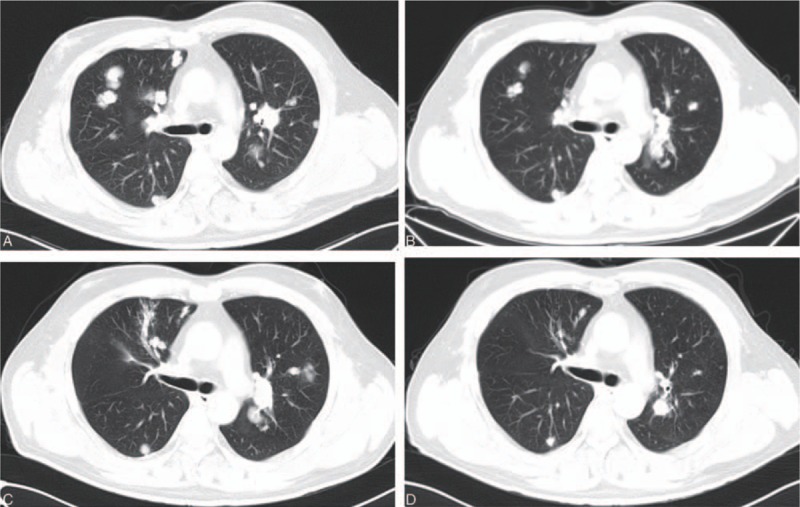
A, The lung metastases before the apatinib treatment (September 18, 2015). B, The lung metastases 3 months after the apatinib treatment (December 23, 2015). C, The lung metastases 7 months after the apatinib treatment (April 12, 2016). D, The lung metastases 14 months after the apatinib treatment (November 18, 2016).
Figure 3.
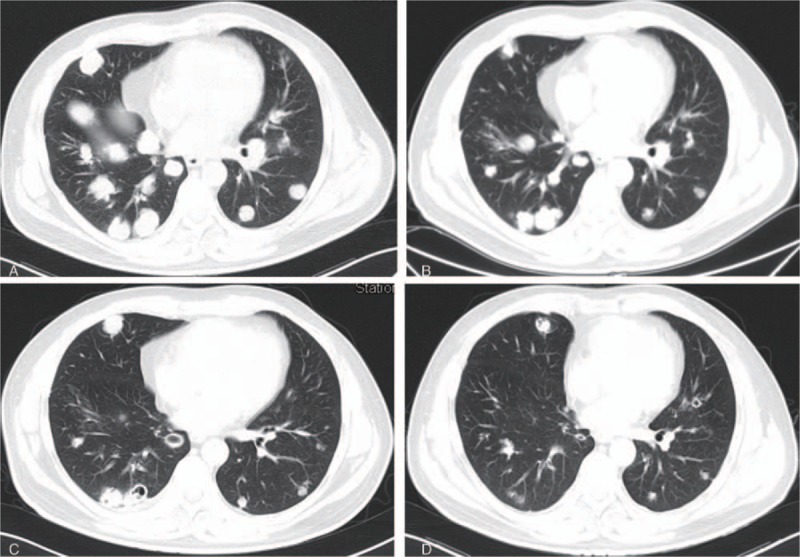
A, The lung metastases before the apatinib treatment (September 18, 2015). B, The lung metastases 3 months after the apatinib treatment (December 23, 2015). C, The lung metastases 7 months after the apatinib treatment (April 12, 2016). D, The lung metastases 14 months after the apatinib treatment (November 18, 2016).
Figure 4.
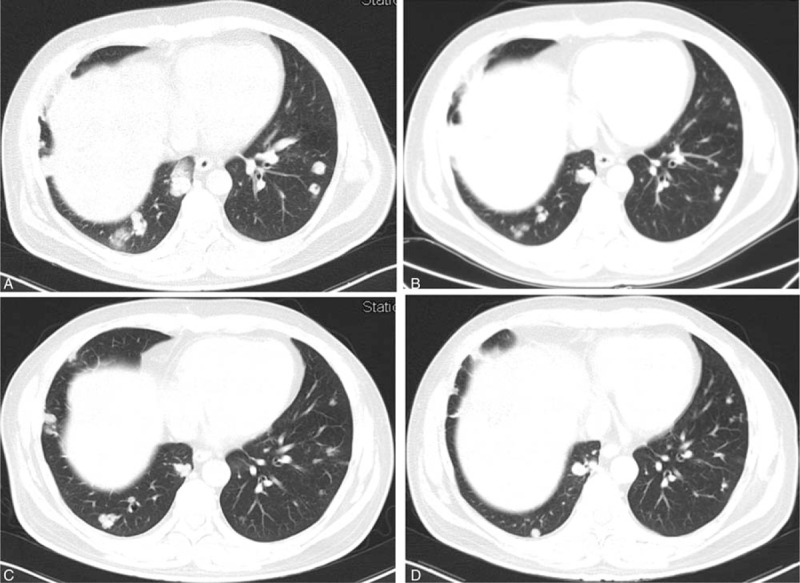
A, The lung metastases before the apatinib treatment (September 18, 2015). B, The lung metastases 3 months after the apatinib treatment (December 23, 2015). C, The lung metastases 7 months after the apatinib treatment (April 12, 2016). D, The lung metastases 14 months after the apatinib treatment (November 18, 2016).
Figure 5.
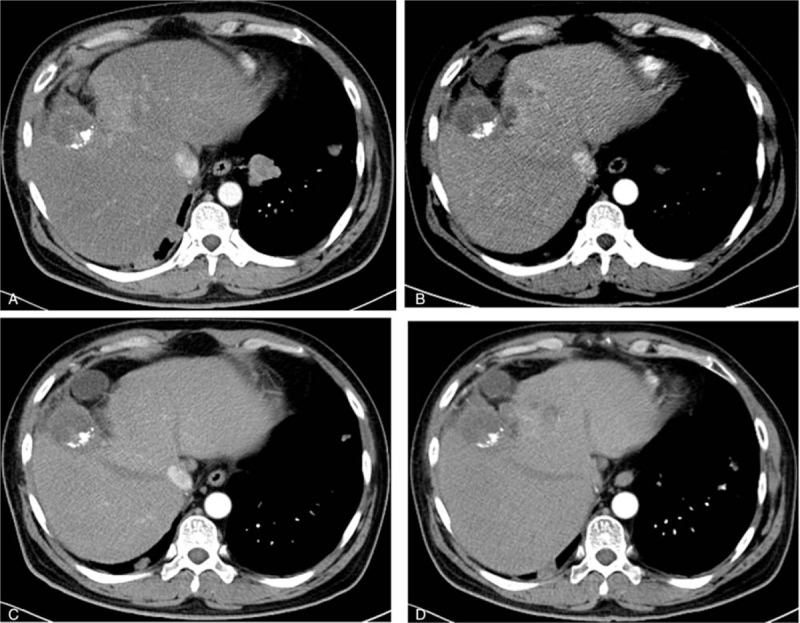
A, The liver mass before the apatinib treatment (September 18, 2015). B, The liver mass 3 months after the apatinib treatment (December 23, 2015). C, The liver mass 7 months after the apatinib treatment (April 12, 2016). D, The liver mass 14 months after the apatinib treatment (November 18, 2016).
During the treatment process, the patient experienced grade 3 hypertension, grade 2 hand-foot syndrome, grade 3 proteinuria, and grade 2 thrombocytopenia. Nevertheless, the symptoms relieved soon after the appropriate treatments were administered. The cough and hemoptysis continued after the apatinib treatment, but they were mild and could be controlled by hemostatic therapy.
3. Discussion
HCC is a leading cause of death in Eastern countries, where hepatitis B and C viruses are prevalent.[3] A minority of patients are diagnosed with resectable HCC, whereas approximately 70% to 85% of HCC patients have locally advanced unresectable or metastatic disease at diagnosis.[1,2] Advanced HCC carries a poor prognosis, and systemic therapy with cytotoxic agents provides a marginal benefit.[10]
HCC is featured by vascular recruitment and rich in vascular vessels always leads to early recurrence after surgical operation.[10–12] Sorafenib, a multikinase inhibitor that targets multiple signaling pathways, including VEGF signaling, is the only systemic agent to demonstrate an overall survival benefit as first-line therapy in advanced HCC.[2,3] However, disease control with sorafenib is short lived.[3,4] Therefore, there is an unmet need for effective systemic therapies for HCC, particularly after treatment with sorafenib.
In this report, the patient used sorafenib for 5 months. However, there were many new metastases of the lung, and the previous lesions of the lung grew. Therefore, the patient experienced disease progression soon after the first-line treatment. It was thought that the patient would die in a short time. As there was no standard treatment after sorafenib, apatinib was suggested. Surprisingly, he came to our hospital 3 months later. The CT results showed the lung metastases had diminished remarkably and a few metastases disappeared (PR). Furthermore, the PR state continued for approximately 1 year. At his last follow-up visit (November 18, 2016), most of the lung metastases had disappeared with several small lesions remaining and the patient had a very good performance status. Regretfully, he died of a lung infection <2 months later.
Apatinib, a new antiangiogenesis small-molecule oral agent, has been recommended for patients with advanced or metastatic gastric cancer for whom 2 or more lines of chemotherapy have failed.[5,6] It has not been reported for other cancers in clinical trials, except for some case reports. Kou et al[13] reported treatment of a patient with HCC using a combination of apatinib and TACE followed by oxaliplatin-based chemotherapy; the patient's progression-free survival (PFS) time was 8 months. Liu et al[9] reported a retrospective study involving 19 patients with HCC treated with apatinib and TACE, with a median PFS time of 8.1 months. In addition, the apatinib was used as first-line treatment in these 2 reports.[9,13] In the present case report, the apatinib was used alone as second-line treatment in a patient with sorafenib-resistant HCC and many lung metastases. Nevertheless, the patient showed PR 3 months after the treatment, and the PR state continued for approximately 1 year. During the entire process, the lung metastases continued to diminish. Finally, only very few lesions remained. If the patient had not gotten a lung infection, he would have had a much longer PFS time. The adverse effects were tolerable and were easily relieved by appropriate treatments.
4. Conclusion
Apatinib alone as second-line treatment of a patient with HCC and many lung metastases who failed treatment with sorafenib showed an excellent antitumor effect. Most of the lung metastases diminished with only a few lesions left. The patient achieved PR soon after the treatment, which lasted for approximately 1 year. Apatinib alone may be a good choice for the second-line treatment of HCC when sorafenib fails; however, further investigation in future prospective clinical studies is warranted.
Author contributions
Conceptualization: Ji Duo.
Data curation: Hong Zhu, Xiaojun Ma.
Formal analysis: Hong Zhu, Ji Duo.
Investigation: Hong Zhu, Xiaojun Ma, Yaqin Zhao.
Resources: Hong Zhu.
Supervision: Ji Duo.
Writing – original draft: Hong Zhu, Xiaojun Ma, Yaqin Zhao.
Writing – review and editing: Hong Zhu, Ji Duo.
Footnotes
Abbreviations: AFP = alpha fetoprotein, CT = computed tomography, HCC = hepatocellular carcinoma, PD = progression disease, PFS = progression-free survival, PR = partial response, TACE = transcatheter arterial chemoembolization, VEGF = vascular endothelial growth factor.
The authors have no conflicts of interest to disclose.
References
- [1].Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27:1485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- [3].Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- [4].Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56–66. [DOI] [PubMed] [Google Scholar]
- [5].Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219–25. [DOI] [PubMed] [Google Scholar]
- [6].Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–54. [DOI] [PubMed] [Google Scholar]
- [7].Zhu H, Zhao Y, Wang X. The radiosensitive effect of apatinib for hepatocellular carcinoma patient with big paraspinal metastasis: a case report. Medicine 2018;97:e9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lu W, Jin XL, Yang C, et al. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: a single-center randomized controlled trial. Cancer Biol Ther 2017;18:433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu C, Xing W, Si T, et al. Efficacy and safety of apatinib combined with transarterial chemoembolization for hepatocellular carcinoma with portal venous tumor thrombus: a retrospective study. Oncotarget 2017;8:100734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Burroughs A, Hochhauser D, Meyer T. Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol 2004;5:409–18. [DOI] [PubMed] [Google Scholar]
- [11].Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol 2013;31:4067–75. [DOI] [PubMed] [Google Scholar]
- [12].Poon RT, Ng IO, Lau C, et al. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol 2002;20:1775–85. [DOI] [PubMed] [Google Scholar]
- [13].Kou P, Zhang Y, Shao W, et al. Significant efficacy and well safety of apatinib in an advanced liver cancer patient: a case report and literature review. Oncotarget 2017;8:20510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


