Abstract
Most previous research investigating osteoporosis in rheumatoid arthritis (RA) has focused on female patients and there is a lack of data regarding clinical characteristics of osteoporosis in male patients with RA.
The aim of this study was to compare the frequency of osteoporosis between male patients with RA and healthy patients, and to identify the risk factors for osteoporosis and low bone mineral density (BMD) in male patients with RA.
We conducted a retrospective, cross-sectional study including 76 South Korean male patients with RA aged over 50 years and 76 age-matched male healthy individuals. BMD was measured at the lumbar spine (L1-4) and left hip (femoral neck and total hip) using dual energy X-ray absorptiometry. Osteoporosis was defined as a T-score of ≤ −2.5 according to the World Health Organization (WHO) classification.
The frequency of osteoporosis at either the spine or the hip among male patients with RA was significantly higher than that among controls (22.4% vs 10.5%, P = .049) and RA patients had a significantly lower total hip BMD than healthy individuals (0.92 ± 0.14 vs 0.96 ± 0.1 g/cm2, P = .027). For male RA patients, the mean 28-joint Disease Activity Scores using erythrocyte sedimentation rate (DAS28-ESR) and body mass index (BMI) were 3.28 and 22 kg/m2, respectively. In multivariable logistic regression models, BMI ≤ 22 kg/m2 (odds ratio = 3.43, P = .043) and DAS28-ESR > 3.2 (odds ratio = 3.85, P = .032) were independent risk factors for osteoporosis at either site in male patients with RA.
Our data demonstrate that male patients with RA had a 2.1 times higher risk for osteoporosis compared with healthy individuals. This suggests that appropriate management of osteoporosis in patients with RA is crucial not only for postmenopausal women but also for men aged over 50 years, especially those with low BMI and higher disease activity.
Keywords: bone density, inflammation, male, osteoporosis, rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by polyarticular small joint arthritis leading to joint destruction and extra-articular manifestations, such as rheumatoid nodules and cardiopulmonary and hematologic disorders. In addition to periarticular bone loss and local erosions, systemic bone loss begins early during the course of the disease and is a well-established extra-articular complication of RA caused by sustained inflammation, glucocorticoids (GCs) use, immobility, disability, sarcopenia, and traditional risk factors such as aging and postmenopausal state.[1–3] In particular, GCs is one of the major risk factor for systemic bone loss in RA. Two-phase of bone loss seems to be induced by GCs: a rapid first phase when 12% of bone mass decrease in the first 6 to 12 months of treatment, followed by a subsequent chronic phase with a slower rate of bone loss. Thus, RA is now considered one of the most common secondary causes of osteoporosis and bone densitometry should be performed routinely in patients with RA. Previous epidemiologic studies showed that patients with RA have a higher prevalence of osteoporosis and higher incidence of vertebral and nonvertebral fracture as compared with the general population,[4–7] contributing to considerable morbidity and mortality as well as medical and societal cost. In particular, the 1-year cumulative mortality rate due to hip fracture in RA patients was reported to be approximately 20% and significantly higher than that in general population.[8] Accordingly, appropriate management of osteoporosis and prevention of fragility fracture in patients with RA are crucial to optimize clinical outcome.[9] However, despite advances in therapeutics for RA disease control, there has been no apparent change in the incidence of osteoporotic fracture in patients with RA in recent decades,[10] which imposes a significant burden in clinical practice.
Osteoporosis has been generally recognized as a disease affecting women, because men do not experience rapid generalized bone loss after menopause.[11] Instead, men have more gradual and less severe bone loss that usually begins by the sixth decade of life and occurs at an average rate of 0.5% to 1% per year.[12] Although male osteoporosis is less frequent than female osteoporosis and seems to be an underdiagnosed and undertreated condition, there has been growing awareness to its clinical importance. One out of 8 men aged older than 50 years will sustain an osteoporotic fracture during their remaining lifetime, and men account for approximately 30% of hip fractures.[11,13,14] Previous studies demonstrated that the mortality rate associated with fragility fracture is much higher in men than in women.[15–17] These data collectively indicate that osteoporosis can be a serious health problem for not only women but also men, and rheumatologists need to pay special attention to osteoporosis in male patients with RA. However, because postmenopausal women are at a greater risk for osteoporosis, most previous research investigating osteoporosis in RA has focused on female patients[4,5] and there is a lack of data regarding clinical characteristics of osteoporosis in male patients with RA. Therefore, it is necessary to determine the clinical importance of systemic bone loss in male RA patients. The purpose of the present study is to compare the frequency of osteoporosis between male patients with RA and healthy individuals and to identify risk factors for osteoporosis and low bone mineral density (BMD) in male patients with RA.
2. Materials and methods
2.1. Study design and patients
We conducted a retrospective cross-sectional study including 76 male patients with RA aged over 50 years and 76 age-matched (±2 years) male healthy individuals who underwent BMD measurement by dual energy X-ray absorptiometry (DEXA) at a university-affiliated hospital in South Korea from January 2012 to December 2015. All patients with RA fulfilled the 2010 American College of Rheumatology/European League Against Rheumatism collaborative initiative classification criteria for RA.[18] The following patients with RA were excluded from our study: patients with rheumatic diseases other than RA; patients with prior spine and/or hip surgery or metal implants that might affect BMD measurement; patients who had previously received or were currently receiving anti-osteoporotic treatments such as bisphosphonates, selective estrogen receptor modulators, teriparatide, or denosumab, except for calcium and/or vitamin D; patients with stage III or higher chronic kidney disease (CKD); and patients with pre-existing hypothyroidism or hyperthyroidism. Healthy controls were selected randomly from applicants for a routine health check up at the same hospital who had no history of rheumatic disease, including RA, no history of chronic diseases that could affect BMD, such as CKD or thyroid diseases, and no history of taking medication for osteoporosis. All patients with RA and healthy controls in our study were South Korean. The present study was approved by the Research and Ethical Review Board of the Pusan National University Hospital, which waived informed consent for study participants due to the retrospective study design (IRB no. 1610-009-048).
2.2. BMD measurements
BMD was measured at the lumbar spine (L1-4) and left hip (femoral neck and total hip) using DEXA equipment (GE-Lunar Prodigy or Lunar Prodigy advance, Madison, MA). BMD was expressed in grams per square centimeter (g/cm2) and the standard deviation (SD) from the young and healthy population (T-score). We used South Korean female reference data provided by the manufacturers when calculating the T-score, as previously recommended.[19–22] All procedures were conducted according to the standardized procedures for BMD measurements provided by the manufacturer and the coefficient of variance was 0.33% at the spine and 0.4% at the hip in our hospital. Osteoporosis in men aged 50 years or older was defined as T-scores of −2.5 or lower, according to the World Health Organization (WHO) classification.[23]
2.3. Clinical assessments
Demographic and anthropometric data, such as age and body mass index (BMI), were collected from both patients with RA and healthy controls. BMI was determined by dividing the body weight by the square of the height in meters (kg/m2). For patients with RA, the following clinical and laboratory markers were recorded: disease duration, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) levels, disease activity score assessed using the 28-joint count for swelling and tenderness (DAS28)-ESR, rheumatoid factor, anti-cyclic citrullinated peptide (CCP) antibody levels, van der Heijde modified total Sharp score (vdH-mTSS), and current medications, such as disease-modifying anti-rheumatic drugs (DMARDs) and GCs. Serum CRP level was determined by particle-enhanced immunoturbidimetry (Tina-quant C-reactive protein assay; Roche Diagnostics, Zurich, Switzerland) with a P800 Module (Roche Diagnostics). DAS28-ESR score was = 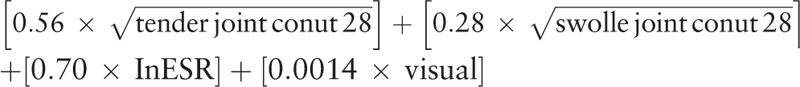 .[24] High disease activity was defined as a DAS28-ESR of > 5.1; moderate disease activity was defined as 3.2 < DAS28-ESR ≤ 5.1; low disease activity was defined as 2.6 < DAS28-ESR ≤ 3.2; remission was defined as DAS28-ESR ≤ 2.6.[25] Serum RF titer was assessed using a particle-enhanced immunoturbidimetric assay (range 0–14 IU/mL) and serum anti-CCP antibody titer was measured by a chemiluminescent microparticle immunoassay (range 0–5 U/mL). The vdH-mTSS of patients with RA was assessed by an experienced rheumatologist who was blind to clinical and laboratory data, using hand and foot X-ray images.[26] Data regarding current use of DMARDs, including methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, tacrolimus, and tumor necrosis factor-α (TNF-α) inhibitor were collected. The cumulative dose (g) of GCs was expressed as prednisone equivalent and was determined by multiplying the current daily dose by the number of days for which patients with RA had been treated with GCs since first prescription.
.[24] High disease activity was defined as a DAS28-ESR of > 5.1; moderate disease activity was defined as 3.2 < DAS28-ESR ≤ 5.1; low disease activity was defined as 2.6 < DAS28-ESR ≤ 3.2; remission was defined as DAS28-ESR ≤ 2.6.[25] Serum RF titer was assessed using a particle-enhanced immunoturbidimetric assay (range 0–14 IU/mL) and serum anti-CCP antibody titer was measured by a chemiluminescent microparticle immunoassay (range 0–5 U/mL). The vdH-mTSS of patients with RA was assessed by an experienced rheumatologist who was blind to clinical and laboratory data, using hand and foot X-ray images.[26] Data regarding current use of DMARDs, including methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, tacrolimus, and tumor necrosis factor-α (TNF-α) inhibitor were collected. The cumulative dose (g) of GCs was expressed as prednisone equivalent and was determined by multiplying the current daily dose by the number of days for which patients with RA had been treated with GCs since first prescription.
2.4. Statistical analyses
Continuous variables are reported as mean ± SD or median (interquartile range; IQR), as appropriate, and categorical variables are reported as the number (percentage). For group comparisons, Student 2-tailed t test or Mann–Whitney U test for continuous variables and Pearson Chi-square test or Fisher exact test for categorical variables were used, as appropriate. To determine the factors associated with BMD in male patients with RA, we conducted a stepwise multivariable linear regression analysis. We conducted stepwise multivariable linear regression models including variables with P < .1 in univariable analysis and a priori confounding factors such as age and BMI. Multivariable logistic regression models with backward selection adjusting for variables with P < .1 in univariable analysis and a priori confounding factors such as age were used to calculate odds ratios (ORs) for the presences of osteoporosis in patients with RA. A 2-sided P < .05 was considered to be statistically significant. All statistical analyses were performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL) and STATA version 11.1 for Windows (StataCorp LP, College Station, TX).
3. Results
Clinical and laboratory characteristics of male patients with RA are summarized in Table 1. Median (IQR) disease duration was 37.5 (1.3–105.8) months and the frequency of patients with RA having positive results for RF and anti-CCP antibody was 91.8% and 80.6%, respectively. Mean DAS28-ESR was 3.28 ± 1.44 and median vdH-mTSS was 4 (1–9). In addition, 38 (50%) patients showed moderate to high disease activity, whereas 30 patients with RA had remission status. The most common concomitant DMARDs was methotrexate (52.6%), followed by hydroxychloroquine (39.5%) and sulfasalazine (22.4%). The majority of patients with RA (77.6%) were treated with GCs and median (IQR) cumulative dose of GCs was 3.11 g (1.65–8.15). Seventeen patients (22.4%) received calcium and/or vitamin D supplementation.
Table 1.
Clinical and laboratory characteristics in male patients with rheumatoid arthritis.
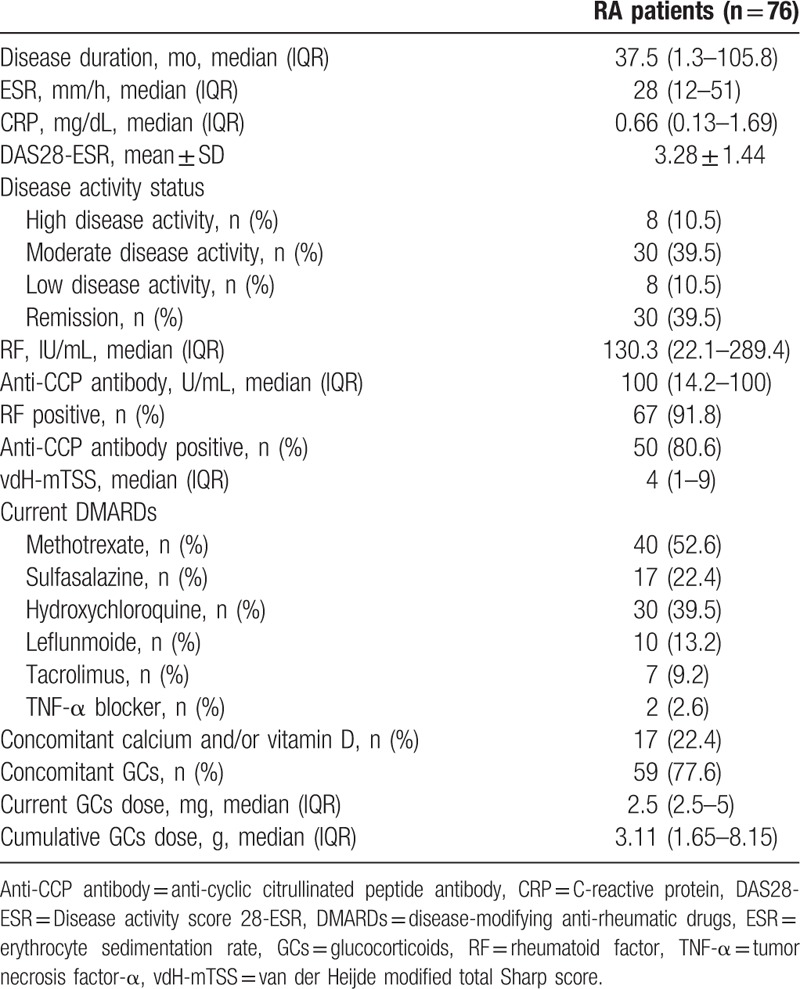
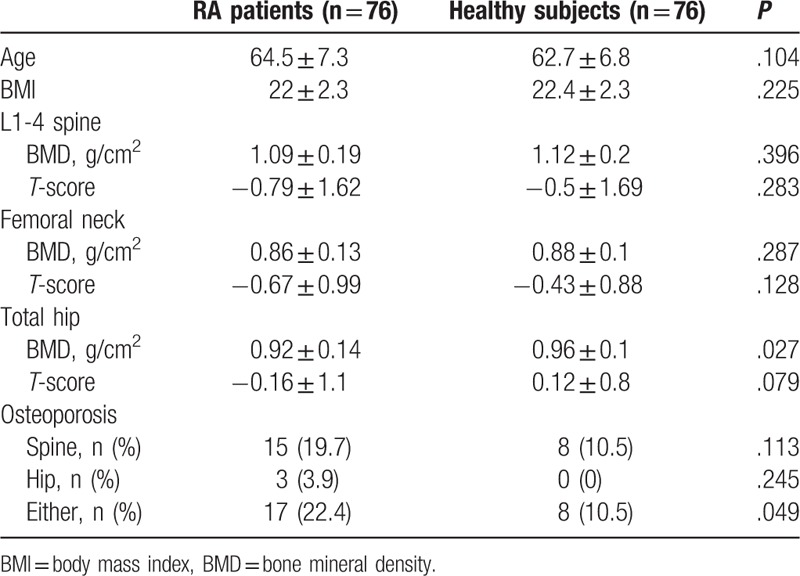
Table 2 summarizes comparisons of BMD and the frequency of osteoporosis between male patients with RA and healthy individuals. Age and BMI in patients with RA were comparable with those of healthy controls. Patients with RA had a significantly lower total hip BMD than healthy individuals (0.92 ± 0.14 vs 0.96 ± 0.1 g/cm2, P = .027), but there was no significant difference in L1-4 and femur neck BMD between patients with RA and controls. In subgroup analyses, no significant differences in L1-4, femoral neck, and total hip BMD between RA patients not treated with GCs and controls were observed (data not shown), whereas RA patients receiving GCs therapy had significantly lower total hip BMD than healthy subjects (0.92 ± 0.14 vs 0.96 ± 0.11 g/cm2, P = .031). The overall frequency of osteoporosis at either the spine or the hip in male patients with RA was significantly higher than healthy controls (22.4% vs 10.5%, respectively; P = .049). However, no significant differences in the prevalence of osteoporosis at the spine (19.7% vs 10.5%, respectively; P = .113) and the hip (3.9% vs 0%, respectively; P = .245) were found between patients with RA and healthy controls.
Table 2.
Comparisons of bone mineral density and the frequency of osteoporosis between patients with rheumatoid arthritis and healthy subjects.
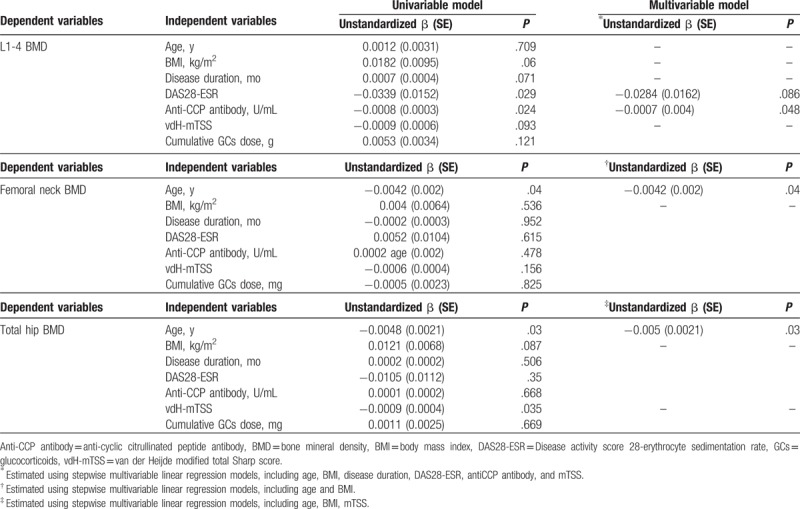
Associated factors for BMD in male patients with RA were analyzed by linear regression analyses (Table 3). Of interest, increased anti-CCP antibody titer significantly correlated with low L1-4 BMD (unstandardized β = −0.0007, P = .048) after adjusting for confounding factors. Higher DAS28-ESR appeared to be associated with lower L1-4 BMD (unstandardized β = −0.0086, P = .086), but did not reach statistical significance in the multivariable linger regression model. As expected, older age was a significant risk factor for low femoral neck and total hip BMD in multivariable linear regression analyses (Table 3). Radiographic damages measured by vdH-mTSS correlated with low total hip BMD in the univariable model, but this association was not statistically significant in the multivariable model (Table 3). In addition, BMI, disease duration, and cumulative GC dose did not show a significant association with BMD in male patients with RA.
Table 3.
Linear regression models for bone mineral density in male patients with rheumatoid arthritis.
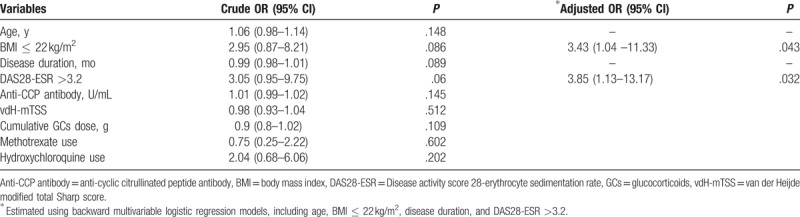
Patients with RA with osteoporosis had significantly lower BMI and higher disease activity than those without osteoporosis (Table 4). No significant differences in age, disease duration, RF titer, anti-CCP antibody titer, vdH-mTSS, and cumulative GC dose according to the presence of osteoporosis were observed (Table 4). Risk factors for osteoporosis in male patients with RA analyzed by logistic regression models are summarized in Table 5. In univariable analysis, BMI ≤ 22 kg/m2 and DAS28-ESR > 3.2 significantly increased the risk for osteoporosis, whereas longer disease duration tended to be associated with the presence of osteoporosis in male patients with RA. Backward multivariable logistic regression models revealed that both BMI ≤ 22 kg/m2 [(OR = 3.43, 95% confidence interval (95% CI) = 1.04–11.33, P = .043] and DAS28-ESR > 3.2 (OR = 3.85, 95% CI = 1.13–13.17, P = .032) were independent risk factors for osteoporosis in male patients with RA (Table 5). However, no statistically significant associations between osteoporosis and age, serum anti-CCP antibody titer, or vdH-mTSS were found in our logistic regression analyses.
Table 4.
Comparisons of the clinical features in male patients with rheumatoid arthritis according to the presence of osteoporosis.
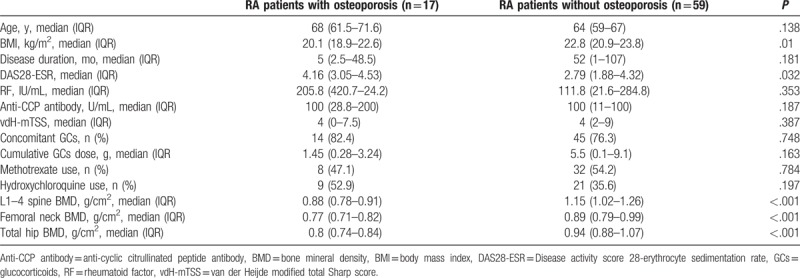
Table 5.
Risk factors for osteoporosis in male patients with rheumatoid arthritis using logistic regression analyses.
4. Discussion
The present study showed that patients with RA aged over 50 years had significantly lower total hip BMD as compared with age- and sex-matched healthy controls, but there were no significant differences in BMD in the L1-4 spine and femoral neck between the 2 groups. Similar to female counterparts, the overall frequency of osteoporosis at either the spine or the hip in male patients with RA was 2.1 times higher than that in controls. This finding suggests that appropriate management for osteoporosis in patients with RA is crucial not only for postmenopausal women but also for men aged 50 years and over. In addition, low BMI and moderate-to-high disease activity measured by DAS28-ESR were independent risk factors for the presence of osteoporosis in male patients with RA. Of interest, higher anti-CCP titer was significantly associated with reduced L1-4 BMD after adjusting for confounding factors. These findings indicate that beyond traditional risk factors, inflammatory status and RA-associated autoimmunity were important determinants for generalized bone loss in male patients with RA. Therefore, careful monitoring for the development of systemic bone loss should be considered for male patients with RA with lower BMI, higher disease activity, or higher anti-CCP antibody titer.
The major finding of our study was that frequency of osteoporosis, defined as T-scores ≤ −2.5, in male patients with RA aged over 50 years was 22.4%, approximately 2 times higher than that of healthy individuals (10.5%). Similar to our results, Haugeberg et al[27] and Nolla et al[28] reported that the prevalence of osteoporosis, defined using T-scores ≤ −2.5, in male patients with RA was 23% and 21%, respectively, in at least 1 of the evaluated sites, although no comparison with healthy controls was performed. The prevalence of osteoporosis in female patients with RA ranged from 11.5% to 22.1% [4,5,29,30] and 2 times higher that of controls in previous studies.[4,5] Thus, unlike general population studies showing that osteoporosis and subsequent fragility fracture are less frequent in men as compared with women,[8,14] our data and previous reports suggest that the frequency of osteoporosis in male patients with RA may be no less than that of female patients with RA. Thus, we assume that osteoporosis may also be an important clinical concern for the management of both male and female patients with RA. Although multifactorial factors such as inflammation, GCs use and functional disability can contribute to bone loss in RA patients, the role of gender in osteoporosis in RA has not been extensively studied. Thus, further researches to investigate distinctive features between male and female osteoporosis in RA patients are obviously needed.
In addition to the overall frequency, osteoporosis in RA has several distinctive clinical features compared with osteoporosis in general population. RA patients have lower bone formation marker such as serum osteocalcin and higher bone resorption markers such as crosslinked N-telopeptidases of type 1 collagen than general population, which may be due to inflammatory status in RA.[31] In addition, unlike non-RA subject, bone loss in peripheral arthritis (periarticular osteopenia) characterized by the trabecular size loss is another feature in RA patients,[31] which correlates with systemic osteoporosis in this population.[32]
Using an appropriate reference database is important for measurement of the T-score in investigating the frequency of osteoporosis. Unlike the diagnosis of osteoporosis in women, there is no consensus regarding appropriate reference data in the assessment of T-scores in male patients. Use of female reference data when calculating T-score in men is supported by the by the WHO,[19] Scientific Advisory Council of Osteoporosis Canada,[20] National Osteoporosis Guideline Group,[21] and International Society for Clinical Densitometry,[22] whereas the Endocrine Society recommends use of male reference data.[33] In the present study, South Korean female reference data provided by the manufacturers were used for calculating T-scores in male patients with RA and healthy patients of the same ethnicity. The same method was also applied in the Osteoporotic Fractures in Men Study, a representative study of male osteoporosis.[34]
Total hip BMD in male patients with RA was significantly lower than that of healthy controls in our study, whereas there was no significant difference in spine BMD between patients with RA and controls. Similar results have been reported in both male and female patients with RA in previous studies.[4,5,35,36] Thus, unlike postmenopausal osteoporosis, marked bone loss in the total hip but relatively preserved axial bone mass may be an clinical feature of osteoporosis in “RA” patients rather than “male” patients. This notion was also pointed out by Vosse and de Vlam.[37] As the proportion of cortical bone in the total hip is higher than that of the spine, it is assumed that cortical bone loss may be more susceptible to RA-specific risk factors, such as inflammation and autoimmunity, compared with trabecular bone loss. This hypothesis may be supported by evidence that high disease activity or high titer of RA antibodies is associated with localized cortical bone erosion, but the underlying mechanisms of RA-associated inflammation directly leading to bone loss in the hip remains elusive.
Risk factors for osteoporosis and low BMD in male patients with RA were analyzed in the present study. First, increased disease activity was an independent risk factor for osteoporosis in our data, similar to a previous study by Dao et al.[36] However, as 50% of RA patients were in remission (39.5%) or low disease activity (10.5%), relationship between disease activity and generalized bone loss in RA in our data needed to be interpreted cautiously. As TNF-α, a key proinflammatory cytokine in RA, can stimulate osteoclastogenesis via receptor activator of nuclear factor kappa B ligand activation, leading to systemic bone loss,[38] it is obvious that inflammation is a considerable contributing factor for osteoporosis in RA. However, bone loss in chronic inflammatory diseases such as RA must be a multifactorial affair and changes in energy balance, calcium metabolism, and hormonal pathways during inflammatory process can also cause low bone mass.[39] Thus, we assumed that disease activity is necessary but not sufficient for development of bone loss in RA. Second, anti-CCP antibody titer inversely correlated with spine BMD in male patients with RA and a significant inverse association between anti-citrullinated protein antibody (ACPA) titer and BMD in patients with RA was observed in previous studies.[40,41] The effect of ACPA on inducing osteoclasts activation, even in the absence of inflammation in RA, was also reported[41] and the binding of ACPAs to citrullinated vimentin in the surface of monocyte/macrophage lineage induces differentiation of these cells into osteoclasts,[42] which supports the effect of ACPA on bone loss in RA. Third, cumulative GC dose did not show a significant association with osteoporosis or BMD in male patients with RA in our study. For female patients with RA, a significant association between GC use and osteoporosis was found in some previous studies,[4,36] but others reported no association.[29,43] As GC use is a major cause of osteoporosis and fragility fracture, the lack of association between cumulative GC dose and reduced BMD in our study is somewhat unexpected. The small sample size and imprecise data collection regarding cumulative dose due to the retrospective design may have led to a lack of power to detect a correlation between GCs and bone loss in our study. Third, lower BMI, which is considered a traditional risk factor for osteoporosis, was also significantly linked with generalized bone loss in male patients with RA. Lastly, DMARD therapy, such as methotrexate and hydroxychloroquine, did not affect the rate of osteoporosis in patients with RA. Of interest, Mok et al[44] reported the protective role of hydroxychloroquine against osteoporosis in patients with systemic lupus erythematosus, but this effect was not found in our study. Further longitudinal studies are needed to confirm the effect of DMARDs on systemic bone loss in RA.
The present study has several limitations. First, the main limitation of the present study is the small sample size leading to low statistical power. As our study was conducted in a single center and RA is more common in female patients than male patients, it is difficult to collect a substantial number of male patients with RA. In particular, although male RA patients had significantly higher overall frequency of osteoporosis at either site compared with controls, there were no differences in the frequency of osteoporosis at the spine or hip between these 2 groups in our study. In addition, because no healthy subjects had hip osteoporosis, the direct comparison of hip osteoporosis between RA patients and controls may not be reasonable in our study. This result may be due to a small sample size and further larger studies are needed. Second, our study participants may not necessarily be representative of all South Korean male patients with RA. The cost of DEXA examination in South Korea is approximately $100 (US dollars) and male patients with RA can receive reimbursement for DEXA from the National Health Insurance Service in South Korean government if age 70 years or older. Thus, male patients with RA aged from 50 to 69 years could be reluctant to check their BMD due to cost, which may cause selection bias in our data based on real clinical practice. Third, we only evaluated T-scores of study participants based on female reference data. Further studies are needed to compare T-scores of male patients with RA according to male and female reference data. Lastly, due to retrospective study design, we could not fully collect information about the functional status in RA patients, which is important for analysis of the risk factors for osteoporosis.
In conclusion, male patients with RA had a 2.1 times higher risk for osteoporosis as compared with healthy controls. Low BMI (BMI ≤ 22 kg/m2) and moderate-to-high disease activity (DAS28-ESR > 3.2) were independent risk factors for the presence of osteoporosis in male patients with RA. Thus, similar to their female counterparts, special attention may also be needed to detect and treat osteoporosis in male patients with RA, especially those with low BMI and higher disease activity. Despite significant advances in the understanding of male osteoporosis in recent years, little work has been done on epidemiology, etiology, and optimal treatment of osteoporosis in male patients with RA. The present study sheds light on the importance of the increased risk of osteoporosis in male patients with RA, but further research is needed to confirm our findings due to limitations of our data, such as the small sample size.
Acknowledgments
We specially thank the late Professor Sung-Il Kim who devoted himself to education, research, and patient care in the Division of Rheumatology, Department of Internal medicine, Pusan National University School of Medicine (1963–2011).
Author contributions
Conceptualization: Dong Hyun Sohn, Seung-Geun Lee.
Data curation: Ji-Heh Park, Jung Hee Koh, Keunyoung Kim, Seung-Geun Lee.
Formal analysis: Seong-Min Kweon, Dong Hyun Sohn, Keunyoung Kim, Seung-Geun Lee.
Funding acquisition: Dong Hyun Sohn
Investigation: Seong-Min Kweon, Dong Hyun Sohn, Ji-Heh Park, Jung Hee Koh, Eun-Kyoung Park, Keunyoung Kim, Seung-Geun Lee.
Methodology: Seong-Min Kweon, Jung Hee Koh, Han-Na Lee, Keunyoung Kim, Seung-Geun Lee.
Project administration: Seung-Geun Lee.
Resources: Seung-Geun Lee.
Software: Seung-Geun Lee.
Supervision: Geun-Tae Kim, Seung-Geun Lee.
Validation: Dong Hyun Sohn, Seung-Geun Lee.
Writing – original draft: Seong-Min Kweon, Dong Hyun Sohn, Seung-Geun Lee.
Writing – review & editing: Yunkyung Kim, Geun-Tae Kim, Seung-Geun Lee.
Footnotes
Abbreviations: BMD = bone mineral density, BMI = body mass index, CCP = cyclic citrullinated peptide, CKD = chronic kidney disease, CRP = C-reactive protein, DAS28 = disease activity score assessed using the 28-joint count for swelling and tenderness, DEXA = dual energy X-ray absorptiometry, DMARDs = disease-modifying anti-rheumatic drugs, ESR = erythrocyte sedimentation rate, GCs = glucocorticoids, IQR = interquartile range, RA = rheumatoid arthritis, SD = standard deviation, TNF-α = tumor necrosis factor-α, vdH-mTSS = van der Heijde modified total Sharp score, WHO = World Health Organization.
S-MK and DHS contributed equally to this work.
Funding/support: This work was supported by Pusan National University Research Grant, 2015.
The authors report no conflicts of interest.
References
- [1].Vis M, Guler-Yuksel M, Lems WF. Can bone loss in rheumatoid arthritis be prevented? Osteoporos Int 2013;24:2541–53. [DOI] [PubMed] [Google Scholar]
- [2].Park JH, Park EK, Koo DW, et al. Compliance and persistence with oral bisphosphonates for the treatment of osteoporosis in female patients with rheumatoid arthritis. BMC Musculoskelet Disord 2017;18:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arain SR, Riaz A, Nazir L, et al. Low bone mineral density among patients with newly diagnosed rheumatoid arthritis. J Ayub Med Coll Abbottabad 2016;28:175–8. [PubMed] [Google Scholar]
- [4].Lee SG, Park YE, Park SH, et al. Increased frequency of osteoporosis and BMD below the expected range for age among South Korean women with rheumatoid arthritis. Int J Rheum Dis 2012;15:289–96. [DOI] [PubMed] [Google Scholar]
- [5].Haugeberg G, Uhlig T, Falch JA, et al. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheum 2000;43:522–30. [DOI] [PubMed] [Google Scholar]
- [6].Xue AL, Wu SY, Jiang L, et al. Bone fracture risk in patients with rheumatoid arthritis: a meta-analysis. Medicine (Baltimore) 2017;96:e6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Meng J, Li Y, Yuan X, et al. Evaluating osteoporotic fracture risk with the Fracture Risk Assessment Tool in Chinese patients with rheumatoid arthritis. Medicine(Baltimore) 2017;96:e6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lin YC, Li YH, Chang CH, et al. Rheumatoid arthritis patients with hip fracture: a nationwide study. Osteoporos Int 2015;26:811–7. [DOI] [PubMed] [Google Scholar]
- [9].Shin K, Park SH, Park W, et al. Monthly oral ibandronate reduces bone loss in korean women with rheumatoid arthritis and osteopenia receiving long-term glucocorticoids: a 48-week double-blinded randomized placebo-controlled investigator-initiated trial. Clin Ther 2017;39:268–78. [DOI] [PubMed] [Google Scholar]
- [10].Ochi K, Inoue E, Furuya T, et al. Ten-year incidences of self-reported non-vertebral fractures in Japanese patients with rheumatoid arthritis: discrepancy between disease activity control and the incidence of non-vertebral fracture. Osteoporos Int 2015;26:961–8. [DOI] [PubMed] [Google Scholar]
- [11].D’Amelio P, Isaia GC. Male osteoporosis in the elderly. Osteoporos Int 2015;2015:907689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Melton LJ, 3rd, Khosla S, Achenbach SJ, et al. Effects of body size and skeletal site on the estimated prevalence of osteoporosis in women and men. Osteoporos Int 2000;11:977–83. [DOI] [PubMed] [Google Scholar]
- [13].Drake MT, Khosla S. Male osteoporosis. Endocrinol Metab Clin North Am 2012;41:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Giusti A, Bianchi G. Male osteoporosis. Reumatismo 2014;66:136–43. [DOI] [PubMed] [Google Scholar]
- [15].Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009;301:513–21. [DOI] [PubMed] [Google Scholar]
- [16].Kannegaard PN, van der Mark S, Eiken P, et al. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing 2010;39:203–9. [DOI] [PubMed] [Google Scholar]
- [17].Morin S, Lix LM, Azimaee M, et al. Mortality rates after incident non-traumatic fractures in older men and women. Osteoporos Int 2011;22:2439–48. [DOI] [PubMed] [Google Scholar]
- [18].Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- [19].Kanis JA, McCloskey EV, Johansson H, et al. A reference standard for the description of osteoporosis. Bone 2008;42:467–75. [DOI] [PubMed] [Google Scholar]
- [20].Papaioannou A, Morin S, Cheung AM, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010;182:1864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Compston J, Bowring C, Cooper A, et al. Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas 2013;75:392–6. [DOI] [PubMed] [Google Scholar]
- [22].Watts NB, Leslie WD, Foldes AJ, et al. 2013 International Society for Clinical Densitometry Position Development Conference: task force on normative databases. J Clin Densitom 2013;16:472–81. [DOI] [PubMed] [Google Scholar]
- [23].Leslie WD, Adler RA, El-Hajj Fuleihan G, et al. Application of the 1994 WHO classification to populations other than postmenopausal Caucasian women: the 2005 ISCD Official Positions. J Clin Densitom 2006;9:22–30. [DOI] [PubMed] [Google Scholar]
- [24].Prevoo ML, van ’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- [25].van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum 1998;41:1845–50. [DOI] [PubMed] [Google Scholar]
- [26].van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000;27:261–3. [PubMed] [Google Scholar]
- [27].Haugeberg G, Orstavik RE, Uhlig T, et al. Clinical decision rules in rheumatoid arthritis: do they identify patients at high risk for osteoporosis? Testing clinical criteria in a population based cohort of patients with rheumatoid arthritis recruited from the Oslo Rheumatoid Arthritis Register. Ann Rheum Dis 2002;61:1085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nolla JM, Roig-Vilaseca D, Gomez-Vaquero C, et al. Frequency of osteoporosis in 187 men with rheumatoid arthritis followed in a university hospital. J Rheumatol 2006;33:1472–5. [PubMed] [Google Scholar]
- [29].Lodder MC, de Jong Z, Kostense PJ, et al. Bone mineral density in patients with rheumatoid arthritis: relation between disease severity and low bone mineral density. Ann Rheum Dis 2004;63:1576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Guler-Yuksel M, Bijsterbosch J, Goekoop-Ruiterman YP, et al. Bone mineral density in patients with recently diagnosed, active rheumatoid arthritis. Ann Rheum Dis 2007;66:1508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Maruotti N, Corrado A, Cantatore FP. Osteoporosis and rheumatic diseases. Reumatismo 2014;66:125–35. [DOI] [PubMed] [Google Scholar]
- [32].Wegierska M, Dura M, Blumfield E, et al. Osteoporosis diagnostics in patients with rheumatoid arthritis. Reumatologia 2016;54:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:1802–22. [DOI] [PubMed] [Google Scholar]
- [34].Cauley JA, Cawthon PM, Peters KE, et al. Risk factors for hip fracture in older men: the Osteoporotic Fractures in Men Study (MrOS). J Bone Miner Res 2016;31:1810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Haugeberg G, Uhlig T, Falch JA, et al. Reduced bone mineral density in male rheumatoid arthritis patients: frequencies and associations with demographic and disease variables in ninety-four patients in the Oslo County Rheumatoid Arthritis Register. Arthritis Rheum 2000;43:2776–84. [DOI] [PubMed] [Google Scholar]
- [36].Dao HH, Do QT, Sakamoto J. Bone mineral density and frequency of osteoporosis among Vietnamese women with early rheumatoid arthritis. Clin Rheumatol 2011;30:1353–61. [DOI] [PubMed] [Google Scholar]
- [37].Vosse D, de Vlam K. Osteoporosis in rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol 2009;27:S62–7. [PubMed] [Google Scholar]
- [38].Osta B, Benedetti G, Miossec P. Classical and paradoxical effects of TNF-alpha on bone homeostasis. Front Immunol 2014;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Straub RH, Cutolo M, Pacifici R. Evolutionary medicine and bone loss in chronic inflammatory diseases—a theory of inflammation-related osteopenia. Semin Arthritis Rheum 2015;45:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bugatti S, Bogliolo L, Vitolo B, et al. Anti-citrullinated protein antibodies and high levels of rheumatoid factor are associated with systemic bone loss in patients with early untreated rheumatoid arthritis. Arthritis Res Ther 2016;18:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Llorente I, Merino L, Ortiz AM, et al. Anti-citrullinated protein antibodies are associated with decreased bone mineral density: baseline data from a register of early arthritis patients. Rheumatol Int 2017;37:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zerbini CAF, Clark P, Mendez-Sanchez L, et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int 2017;28:429–46. [DOI] [PubMed] [Google Scholar]
- [43].Laan RF, Buijs WC, Verbeek AL, et al. Bone mineral density in patients with recent onset rheumatoid arthritis: influence of disease activity and functional capacity. Ann Rheum Dis 1993;52:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mok CC, Mak A, Ma KM. Bone mineral density in postmenopausal Chinese patients with systemic lupus erythematosus. Lupus 2005;14:106–12. [DOI] [PubMed] [Google Scholar]


