Supplemental Digital Content is available in the text.
Keywords: endothelial cells, eugenol, inflammation, nitric oxide, tobacco
Abstract
Objective—
Use of alternative tobacco products including electronic cigarettes is rapidly rising. The wide variety of flavored tobacco products available is of great appeal to smokers and youth. The flavorings added to tobacco products have been deemed safe for ingestion, but the cardiovascular health effects are unknown. The purpose of this study was to examine the effect of 9 flavors on vascular endothelial cell function.
Approach and Results—
Freshly isolated endothelial cells from participants who use nonmenthol- or menthol-flavored tobacco cigarettes showed impaired A23187-stimulated nitric oxide production compared with endothelial cells from nonsmoking participants. Treatment of endothelial cells isolated from nonsmoking participants with either menthol (0.01 mmol/L) or eugenol (0.01 mmol/L) decreased A23187-stimulated nitric oxide production. To further evaluate the effects of flavoring compounds on endothelial cell phenotype, commercially available human aortic endothelial cells were incubated with vanillin, menthol, cinnamaldehyde, eugenol, dimethylpyrazine, diacetyl, isoamyl acetate, eucalyptol, and acetylpyrazine (0.1–100 mmol/L) for 90 minutes. Cell death, reactive oxygen species production, expression of the proinflammatory marker IL-6 (interleukin-6), and nitric oxide production were measured. Cell death and reactive oxygen species production were induced only at high concentrations unlikely to be achieved in vivo. Lower concentrations of selected flavors (vanillin, menthol, cinnamaldehyde, eugenol, and acetylpyridine) induced both inflammation and impaired A23187-stimulated nitric oxide production consistent with endothelial dysfunction.
Conclusions—
Our data suggest that short-term exposure of endothelial cells to flavoring compounds used in tobacco products have adverse effects on endothelial cell phenotype that may have relevance to cardiovascular toxicity.
Electronic cigarettes (e-cigarettes) came on the market in 2003, and since that time, their popularity has increased dramatically. The majority of adult e-cigarette users are current or former combustible cigarette smokers, which has garnered interest on whether e-cigarettes may aid in smoking cessation or be a harm-reduction tool in smokers.1–3 In addition, e-cigarette use by youth is rising rapidly with ≈16% of high school students having used an e-cigarette in the past 30 days, whereas 37% of high schoolers reported ever use of an e-cigarette in 2015.4–7 Importantly, studies have shown that youth who try e-cigarettes are at a 3- to 5-fold greater risk for combustible cigarette smoking, suggesting that e-cigarettes are serving as a gateway to other tobacco product use.6,8 Further, e-cigarettes are marketed and perceived as being safer than combustible cigarettes because of the limited number of ingredients in the electronic liquid (primarily nicotine, propylene glycol/glycerin, and often contain flavorings).
Although combustible cigarettes are prohibited from containing characterizing flavors, with the exception of menthol, other tobacco products including e-cigarettes, cigars, little cigars, cigarillos, smokeless tobacco, and hookah are unrestricted regarding flavoring addition. Electronic liquids are available in a wide variety of flavorings with ≈7000 on the market with menthol, sweet, and fruity electronic liquids being the most popular.9 The flavorings used in tobacco products, including electronic liquids, greatly increase the appeal of tobacco products and mask the harshness associated with use.9–13 In 2014, of the high school students who reported use of a tobacco product, an estimated 73% reported using a flavored tobacco product,10 and among youth, flavorings are cited as a primary reason for use of alternative tobacco products, including e-cigarettes, hookah, and cigars.10,14,15 Although the majority of the morbidity and mortality burden of combustible cigarette smoking is attributable to cardiovascular disease, the effects of tobacco product flavorings on the cardiovascular system are largely unknown.
The cardiovascular system is exposed to circulating toxins, and measures of vascular function are rapidly altered in response to environmental exposures. The endothelium plays a key role in maintaining vascular homeostasis, which has been shown to be disrupted by several cardiovascular risk factors, including combustible cigarette smoking.16–18 Endothelial dysfunction not only precedes the development of atherosclerosis but also predictive of worse outcomes, including myocardial infarction and cardiac death.19,20 Combustible cigarette smoking has been shown to induce endothelial dysfunction characterized by increased oxidative stress, a loss of nitric oxide signaling, inflammation, oxidative stress, and a prothrombotic phenotype.16–18 Several studies in endothelial cells suggest that acrolein, an aldehyde found in combustible cigarette smoke and e-cigarette aerosol, induces inflammation and oxidative stress.21–23 A recent study showed that flow-mediated vasodilation was impaired in young, healthy tobacco naïve individuals and combustible cigarette smokers 30 minutes after the use of an e-cigarette, suggesting that acute e-cigarette use impairs endothelial function.24 However, the mechanisms underlying e-cigarette–induced vascular injury are largely unknown, and whether tobacco product flavorings induce endothelial dysfunction is unclear.
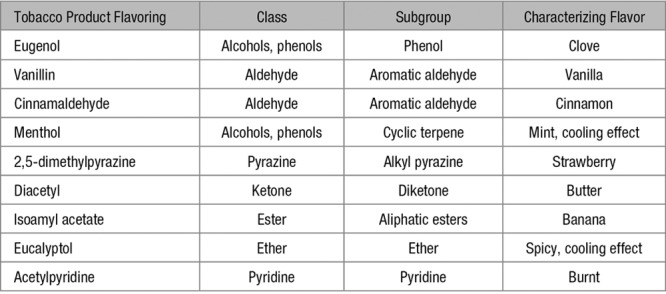
In this study, we developed an in vitro screening panel to identify whether flavorings added to tobacco products are toxic to endothelial cells and, if so, what levels induce toxicity. We selected a panel of measures of endothelial function, including measures of cell death, oxidative stress, inflammation, and nitric oxide bioavailability. Under pathological conditions, endothelial cells undergo cell death, have increased oxidative stress, decrease production or lose bioavailable nitric oxide, and become proinflammatory. We tested the vascular endothelial cell toxicity of common flavoring compounds in tobacco products across many different chemical classes. The flavorings vanillin, cinnamaldehyde, eugenol, acetylpyridine, and menthol impaired A23187-induced nitric oxide production and increased expression of the proinflammatory mediator, IL-6 (interleukin-6), suggesting that these flavors are harmful to the endothelium (Table 1).
Table 1.
Tobacco Product Flavorings Tested
Materials and Methods
Data available upon request from the authors.
Study Participants
We enrolled age- and sex-matched nonsmokers who do not use any tobacco products, nonmenthol cigarette smokers, and menthol cigarette smokers. All participants enrolled had no risk factors (diabetes mellitus, smoking, hypertension, dyslipidemia) or known cardiovascular disease. All participants provided written consent, and all study protocols were approved by the Boston Medical Center Institutional Review Board.
Flow-Mediated Vasodilation
Endothelial function was evaluated using flow-mediated vasodilation in which hyperemic flow stimulates endothelial nitric oxide production and subsequent vasodilation. As previously described, hyperemic flow was induced by proximal forearm cuff occlusion of the upper arm for 5 minutes, and a Toshiba SSH-140A ultrasound system was used to measure brachial artery diameter at baseline and 1 minute after the 5-minute occlusion.25 The commercially available software, Brachial Analyzer version 3.2.3 (Medical Imaging Applications), was used to assess flow-mediated dilation data. Flow-mediated vasodilation is expressed as percent dilation.
Venous Endothelial Cell Biopsy
Venous endothelial cells were freshly isolated from nonsmokers, nonmenthol cigarette smokers, and menthol cigarette smokers without cardiovascular disease, as previously described.26–28 A 0.018-inch J-wire (Arrow International, Reading, PA) was inserted through a 20 or 22 gauge catheter in a vein of the forearm and used to gently rub the inside of the vessel. After removal, the J-wire was rinsed several times with red blood cell lysis and dissociation buffer. The sample was then centrifuged and cells applied to poly-l-lysine–coated slides (Sigma, St Louis, MO). Nitric oxide production and bioavailability were then assessed immediately after isolation as outlined below.
Cell Culture Conditions and Tobacco Flavorings Exposures
Commercially available (Lonza Inc, Walkersville, MD) human aortic endothelial cells (HAECs) were cultured from passage 4 to 7 (EGM-2 [endothelial cell growth medium] complete media, Lonza). When the cells were near confluent, serum was withdrawn for 4 hours, and the cells were exposed to a flavoring compound diluted in media (phenol red–free EGM-2, Lonza) for 90 minutes at 37°C before measurement of apoptosis, oxidative stress, inflammation, and nitric oxide production as outlined below. Controls were vehicle matched to flavoring. All flavors are food safe grade, production lot consistent, and obtained from Sigma-Aldrich (St Louis, MO; Table I in the online-only Data Supplement for catalog numbers).
The flavoring compounds were heated at temperatures achieved using e-cigarette tank devices to test the potential toxicity of thermal degradation productions. A drop-tube furnace consisting of a quartz tube was configured in a vertical position and set to temperatures 200°C±50°C or 700°C±50°C. Vanillin, menthol, or eugenol were added dropwise into the heated area of the furnace where the flavoring compound was quickly aerosolized. The aerosol then moved through a glass impinge for collection in an ethanol solution (55% in PBS). Test concentrations of thermal product solutions were determined from the volume of flavoring compound initially added to the furnace before heating and collection. All cell exposures to the aerosolized flavoring compounds were compared with ethanol vehicle control.
Measurement of Nitric Oxide Bioavailability
HAECs were grown on 8-well slides, and after a 90-minute flavoring exposure, the cells were incubated with 3 µmol/L 4,5-diaminofluorescein diacetate (Calbiochem) for 30 minutes. After 2 washes with Hanks’ balanced salt solution, cells were stimulated with 1 µmol/L A23187 (Sigma) for 15 minutes and fixed with 2% paraformaldehyde. Mean fluorescence intensity (excitation of 498 nm) of individual cells (20 cells per condition) was measured on a fluorescence microscope (Nikon Eclipse TE2000). Data are expressed as percent increase in 4,5-diaminofluorescein diacetate fluorescence stimulated by A23187 compared with unstimulated cells.
Quantification of Cell Death
HAECs were grown on 8-well slides, incubated with flavoring compounds for 90 minutes, fixed with 2% paraformaldehyde, and stored at −80°C. A commercially available TUNEL assay (terminal deoxynucleotidyl transferase dUTP nick-end labeling; Roche) was performed, and cells were imaged on a fluorescence microscope (Nikon Eclipse TE2000) for fluorescein and DAPI (4’,6-diamidino-2-phenylindole; Vector Laboratories). DNAse 1 (Sigma) was used as a positive control for apoptosis. A minimum of 50 cells were quantitated for each condition. Data are presented as % TUNEL-positive cells.
Assessment of Oxidative Stress
HAECs were grown on 96-well plates and, after flavoring exposure, were incubated with dihydroethidium (DHE, 10 µmol/L, Thermo Fisher) for 30 minutes. Cells were washed 3× to remove DHE with Hanks’ balanced salt solution. Fluorescence was measured on a plate reader with an excitation of 518 nm and emission of 606 nm (Molecular Devices). Antimycin A (50 µmol/L; Sigma) treatment for 30 minutes was used as a positive control. Data are presented as fold change in DHE fluorescence compared with vehicle control.
Quantification of IL-6 and ICAM-1 (Inflammatory) Activation
HAECs were grown on 6-well plates and were incubated an additional 90 minutes in media after flavoring exposure, allowing for a total time of 180 minutes for changes in RNA expression. Cells were scraped into Qiazol (Qiagen), frozen, thawed, chloroform (1/5 of the Qiazol volume) extracted, and shaken for 15 seconds. After a 5-minute incubation at room temperature, the samples were centrifuged at 12 000g for 15 minutes at 4°C. The aqueous phase was collected and RNA was extracted with a kit (miRNeasy Micro Kit, Qiagen) according to the manufacturer’s instructions. RNA was eluted in 14 µL of water and quantified with a Nanodrop spectrophotometer (Thermo Fisher, average of 200 ng RNA/µL). cDNA synthesis of mRNA was performed with a cDNA reverse transcription kit (Quanta Bio, Beverly, MA). Reverse transcription-quantitative polymerase chain reaction was performed with a Viia7 (Applied Biosystems) thermal cycler using TaqMan Master Mix and TaqMan primers for IL-6 and ICAM-1 (intercellular adhesion molecule-1; Thermo Fisher). The 2−ΔCt was calculated from threshold Ct values using GAPDH as a reference gene. Data are expressed as relative quantification to matched control.
Statistical Analysis
Statistical analyses were performed using SPSS version 24.0 (IBM Corp, Armonk, NY). Data are expressed as mean±SD, unless otherwise indicated. We evaluated each measure for normality using the Shapiro–Wilk test. For between group comparisons, variables with normal distribution were compared using a 1-way ANOVA using post hoc Dunnett 2-sided tests with contrasts to control (vehicle alone) or χ2 testing for continuous or categorical data, respectively. For variables that were not normally distributed, we used Kruskal–Wallis tests. For 2-group comparisons before and after treatment, we used paired t tests, and for variables not normally distributed, we used Wilcoxon signed-rank tests. A P<0.05 was considered to be statistically significant.
Results
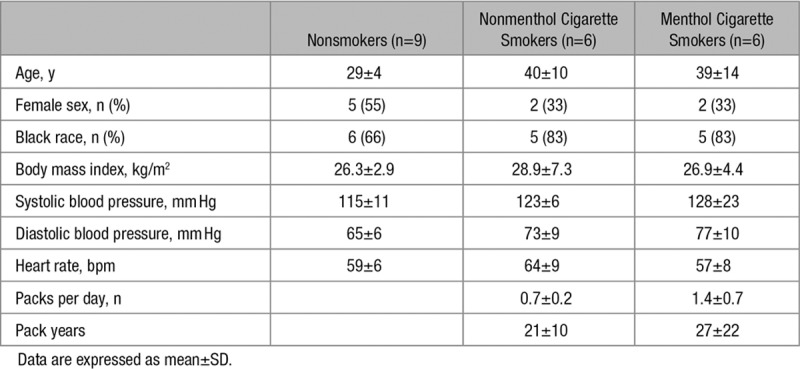
In a healthy endothelium, stimulation with eNOS (endothelial NO synthase) agonists, such as A23187, induces an increase in nitric oxide which in vivo results in vasodilation and is indicative of cardiovascular health.19,29 Venous endothelial cells were freshly isolated by venous biopsy from nonsmokers (n=9), nonmenthol cigarette smokers (n=6), and menthol cigarette smokers (n=6) of similar age and sex (Table 2). Nonmenthol and menthol cigarette smokers had a similar number of packs smoked per day (0.7±0.2 versus 1.4±0.7; P=0.4) and pack years (21±10 versus 27±22; P=0.7). As part of larger, on-going study of vascular function and smoking, we evaluated flow-mediated vasodilation in the patients recruited for endothelial cell biopsy. We found a trend for lower flow-mediated vasodilation between the smoking groups and nonsmokers (Figure I in the online-only Data Supplement; P=0.12 between groups).
Table 2.
Clinical Characteristics
Freshly isolated endothelial cells collected from nonmenthol and menthol cigarette smokers had lower nitric oxide production in response to A23187 stimulation compared with cells from nonsmokers (P=0.003 nonsmokers versus nonmenthol cigarette smokers; P=0.012 nonsmokers versus menthol cigarette smokers; Figure 1A). The impairment in A23187-stimulated nitric oxide production was similar between nonmenthol cigarette smokers and menthol cigarette smokers (P=0.86; Figure 1A). The absence of a difference in A23187-stimulated nitric oxide production is likely because of the overwhelming toxicity of the many components of tobacco smoke that are already maximally impairing nitric oxide bioavailability. Consistent with this, we observed that the treatment of endothelial cells from nonmenthol cigarette smokers with 0.01 mmol/L menthol did not further impair nitric oxide production in response to A23187 (2.1±2.4 versus −2.6±5.1; P=0.5). Treatment of freshly isolated endothelial cells from healthy participants with 0.01 mmol/L menthol (Figure 1B) or 0.01 mmol/L eugenol (Figure 1C) impaired nitric oxide production in response to A23187 stimulation. These findings suggest that flavoring compounds induce endothelial cell dysfunction in human cells similarly to the abnormal function in active cigarette smokers.
Figure 1.
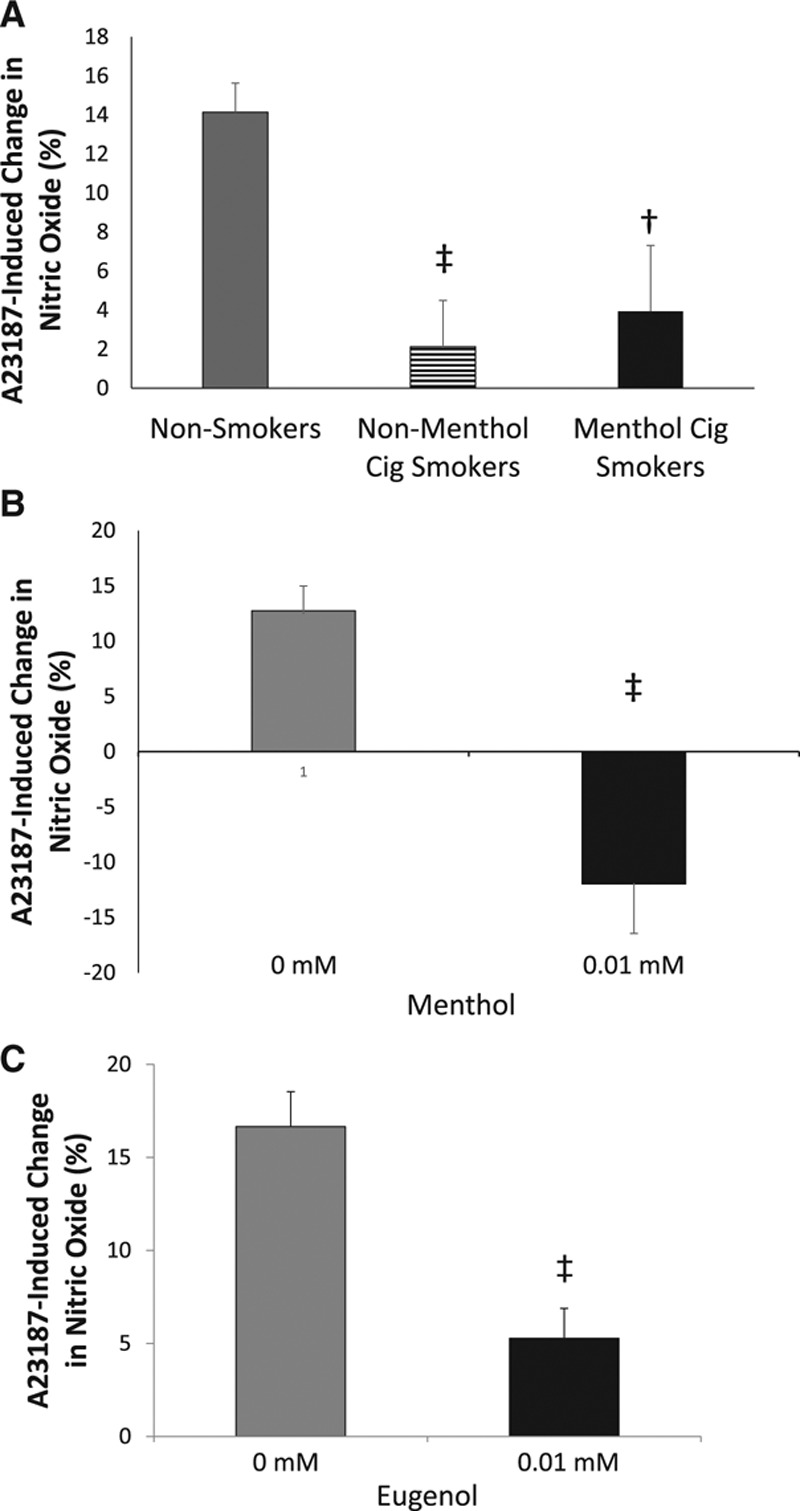
Menthol and eugenol impair nitric oxide production in freshly isolated endothelial cells from human participants. Endothelial cells from menthol (n=6) and nonmenthol cigarette smokers (n=6) had a lower change in nitric oxide measured by 4,5-diaminofluorescein diacetate (DAF-2DA) fluorescence in response to A23187 stimulation compared with endothelial cells from nonsmokers (n=9, ‡P<0.01, †P<0.05; A). Treatment of endothelial cells freshly isolated from healthy participants with 0.01 mmol/L menthol (B) or eugenol (C) decreased DAF-2DA fluorescence in response to A23187 stimulation (n=5, ‡P<0.01 for menthol; n=5, ‡P<0.01 for eugenol). Data are expressed as mean±SEM.
To further characterize the acute effects of several flavoring compounds on a broad set of endothelial phenotypes, we studied commercially available endothelial cells. Several tobacco product flavorings and doses induced cell death measured by TUNEL assay (Figure 2). Specifically, all flavorings tested induced cell death at the highest concentration tested (10–100 mmol/L). Cinnamaldehyde precipitated out of solution at concentrations >10 mmol/L; therefore, all experiments were performed at 10 mmol/L or lower concentrations. Cinnamaldehyde, eugenol, dimethylpyrazine, isoamyl acetate, and eucalyptol treatment at 10 mmol/L increased cell death compared with vehicle control. Treatment of HAECs with 1 mmol/L dimethylpyrazine also increased cell death, suggesting that endothelial cells are especially sensitive to dimethylpyrazine exposure.
Figure 2.
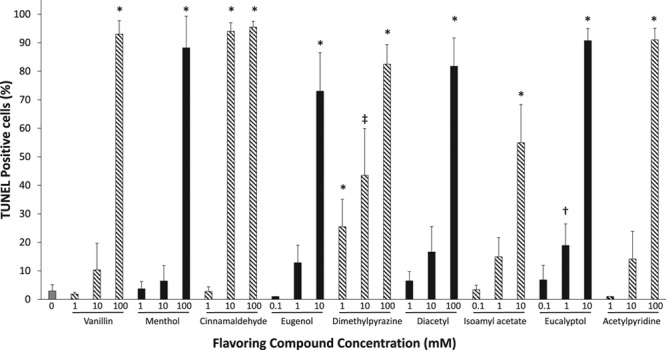
Tobacco flavoring compounds induce cell death. The percentage of cells staining positive for DNA strand breaks (TUNEL positive [terminal deoxynucleotidyl transferase dUTP nick-end labeling]) after a 90 min incubation with varying concentrations of flavor compounds were detected using immunofluorescence (n=3–4, *P<0.001, ‡P<0.01, †P<0.05 compared with vehicle control). Data are expressed as mean±SEM.
Oxidative stress was assessed using the fluorescent dye DHE after treatment of HAECs with flavorings across several concentrations (Figure 3). As expected, the positive control, antimycin A, increased the levels of oxidants as measured by an increase in DHE fluorescence. Vanillin and eugenol increased oxidative stress at the highest concentration tested (10 mmol/L vanillin, 10 mmol/L eugenol), whereas all other flavorings tested had no effect on oxidative stress. The concentrations of vanillin and eugenol that increased oxidative stress were also the same concentrations that caused cell death, suggesting significant damage to endothelial cells at these levels. The concentrations of flavorings for all additional assays tested were performed at concentrations below the dose observed to induce cell death.
Figure 3.
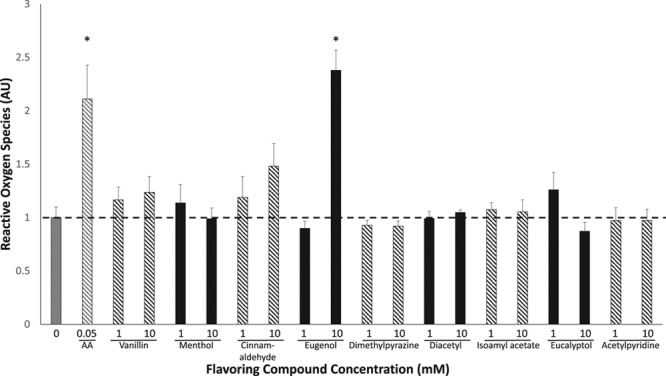
Tobacco flavoring compounds increase oxidative stress. Oxidative stress was measured by quantifying the fluorescent dye dihydroethidium after exposure of human aortic endothelial cells to flavoring compounds at varying concentrations (n=3, *P<0.001). Antimycin A (AA), a stimulus for mitochondrial oxidant generation, served as a positive control. Data are expressed as mean±SEM.
Expression of a proinflammatory mediator, IL-6, was quantified in HAECs 3 hours after exposure to flavoring compounds (Figure 4). Vanillin, cinnamaldehyde, eugenol, and acetylpyridine increased IL-6 expression at most concentrations tested, even in the 0.001 to 0.01 mmol/L range. Menthol increased IL-6 expression at 10 and 100 mmol/L but not at the lower concentrations (0.01–1 mmol/L). Dimethylpyrazine, diacetyl, isoamyl acetate, and eucalyptol had no effect on IL-6 expression in HAECs. Expression of the adhesion molecule, ICAM-1, was quantified in HAECs after a 3-hour exposure to flavoring compounds (Figure II in the online-only Data Supplement). Vanillin at a concentration of 10 mmol/L increased ICAM-1 expression in HAECs whereas other concentrations and flavoring compounds had no effect on ICAM-1 expression.
Figure 4.
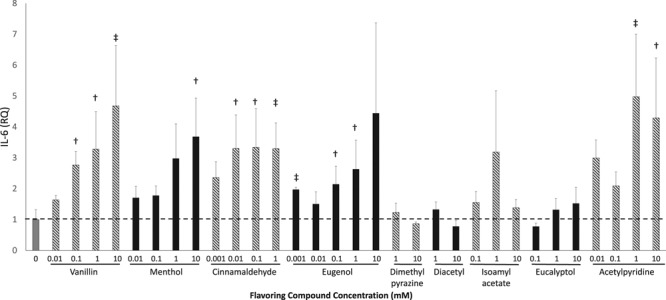
Tobacco flavoring compounds increase endothelial cell inflammation. IL-6 (interleukin-6) expression was quantified using reverse transcription-quantitative polymerase chain reaction 3 h after initial flavoring exposure in human aortic endothelial cells. All data are expressed as the relative quantification compared with vehicle alone treated cells (n=3–6, ‡P<0.01, †P<0.05). Data are expressed as mean±SEM.
HAECs treated with the selected flavorings vanillin, menthol, cinnamaldehyde, eugenol, or acetylpyridine displayed a loss of nitric oxide production in response to A23187 stimulation at all the tested concentrations of tobacco product flavoring (Figure 5). In HAECs exposed to varying concentrations of eugenol for 90 minutes, phosphorylation of eNOS at its activation site, Serine 1177, in response to A23187 was impaired (Figure III in the online-only Data Supplement), suggesting that eugenol-induced decrease in nitric oxide bioavailability is due, in part, to an impairment in eNOS activation. Further, HAECs were treated with vanillin, eugenol, and menthol that had been aerosolized at temperatures designed to simulate those achieved using e-cigarette devices (200°C and 700°C) for 90 minutes and then A23187-stimulated nitric oxide was assessed. Treatment of HAECs with vanillin aerosolized at 200°C but not 700°C decreased nitric oxide production in response to A23187 stimulation (Figure 6). HAECs treated with eugenol at 200°C and 700°C impaired nitric oxide production in response to A23187 stimulation while aerosolized menthol treatment had no effect (Figure 6). Collectively, these data suggest that heating of the flavoring compounds alters their toxicity.
Figure 5.
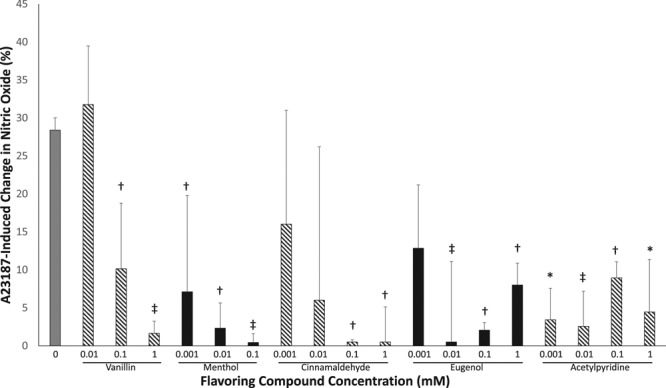
Tobacco flavoring compounds impair nitric oxide production in human aortic endothelial cells (HAECs). Nitric oxide production (4,5-diaminofluorescein diacetate [DAF-2DA] fluorescence) in response to A23187 stimulation was decreased in HAECs treated with flavoring compounds (n=3, *P<0.001, ‡P<0.01, †P<0.05 compared with untreated control). Data are presented as percent change in DAF-2DA fluorescence in response to A23187 stimulation. Data are expressed as mean±SEM.
Figure 6.
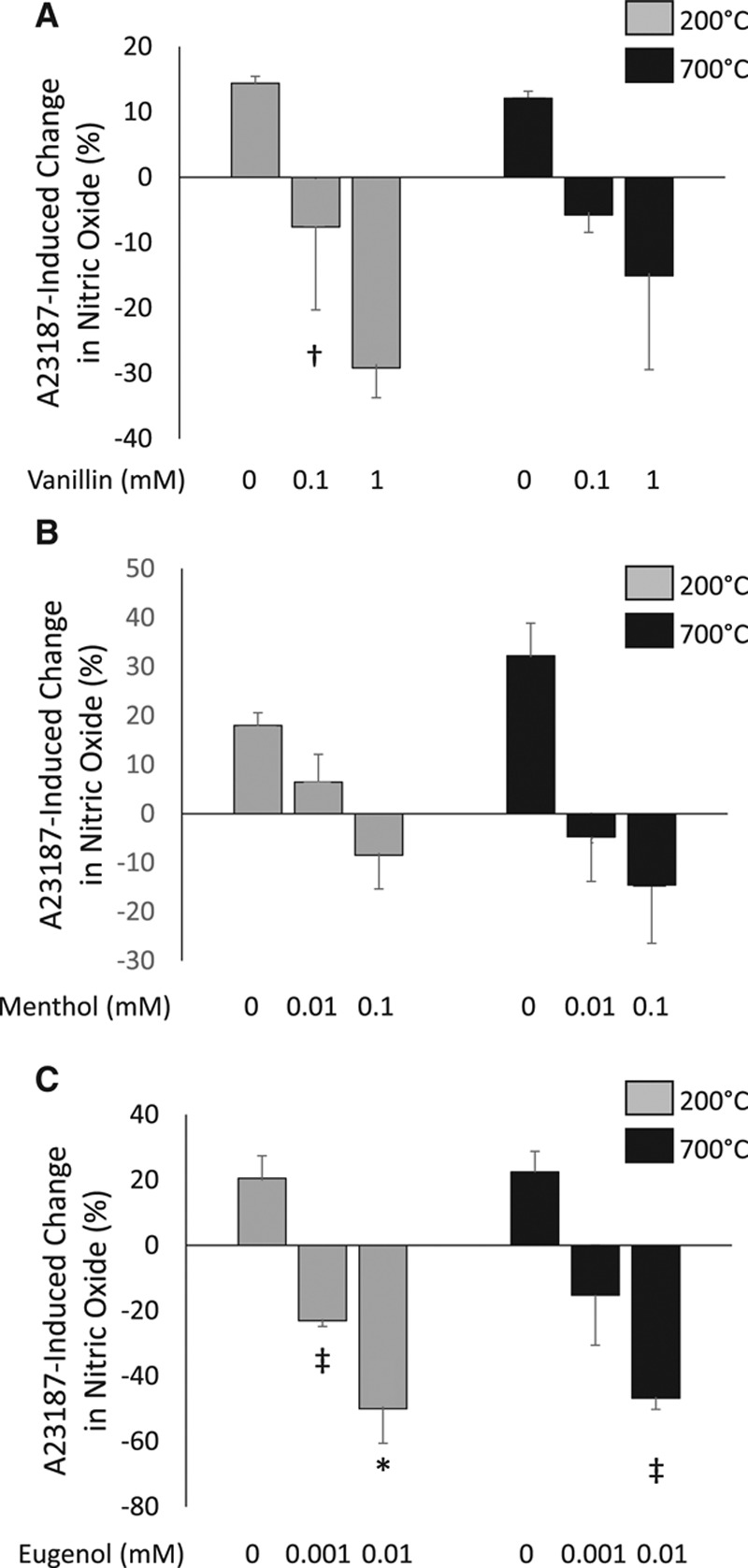
Differential effects of aerosolizing tobacco flavoring compounds on A23187-stimulated nitric oxide production in human aortic endothelial cells (HAECs). Treatment of HAECs with vanillin aerosolized at 200°C impaired A23187-stimulated nitric oxide production (4,5-diaminofluorescein diacetate [DAF-2DA] fluorescence), but this impairment was not observed with vanillin aerosolized at 700°C (n=3, †P<0.05 compared with untreated vehicle control, A). HAECs treated with menthol aerosolized at 200°C or 700°C had no effect on A23187-stimulated nitric oxide production (n=3, B). Treatment of HAECs with eugenol aerosolized at 200°C and 700°C impaired nitric oxide production in response to A23187 stimulation (n=3, *P<0.001, ‡P<0.01, compared with vehicle control, C). Data are presented as percent change in DAF-2DA fluorescence in response to A23187 stimulation. Data are expressed as mean±SEM.
Discussion
Our study provides evidence that flavoring additives in tobacco products induce acute alterations in endothelial function. Treatment of endothelial cells from nonsmokers with menthol and eugenol resulted in a loss of nitric oxide signaling, recapitulating the phenotype observed in endothelial cells from nonmenthol and menthol cigarette smokers. We found a similar degree of impairment in nitric oxide bioavailability in endothelial cells from nonmenthol and menthol cigarette smokers, which is consistent with prior literature showing that nonmenthol and menthol cigarette smokers have a similar degree of cardiovascular risk.30–34 We then systematically assessed the impact of several flavoring compounds, in the absence of combustion products, on a panel of measures of endothelial function, including nitric oxide production, oxidative stress, inflammation, and cell death. The unique chemical characteristics of each flavoring compound led to variable changes in different outcomes whereas at some concentrations, all tobacco product flavoring compounds induced cell death. Oxidative stress was induced by the flavoring compounds but at concentrations that are likely supraphysiological. Notably, the flavorings vanillin, cinnamaldehyde, eugenol, and acetylpyridine impaired A23187-induced nitric oxide production and increased expression of the proinflammatory mediator, IL-6, across all concentrations tested, suggesting that the endothelium is particularly sensitive to these flavors. Menthol also increased IL-6 expression at the higher concentrations tested (10 and 100 mmol/L) and impaired nitric oxide production in response to A23187 at doses as low as 0.01 mmol/L. Treatment of HAECs with flavoring additives vanillin and eugenol aerosolized at temperatures achieved using e-cigarettes, impaired nitric oxide production in response to A23187 stimulation whereas aerosolized of menthol had no effect on nitric oxide production. The impairment in nitric oxide bioavailability observed after treatment with native and aerosolized flavoring additives suggests that the heating process alters the flavoring-induced endothelial dysfunction for some flavors but not others. Collectively, our data suggest that acute exposure to flavoring additives used in tobacco products induce characteristics of endothelial dysfunction at potentially physiologically relevant concentrations.
Although several studies have investigated the toxicity of tobacco product flavorings on pulmonary epithelial cells, few studies have assessed flavoring toxicity on the vascular endothelium. In human umbilical vein endothelial cells, treatment with vapor extract from different e-cigarette devices for 24 to 48 hours decreased endothelial cell viability and proliferation and altered endothelial cell morphology to varying degrees across the products, but to less of an extent that combustible cigarette smoke extract.35 Among the e-cigarette products used to generate the vapor extracts, all contained flavorings, but the authors did not provide product information.35 Thus, differentiating which flavored products were toxic on endothelial cells in this study was not possible.35 In another study, e-cigarette condensate treatment of rodent pulmonary endothelial cell lines increased monolayer permeability, decreased cellular metabolic activity, and reduced cellular proliferation.36 Interestingly, exposure to e-cigarette condensate without nicotine had a similar effect on endothelial barrier function as the e-cigarette condensate with nicotine, suggesting the effects of e-cigarette condensate are independent of nicotine.36 In healthy nonsmokers and combustible cigarette smokers, use of an e-cigarette with unflavored electronic liquid impaired flow-mediated vasodilation, a measure of endothelial function and nitric oxide bioavailability, and increased measures of oxidative stress.24 However, measures of oxidative stress (serum Nox2 [NADPH oxidase 2]-derived peptide and 8-isoPGF2α [8-iso-prostaglandin F2 alpha]) and nitric oxide bioavailability were less impacted by a single e-cigarette use compared with smoking a single combustible cigarette, suggesting that e-cigarettes could be a reduced harm tobacco product.24 Hence, several studies have tested the toxicity of e-cigarette generated vapor on endothelial cell phenotype, but few have determined whether the flavor additives induce endothelial cell toxicity.
We measured the acute effects of flavoring compounds used in tobacco products on a select panel of measures of endothelial cell function in vitro to rapidly screen for flavor toxicity. Moreover, acute endothelium dysfunction is often observed immediately after smoking (cigarettes and e-cigarettes) and is recognized as a predictor of increased cardiovascular risk and disease.19,20,24,37 We found differential effects of the flavoring compounds on nitric oxide bioavailability, cell death, oxidative stress, and IL-6 expression that may be related to their different chemical properties. All of the flavoring compounds tested impaired nitric oxide production, which may be the result of reactive oxygen species scavenging nitric oxide and reduced eNOS activation. Nitric oxide is a cardioprotective signaling molecule that inhibits vascular inflammation and thrombosis and plays a key role in regulating vascular tone.19,29 The loss of nitric oxide signaling is known to promote a proinflammatory and prothrombotic endothelium, resulting in vascular dysfunction and atherosclerotic plaque formation.19,38 The flavoring compounds that impaired nitric oxide production also upregulated IL-6 which is consistent with oxidative stress, a known stimulus of inflammatory signaling pathways. Treatment of freshly isolated endothelial cells from nonsmokers with menthol or eugenol recapitulated the loss of nitric oxide bioavailability observed in endothelial cells from nonmenthol and menthol cigarette smokers, suggesting an effect of menthol exposure separate from cigarette smoke. Our studies are strengthened by the application of only the flavoring compounds to the cells, allowing us to isolate the effects of individual flavors on endothelial function. Other pathways may be impacted in endothelial cells by flavoring compound exposure, and the mechanisms are yet to be explored.
Our study has many limitations. We incubated endothelial cells with tobacco product flavoring compounds suspended in media without heating or addition of other typical electronic liquid constituents, such as the solvents propylene glycol and glycerol. Heating or combustion of the flavoring compounds likely alters the compounds, making them more or less toxic.39 Consistent with this hypothesis, aerosolization of select flavoring compounds, vanillin and eugenol, did not alter their effects on nitric oxide bioavailability. However, aerosolization of menthol reduced menthol’s inhibition of A23187-induced nitric oxide production. Similarly, we studied only the acute effects of flavoring compounds on endothelial cell function, and the effects associated with chronic use of flavored tobacco products need to be addressed. In addition, the in vitro effects may be different than the in vivo effects observed after flavored tobacco product use. The impairment in nitric oxide production in the endothelial cells from menthol cigarette smokers may not be directly attributable to the presence of menthol and is likely the result of the combined effects of multiple constituents in menthol cigarette smoke. This is consistent with the epidemiological evidence, showing that both menthol and nonmenthol cigarettes increase cardiovascular risk to a similar extent.30–34 A study evaluating the pharmacokinetics of menthol estimated the daily exposure of menthol to be ≈80 µmol for an individual who smokes 20 mentholated cigarettes and estimated an absorption of ≈20% of the menthol in a combustible cigarette.40 In our study, at a dose comparable to this estimated daily exposure, we found that treatment of endothelial cells from healthy, nonsmokers with 10 µmol/L menthol impaired A23187-stimulated nitric oxide production, suggesting that the concentrations evaluated in vitro are likely to be achieved in vivo. However, further work is needed to evaluate the levels of flavoring compounds and their metabolites in the circulation after use of flavored tobacco products.
We provide evidence that flavoring additives to tobacco products impair stimulated nitric oxide production and inflammation suggestive of endothelial dysfunction across a range of concentrations likely to be achieved in vivo. All flavorings tested impaired A23187-induced nitric oxide production, suggesting that measures of eNOS activation and nitric oxide production are sensitive measures of endothelial cell toxicity in vitro. The toxicity data generated herein, using a variety of common flavorings, provide quantitative support for the regulatory prohibition or the establishment of limitations on allowable levels of these flavorings in electronic liquids and other tobacco products. Future studies will focus on how the toxicity of the flavorings is altered with heating and characterization of the levels obtained in the circulation after use of an e-cigarette.
Sources of Funding
Research reported in this work was supported by grant number 5P50HL120163-S1 (all authors) from the National Heart, Lung and Blood Institute and Food and Drug Administration (FDA) Center for Tobacco Products and an American Heart Association Mentored Clinical and Population Research Award 17MCPRP32650002 (Dr Fetterman). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the FDA.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- DHE
- dihydroethidium
- e-cigarettes
- electronic cigarettes
- eNOS
- endothelial NO synthase
- HAEC
- human aortic endothelial cell
- ICAM-1
- intercellular adhesion molecule-1
- IL-6
- interleukin-6
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.118.311156/-/DC1.
Highlights
The cardiovascular health effects of flavoring additives used in tobacco products, including electronic cigarettes, have not yet been studied.
Our data suggest that short-term exposure of endothelial cells to flavoring compounds used in tobacco products have adverse effects on endothelial cell phenotype that may have relevance to cardiovascular toxicity.
The toxicity data generated herein, using a variety of common flavorings, provide quantitative support for the regulatory prohibition or the establishment of limitations on allowable levels of these flavorings in electronic liquids and other tobacco products.
Future studies will focus on how the toxicity of the flavorings is altered with heating and characterization of the levels obtained in the circulation after use of an electronic cigarette.
References
- 1.Bhatnagar A. Cardiovascular perspective of the promises and perils of e-cigarettes. Circ Res. 2016;118:1872–1875. doi: 10.1161/CIRCRESAHA.116.308723. doi: 10.1161/CIRCRESAHA.116.308723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green SH, Bayer R, Fairchild AL. Evidence, policy, and e-cigarettes—will England reframe the debate? N Engl J Med. 2016;374:1301–1303. doi: 10.1056/NEJMp1601154. doi: 10.1056/NEJMp1601154. [DOI] [PubMed] [Google Scholar]
- 3.Fairchild AL, Lee JS, Bayer R, Curran J. E-cigarettes and the harm-reduction continuum. N Engl J Med. 2018;378:216–219. doi: 10.1056/NEJMp1711991. doi: 10.1056/NEJMp1711991. [DOI] [PubMed] [Google Scholar]
- 4.Singh T, Arrazola RA, Corey CG, Husten CG, Neff LJ, Homa DM, King BA. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65:361–367. doi: 10.15585/mmwr.mm6514a1. doi: 10.15585/mmwr.mm6514a1. [DOI] [PubMed] [Google Scholar]
- 5.Neff LJ, Arrazola RA, Caraballo RS, Corey CG, Cox S, King BA, Choiniere CJ, Husten CG. Frequency of tobacco use among middle and high school students—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:1061–1065. doi: 10.15585/mmwr.mm6438a1. doi: 10.15585/mmwr.mm6438a1. [DOI] [PubMed] [Google Scholar]
- 6.Barrington-Trimis JL, Urman R, Berhane K, Unger JB, Cruz TB, Pentz MA, Samet JM, Leventhal AM, McConnell R. E-cigarettes and future cigarette use. Pediatrics. 2016;138:e20160379. doi: 10.1542/peds.2016-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrington-Trimis JL, Urman R, Leventhal AM, Gauderman WJ, Cruz TB, Gilreath TD, Howland S, Unger JB, Berhane K, Samet JM, McConnell R. E-cigarettes, cigarettes, and the prevalence of adolescent tobacco use. Pediatrics. 2016;138:e20153983. doi: 10.1542/peds.2015-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, Riggs NR, Stone MD, Khoddam R, Samet JM, Audrain-McGovern J. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA. 2015;314:700–707. doi: 10.1001/jama.2015.8950. doi: 10.1001/jama.2015.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg CJ. Preferred flavors and reasons for e-cigarette use and discontinued use among never, current, and former smokers. Int J Public Health. 2016;61:225–236. doi: 10.1007/s00038-015-0764-x. doi: 10.1007/s00038-015-0764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corey CG, Ambrose BK, Apelberg BJ, King BA. Flavored tobacco product use among middle and high school students—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:1066–1070. doi: 10.15585/mmwr.mm6438a2. doi: 10.15585/mmwr.mm6438a2. [DOI] [PubMed] [Google Scholar]
- 11.Goldenson NI, Kirkpatrick MG, Barrington-Trimis JL, Pang RD, McBeth JF, Pentz MA, Samet JM, Leventhal AM. Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: application of a novel methodology. Drug Alcohol Depend. 2016;168:176–180. doi: 10.1016/j.drugalcdep.2016.09.014. doi: 10.1016/j.drugalcdep.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostygina G, Ling PM. Tobacco industry use of flavourings to promote smokeless tobacco products. Tob Control. 2016;25(s)(uppl 2):ii40–ii49. doi: 10.1136/tobaccocontrol-2016-053212. doi: 10.1136/tobaccocontrol-2016-053212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kostygina G, Glantz SA, Ling PM. Tobacco industry use of flavours to recruit new users of little cigars and cigarillos. Tob Control. 2016;25:66–74. doi: 10.1136/tobaccocontrol-2014-051830. doi: 10.1136/tobaccocontrol-2014-051830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrose BK, Day HR, Rostron B, Conway KP, Borek N, Hyland A, Villanti AC. Flavored tobacco product use among US youth aged 12-17 years, 2013-2014. JAMA. 2015;314:1871–1873. doi: 10.1001/jama.2015.13802. doi: 10.1001/jama.2015.13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delnevo CD, Giovenco DP, Ambrose BK, Corey CG, Conway KP. Preference for flavoured cigar brands among youth, young adults and adults in the USA. Tob Control. 2015;24:389–394. doi: 10.1136/tobaccocontrol-2013-051408. doi: 10.1136/tobaccocontrol-2013-051408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEvoy JW, Nasir K, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, Barr RG, Budoff MJ, Szklo M, Navas-Acien A, Polak JF, Blumenthal RS, Post WS, Blaha MJ. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:1002–1010. doi: 10.1161/ATVBAHA.114.304960. doi: 10.1161/ATVBAHA.114.304960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powell JT. Vascular damage from smoking: disease mechanisms at the arterial wall. Vasc Med. 1998;3:21–28. doi: 10.1177/1358836X9800300105. doi: 10.1177/1358836X9800300105. [DOI] [PubMed] [Google Scholar]
- 18.Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34:509–515. doi: 10.1161/ATVBAHA.113.300156. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 19.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 20.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 21.O’Toole TE, Abplanalp W, Li X, Cooper N, Conklin DJ, Haberzettl P, Bhatnagar A. Acrolein decreases endothelial cell migration and insulin sensitivity through induction of let-7a. Toxicol Sci. 2014;140:271–282. doi: 10.1093/toxsci/kfu087. doi: 10.1093/toxsci/kfu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeJarnett N, Conklin DJ, Riggs DW, et al. Acrolein exposure is associated with increased cardiovascular disease risk. J Am Heart Assoc. 2014;3:e000934. doi: 10.1161/JAHA.114.000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haberzettl P, Vladykovskaya E, Srivastava S, Bhatnagar A. Role of endoplasmic reticulum stress in acrolein-induced endothelial activation. Toxicol Appl Pharmacol. 2009;234:14–24. doi: 10.1016/j.taap.2008.09.019. doi: 10.1016/j.taap.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AG, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G, Frati G. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest. 2016;150:606–612. doi: 10.1016/j.chest.2016.04.012. doi: 10.1016/j.chest.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 25.McMackin CJ, Vita JA. Update on nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2005;396:541–553. doi: 10.1016/S0076-6879(05)960-16-1. doi: 10.1016/S0076-6879(05)96046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shenouda SM, Widlansky ME, Chen K, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabit CE, Shenouda SM, Holbrook M, Fetterman JL, Kiani S, Frame AA, Kluge MA, Held A, Dohadwala MM, Gokce N, Farb MG, Rosenzweig J, Ruderman N, Vita JA, Hamburg NM. Protein kinase C-β contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation. 2013;127:86–95. doi: 10.1161/CIRCULATIONAHA.112.127514. doi: 10.1161/CIRCULATIONAHA.112.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fetterman JL, Holbrook M, Flint N, Feng B, Bretón-Romero R, Linder EA, Berk BD, Duess MA, Farb MG, Gokce N, Shirihai OS, Hamburg NM, Vita JA. Restoration of autophagy in endothelial cells from patients with diabetes mellitus improves nitric oxide signaling. Atherosclerosis. 2016;247:207–217. doi: 10.1016/j.atherosclerosis.2016.01.043. doi: 10.1016/j.atherosclerosis.2016.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 30.Vozoris NT. Mentholated cigarettes and cardiovascular and pulmonary diseases: a population-based study. Arch Intern Med. 2012;172:590–591. doi: 10.1001/archinternmed.2012.320. doi: 10.1001/archinternmed.2012.320. [DOI] [PubMed] [Google Scholar]
- 31.Jones MR, Tellez-Plaza M, Navas-Acien A. Smoking, menthol cigarettes and all-cause, cancer and cardiovascular mortality: evidence from the National Health and Nutrition Examination Survey (NHANES) and a meta-analysis. PLoS One. 2013;8:e77941. doi: 10.1371/journal.pone.0077941. doi: 10.1371/journal.pone.0077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munro HM, Tarone RE, Wang TJ, Blot WJ. Menthol and nonmenthol cigarette smoking: all-cause deaths, cardiovascular disease deaths, and other causes of death among blacks and whites. Circulation. 2016;133:1861–1866. doi: 10.1161/CIRCULATIONAHA.115.020536. doi: 10.1161/CIRCULATIONAHA.115.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park SJ, Foreman MG, Demeo DL, Bhatt SP, Hansel NN, Wise RA, Soler X, Bowler RP. Menthol cigarette smoking in the COPDGene cohort: relationship with COPD, comorbidities and CT metrics. Respirology. 2015;20:108–114. doi: 10.1111/resp.12421. doi: 10.1111/resp.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pletcher MJ, Hulley BJ, Houston T, Kiefe CI, Benowitz N, Sidney S. Menthol cigarettes, smoking cessation, atherosclerosis, and pulmonary function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arch Intern Med. 2006;166:1915–1922. doi: 10.1001/archinte.166.17.1915. doi: 10.1001/archinte.166.17.1915. [DOI] [PubMed] [Google Scholar]
- 35.Putzhammer R, Doppler C, Jakschitz T, Heinz K, Förste J, Danzl K, Messner B, Bernhard D. Vapours of US and EU market leader electronic cigarette brands and liquids are cytotoxic for human vascular endothelial cells. PLoS One. 2016;11:e0157337. doi: 10.1371/journal.pone.0157337. doi: 10.1371/journal.pone.0157337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schweitzer KS, Chen SX, Law S, et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol. 2015;309:L175–L187. doi: 10.1152/ajplung.00411.2014. doi: 10.1152/ajplung.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puranik R, Celermajer DS. Smoking and endothelial function. Prog Cardiovasc Dis. 2003;45:443–458. doi: 10.1053/pcad.2003.YPCAD13. doi: 10.1053/pcad.2003.YPCAD13. [DOI] [PubMed] [Google Scholar]
- 38.Vita JA. Endothelial function. Circulation. 2011;124:e906–e912. doi: 10.1161/CIRCULATIONAHA.111.078824. doi: 10.1161/CIRCULATIONAHA.111.078824. [DOI] [PubMed] [Google Scholar]
- 39.Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, Duell AK, Peyton DH. Benzene formation in electronic cigarettes. PLoS One. 2017;12:e0173055. doi: 10.1371/journal.pone.0173055. doi: 10.1371/journal.pone.0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benowitz NL, Herrera B, Jacob P., 3rd Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004;310:1208–1215. doi: 10.1124/jpet.104.066902. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]


