Abstract
Objective
Coronary artery disease (CAD) is a major cause of morbidity and mortality, and despite important advances in our understanding of this disorder, the underlying mechanisms remain under investigation. Recently, increased attention has been placed to the role of behavioral factors like emotional stress on CAD risk. Brain areas involved in memory and the stress response, including medial prefrontal cortex, insula and parietal cortex, also have outputs to the peripheral cardiovascular system. The purpose of this study was to assess the effects of mental stress on brain and cardiac function in patients with CAD.
Methods
CAD patients (N=170) underwent cardiac imaging with [Tc-99m] sestamibi single photon emission tomography (SPECT) at rest and during a public speaking mental stress task. On another day they underwent imaging of the brain with [O-15] water positron emission tomography (PET) during mental stress (arithmetic and public speaking) and control conditions.
Results
Patients with mental stress-induced myocardial ischemia (MSI) showed increased activation with stress in anterior cingulate, inferior frontal gyrus, and parietal cortex (p<0.005). This was seen with both arithmetic stress and public speaking stress. Arithmetic stress was additionally associated with left insula activation, and public speaking with right pre/post-central gyrus and middle temporal gyrus activation (p<0.005).
Conclusions
These findings suggest that mental stress-induced myocardial ischemia is associated with activation in brain areas involved in the stress response and autonomic regulation of the cardiovascular system. Altered brain reactivity to stress could possibly represent a mechanism through which stress leads to increased risk of CAD-related morbidity and mortality.
Keywords: stress, PTSD, cardiovascular disease, depressive disorders
Introduction
Coronary artery disease (CAD) is associated with considerable morbidity and represents the leading cause of mortality world-wide (1). Traditional risk factors, like smoking, diabetes, and hypertension, have only been able to explain a portion of the risk for CAD. In an effort to reduce the prevalence of this disorder, other potentially modifiable risk factors have been examined, including behavioral factors.
Emotional factors, such as anger and stress sensitivity, are increasingly recognized as potential contributors to CAD (2–10). For example, anger can trigger acute episodes of Acute Coronary Syndrome (ACS) secondary to increased hemodynamic responses (11–14). Other factors may play a role in the mechanism of anger-provoked ACS, including effects on platelet function and cardiac conductivity. conduction/arrhythmias (4, 15–17). These effects are likely mediated by brain areas involved in both emotion and cardiovascular regulation, like the anterior cingulate (18).
A number of studies showed that acute psychological stress can induce myocardial ischemia in patients with CAD (19–34). An increase in CAD is seen in patients with stress-related mental disorders, including major depression (35–37) and posttraumatic stress disorder (PTSD) (4, 38). Negative affect, which is commonly seen in depression, is associated with an increased perception of anginal chest pain (39). Many CAD patients experience asymptomatic episodes of stress-induced myocardial ischemia on a daily basis (40–43). Mental stress-induced myocardial ischemia (MSI) can occur in patients without exercise-induced myocardial ischemia, and is not consistently related to atherosclerotic CAD (22, 25, 26, 28–30, 33, 44–48). MSI is twice as common in women under 50 than similar aged men (28), is more common in patients with depression (49), and is associated with increased long-term risk for adverse cardiac events with similar effect size as exercise-induced myocardial ischemia (30, 50–56).
Mechanisms of MSI remain unclear. One idea is that an increase in stress-induced vasonconstriction mediates MSI. Stress also activates inflammatory pathways, and an increase in inflammation has been associated with coronary artery vasoconstriction, as seen in Kounis Syndrome (57). Mental stress must, however, act through the brain to induce myocardial ischemia, whether it is mediated by inflammatory, sympathetic, or other responses (17, 23, 58, 59), however the mechanisms by which this occurs are not known. Brain areas involved in memory, emotion, and peripheral cardiovascular regulation include the medial prefrontal cortex, insula, and parietal cortex (17, 60, 61). We hypothesized that patients with MSI would show stress-induced changes in brain areas involved in the regulation of memory, emotion, and/or peripheral cardiovascular responses to stress, including medial prefrontal cortex, insula, dorsolateral prefrontal cortex, and parietal cortex. Understanding brain mechanisms in MSI may lead to new treatment interventions in CAD.
Methods
Study Sample
Patients between the ages of 30 and 79 with known coronary artery disease (CAD) (N=186) from the Mental Stress Ischemia Prognosis Study (MIPS) were included in the study. MIPS patients were recruited from Emory University Hospital, Grady Memorial Hospital and the Atlanta VA Medical Center from September 2010 to September 2016 (56). CAD was defined based on a previous cardiac catheterization showing atherosclerosis, history of prior myocardial infarction, a history of percutaneous coronary intervention or coronary artery bypass grafting at least one year prior to the study, or a positive nuclear stress test. Patients were excluded if they had had a recent acute coronary syndrome, or decompensated congestive heart failure within 1 week of the enrollment visit, pregnancy based on pregnancy testing, systolic blood pressure greater than 180 mm Hg or diastolic blood pressure greater than 110 mm Hg on the day of the test, a history based on the Structured Clinical Interview for the Diagnostic and Statistical Manual IV (SCID) of a severe mental disorder including schizophrenia, psychotic depression, bipolar disorder, or alcohol or substance dependence in the past year, history of loss of consciousness of more than one minute, history of neurological disorder, such as dementia, stroke, or Parkinson’s Disease, or contraindications to regadenoson administration. Beta-adrenergic antagonists were held for 24 hours and calcium channel blockers and nitrates for at least 12 hours prior to the stress test. Patients for whom withholding medications was considered unsafe were excluded. All patients provided written informed consent, and the study was approved by the Emory University Investigational Review Board (IRB).
Psychometric Assessment
All patients were assessed with a number of psychometric instruments, including the Beck Depression Inventory (BDI), a reliable and validated self-report measure of depressive symptoms (62). Information about medications and other clinical data were obtained through questionnaires and medical chart review. The Subjective Units of Distress Scale (SUDS) was used to assess stress before and after the stress procedures. Psychiatric diagnosis was assessed using the Structured Interview for the Diagnostic and Statistical Manual-IV (SCID) (63).
Measurement of Vasoconstriction using Peripheral Arterial Tonometry
The EndoPAT™ Peripheral Arterial Tonometry (PAT) device (Itamar-Medical, Israel) was used to measure peripheral vascular reactivity to stress. The device measured finger pulse wave amplitude with a probe using a robust modified form of volume plethysmography as a means of estimating pulsatile arterial volume changes independently of venous pulsations/pooling (64). Pressure changes accompanying peripheral volume changes are fed to a personal computer by which the signal is bandpass filtered (0.3 to 30 Hz), amplified, displayed and stored. After eliminating areas of artifact, microvascular vasoconstriction was measured by pulse wave amplitude at stress (mean during 4 stress tasks) compared to baseline rest (mean during 4 control tasks). We have found PAT measurement of stress-induced vasoconstriction to be highly reproducible in our lab (65).
Mental Stress Testing
Participants underwent mental stress testing in conjunction with imaging of the heart and brain. Participants initially underwent cardiac imaging of the heart (described below) at rest and during a public speaking task. On a separate day participants returned for imaging of the brain during two mental stress tasks. There were four conditions, two control and two stress conditions (mental arithmetic and public speaking), and participants underwent scanning of the brain twice for each condition, for a total of eight brain scans (Figure 1). Mental stress testing was performed by trained staff using mental arithmetic and public speaking. First, participants were asked to count out loud for the mental arithmetic control condition for two scans, then to read a paragraph out loud for the public speaking control for two scans. For the mental arithmetic stress condition they were asked to perform a series of increasingly complicated mathematical calculations under time pressure, including addition, subtraction, multiplication and division, while they received negative feedback on their performance from a staff member performing the test who was wearing a white coat. For the public speaking mental stress task, patients listened to a scripted message with instructions for the mental stress task. For the cardiac imaging day (which occurred first), participants were given a situation where an elderly relative was being mistreated in a nursing home, and they had to meet with the administrator to try and rectify the situation. For the brain imaging day, participants were given two stressful situations, one involving a long-term house guest who had overstayed her welcome, and the other an uncomfortable situation in which an elderly sister was unfairly hit while driving in a parking lot. They were then asked to prepare and give a speech for each situation that was two minutes in duration. They were told the speech would be evaluated for content.
Figure 1.

Brain and cardiac imaging with mental stress protocol. Participants underwent eight brain scans following injection of radiolabeled water in conjunction with exposure to control and mental stress conditions. Control conditions involving counting out loud for two scans, then reading a neutral paragraph out loud for two scans. Mental stress conditions included performing a series of increasingly complicated mathematical calculations under time pressure for two scans, followed by a public speaking task in which patients were given two stressful situations and then asked to prepare and give a speech for each situation that was two minutes in duration.
Cardiac Imaging at Rest and with Mental Stress
Participants underwent cardiac single photon emission computed tomography (SPECT) imaging of the heart on a separate day for assessment of myocardial perfusion at rest and with mental stress. For the rest image they received 10–14 mCi of [Tc-99m] sestamibi intravenously. Thirty to 40 minutes later resting SPECT images of the heart were obtained at rest. Participants then underwent a public speaking task (nursing home scenario) following which they were injected with 10–14 mCi [Tc-99m]sestamibi at the time of peak stress followed in 30–40 minutes by SPECT imaging of the heart with mental stress. We have found these methods of measuring mental stress-induced myocardial ischemia to be highly reproducible (65).
Brain Imaging with Mental Stress
Patients underwent high resolution positron emission tomography (HRPET) imaging of the brain in conjunction with control and mental stress tasks. HRPET imaging of the brain was performed with the High Resolution Research Tomograph (HRRT) (Siemens, Inc, Erlangen, Germany) (66). This device has 2 mm spatial resolution and significantly higher sensitivity than conventional PET cameras.(66, 67). Blood pressure and heart rate were recorded at 5-minute intervals during the resting phase and at 1-minute intervals during the stress phases using an automatic oscillometric device.
Participants underwent eight PET scans of the brain in conjunction with control and stressful tasks. During each scan, radiolabelled water (H2[15O]O), produced in an on-site cyclotron, was injected for measurement of brain blood flow. During the first 4 scans, patients were asked to count out loud (2 scans) and talk about a neutral event (2 scans). The last 4 scans were performed during an acute mental stress challenge involving mental arithmetic (2 scans) and public speaking (2 scans). All sessions lasted for 2 minutes and 20mCi of O-15 water were injected 10 seconds after each task started. A physician was present during the study and electrocardiogram and vital signs were continuously monitored.
Of the 186 patients who started the study, 170 were able to finish the protocol with usable data. Reasons for non-completion included failure to complete the PET imaging procedure due to elevated blood pressure during the procedure (N=1), claustrophobia in the scanner (N=3), failure to obtain venous access (N=3), inability to lie flat or remain on the scanner table due to pain or physical limitations (N=4), and failure of image acquisition or lost or corrupted image data (N=5).
Image Analysis
Myocardial perfusion images were interpreted by two experienced readers blinded to the condition and without prior knowledge of the medical history or angiographic data. Discrepancies in interpretation of SPECT images were resolved by consensus. Rest and stress images were visually compared for the number and severity of perfusion defects using a 17-segment model (68). Each segment was scored from 0 to 4, with 0 being normal uptake, 1 possibly normal perfusion, 2 definitely abnormal perfusion, 3 severe perfusion defect and 4 no perfusion. A um rest score was calculated by adding up the perfusion scores across the 17 myocardial segments. Sum stress scores were calculated by adding up the perfusion scores across segments during stress and subtracting out sum rest scores. Ischemia was defined as a new myocardial perfusion defect with a score of ≥ 2 in any segment, or worsening of a pre-existing impairment of at least 2 points in a single segment, or worsening of at least 1 point in 2 or more contiguous segments (68).
PET images of the brain were realigned and analyzed using statistical parametric mapping (SPM8) (69) and methods previously described (70). Images were realigned to the first scan of the study session. The mean concentration of radioactivity in each scan was obtained as an area-weighted sum of the concentration of each slice and was adjusted to a nominal value of 50 ml/minute per 100 g. The data underwent transformation into a common anatomical space and were smoothed with a three-dimensional Gaussian filter to 8-mm FWHM. A post hoc analysis involved placement of circular regions of interest (ROIs) over brain areas implicated in the spm analysis to assess relationship between brain activation with stress and myocardial perfusion.
Statistical Analysis
Analysis of variance (ANOVA) was used to compare baseline demographic and risk factors and sum rest and stress scores obtained from cardiac SPECT images between CAD patients with and without MSI. Brain regional blood flow was compared for mental stress and neutral conditions. Statistical analyses yielded image data sets in which the values assigned to individual voxels correspond to the t-statistic of the difference in brain blood flow between conditions. Statistical images were displayed with values of z score units. A threshold z score of 2.68 (p < .005, uncorrected) was used to define significant activation within voxels. This value has been shown to minimize the possibility of both Type 1 and Type 2 errors in brain imaging studies (71, 72). Additionally, a minimum cluster of 11 voxels was used to define areas of significant activation within hypothesized areas (medial prefrontal, inferior frontal, and parietal cortex and insula). Location of areas of activation was identified as the distance from the anterior commissure in millimeters, with x, y, and z coordinates, transformed from Montreal Neurological Institute coordinates to those of the Talairach stereotaxic atlas, a commonly used atlas for the expression of stereotaxic coordinates (73).
Results
There were no significant differences between patients with CAD with and without mental stress ischemia (MSI) for any demographic or risk factors, including age, sex, race, depressive symptoms, body mass index (BMI), or history of smoking, diabetes, hypertension, or dyslipidemia (Table 1). The groups also showed similar patterns for use of medications including vasodilators, angiotensin receptor blocker, angiotensin converting enzyme inhibitors, diuretics and beta-blockers (Table 1).
Table 1.
Demographic and Risk Factors for CAD Patients with and without Mental Stress Ischemia1
| MSI- (N=125 (74%)) | MSI+ (N=45 (26%)) | |
|---|---|---|
| Age | 62 (8 SD) | 63 (9 SD) |
| Sex | 45 F/80 M | 9 F/36 M |
| Race | 38% AA/59% Cauc/3% Asian | 31% AA/65% Cauc/2% Asian/2% NA |
| BMI (kg/m2) | 30 (6 SD) | 30 (6 SD) |
| BDI Score | 13 (11 SD) | 11 (11 SD) |
| Hypertension | 76% | 76% |
| Dyslipidemia | 81% | 87% |
| Diabetes | 34% | 33% |
| Smoking (current) | 13% | 7% |
| Smoking (lifetime) | 63% | 67% |
| Percentage of patients taking: | ||
| Antidepressants | 36% | 22% |
| ACE Inhibitors | 42% | 47% |
| Angiotensin Receptor Inhibitors | 14% | 18% |
| Diuretics | 31% | 29% |
| Vasodilators | 12% | 7% |
| Anxiolytics | 6% | 7% |
| Beta Blockers | 77% | 73% |
| Statins | 89% | 83% |
CAD=coronary artery disease; F=female; M=male; AA=African American; NA=Native American; BMI=body mass index; BDI=Beck Depression Inventory; ACE=angiotensin converting enzyme.
Participants showed increased heart rate, blood pressure, and subjective distress during the cardiac imaging stress compared to the brain imaging stress. As measured by the delta of increase over baseline, there was an increase in heart rate (11 (9 SD) v. 3 (4 SD), p<0.001), systolic blood pressure (25 (17 SD) v. 8 (9 SD), p<0.001),, and subjective distress as measured with the SUDS (10 (20 SD) v. 2 (29 SD), p<0.001), on the cardiac day compared to the brain imaging day. There was no difference in peripheral vasoconstriction, however, as measured with Peripheral Arterial Tonometry (PAT) for the cardiac versus brain imaging day (0.72 (0.35 SD) v. 0.74 (0.45 SD), p=0.78).
Patients with MSI compared to CAD patients without MSI had an increase in mean myocardial perfusion defects at rest as measured by the summed rest scores ((8 (5 SD) versus 6 (5 SD); p=0.016) and an increase in mental stress-induced myocardial ischemia as measured with the summed stress scores ((4 (3 SD) versus 2 (2 SD); p<0.001).
When the group of CAD patients were looked at as a whole there was increased activation with stress in the inferior frontal gyrus and parietal cortex (inferior and superior parietal lobules). Decreased activation was seen in all patients in the pre- and post-central gyrus, cerebellum, fusiform gyrus, and lingual gyrus. Both activations and deactivations were seen in different parts of the medial frontal gyrus and superior temporal gyrus in the group as a whole.
When MSI+ patients were compared to MSI-, the former had increased activations during stress in the parietal cortex (inferior parietal lobule and supramarginal gyrus), middle temporal gyrus and anterior cingulate (Tables 2, 4), and decreased activation in the cerebellum, cingulate and medial frontal gyrus (Tables 3, 5). In addition to the brain areas listed above, during arithmetic stress MSI+ patients also had increased activation in the left insula, medial frontal, inferior and middle frontal gyrus (Figure 2, Table 2). During speech stress there was additional activation in right pre- and post-central gyrus and posterior cingulate (Table 4, Figure 3). There were no significant correlations between resting myocardial perfusion and brain activation with stress in anterior cingulate, parietal cortex, amygdala, hippocampus, orbitofrontal, medial prefrontal, lingual, or fusiform gyrus in the MSI+ patients.
Table 2.
Areas of Increased Activation During Mental Arithmetic Stress in Patients with Mental Stress-Induced Myocardial Ischemia (MSI) compared to non-MSI Patients
| Talairach coordinants
|
||||||
|---|---|---|---|---|---|---|
| Z score | Voxel Number | x | y | Z | Brain Region | Brodman’s Area |
| 6.43 | 8112 | −6 | 16 | 43 | L. Medial Frontal Gyrus | 6 |
| 6.10 | 48 | 30 | 24 | R. Middle Frontal Gyrus | 46 | |
| 6.08 | −4 | 40 | 20 | L. Anterior Cingulate | 32 | |
| 5.31 | 208 | 50 | −41 | 41 | R. Inferior Parietal Lobule | 40 |
| 4.26 | 61 | −49 | 28 | R. Supramarginal Gyrus | 40 | |
| 3.49 | 57 | −45 | 35 | R. Supramarginal Gyrus | 40 | |
| 5.28 | 481 | −32 | 21 | −5 | L. Inferior Frontal Gyrus | 47 |
| 4.38 | −36 | 17 | −12 | L. Inferior Frontal Gyrus | 47 | |
| 4.04 | −40 | 16 | 7 | L. Insula | 13 | |
| 4.03 | 50 | −57 | −33 | −4 | L. Middle Temporal Gyrus | * |
| 3.67 | 27 | −48 | −47 | 36 | L. Inferior Parietal Lobule | 40 |
| 3.45 | 72 | 0 | 43 | 3 | Anterior Cingulate | * |
| 2.91 | 8 | 50 | −3 | R. Anterior Cingulate | 10 | |
| 3.16 | 15 | 34 | −83 | −23 | R. Middle Temporal Gyrus | 22 |
z>2.68, p<.005
Table 4.
Areas of Increased Activation During Speech Mental Stress in Patients with Mental Stress-induced Myocardial Ischemia (MSI) versus non-MSI Patients
| Talairach coordinates
|
||||||
|---|---|---|---|---|---|---|
| Z score | Voxel Number | x | y | z | Brain Region | Brodmann’s Area |
| 7.82 | 15715 | −4 | 18 | 41 | Cingulate Gyrus | 32 |
| 7.82 | −6 | 27 | 34 | Cingulate Gyrus | 32 | |
| 7.82 | −6 | 30 | 24 | Anterior Cingulate | 32 | |
| 7.82 | 241 | 42 | −52 | 43 | Inferior Parietal Lobule | 40 |
| 6.31 | 50 | −41 | 41 | Inferior Parietal Lobule | 40 | |
| 4.34 | 55 | −29 | 40 | Postcentral Gyrus | 2 | |
| 5.51 | 72 | −40 | −48 | 41 | Inferior Parietal Lobule | 40 |
| 5.44 | −46 | −43 | 39 | Inferior Parietal Lobule | 40 | |
| 5.05 | 48 | 61 | −47 | 26 | Supramarginal Gyrus | 40 |
| 4.06 | 35 | −59 | −37 | −9 | Middle Temporal Gyrus | 21 |
| 3.84 | −57 | −43 | −3 | Middle Temporal Gyrus | 21 | |
| 3.93 | 93 | −32 | −69 | −26 | Cerebellum | * |
| 3.90 | 15 | 40 | −26 | 53 | Postcentral Gyrus | 3 |
| 2.88 | 46 | −19 | 51 | Postcentral Gyrus | 3 | |
| 3.68 | 53 | 59 | −10 | −7 | Middle Temporal Gyrus | 21 |
| 3.38 | 57 | −20 | −5 | Middle Temporal Gyrus | 21 | |
| 3.49 | 44 | 30 | −64 | −30 | Cerebellum | * |
| 3.04 | 14 | 12 | −20 | −67 | R. Precentral Gyrus | 6 |
z>2.68, p<.005
Table 3.
Areas of Decreased Activation During Mental Arithmetic Stress in Patients with Mental Stress-induced Myocardial Ischemia (MSI) versus non-MSI Patients
| Talairach coordinates
|
||||||
|---|---|---|---|---|---|---|
| Z score | Voxel Number | x | y | z | Brain Region | Brodmann’s Area |
| 4.02 | 33 | −22 | −71 | −19 | Cerebellum | * |
| 3.57 | 31 | −44 | 26 | 6 | Inferior Frontal Gyrus | 13 |
| 3.51 | 20 | 24 | −69 | −20 | Cerebellum | * |
| 3.44 | 16 | −12 | 14 | 38 | Cingulate Gyrus | 32 |
| 3.23 | 16 | −16 | 39 | 37 | Superior Frontal Gyrus | 9 |
| 3.21 | 11 | 10 | 6 | 42 | Cingulate Gyrus | 24 |
| 3.19 | 19 | 0 | 23 | 30 | Cingulate Gyrus | 32 |
| 3.16 | 13 | −10 | 32 | 24 | Cingulate Gyrus | 32 |
| 3.12 | 15 | 2 | 42 | 29 | Medial Frontal Gyrus | 9 |
| 2.96 | 25 | −36 | 45 | 0 | R. Inferior Frontal Gyrus | 10 |
| 2.91 | 13 | −50 | 9 | 24 | L. Inferior Frontal Gyrus | 44 |
z>2.68, p<.005
Table 5.
Areas of Decreased Activation During Speech Mental Stress in Patients with Mental Stress-induced Myocardial Ischemia (MSI) versus Non-MSI Patients
| Talairach coordinates
|
||||||
|---|---|---|---|---|---|---|
| Z score | Voxel Number | x | y | z | Brain Region | Brodmann’s Area |
| 7.14 | 107 | 22 | −65 | −13 | Cerebellum | * |
| 6.82 | 395 | −55 | 0 | 4 | Superior Temporal Gyrus | 22 |
| 5.83 | −51 | −20 | 18 | Insula | 40 | |
| 5.68 | −46 | −8 | −11 | L. Sup. Temporal Gyrus | 21 | |
| 6.71 | 165 | 40 | −4 | −2 | Insula | * |
| 6.42 | 51 | −5 | 11 | Precentral Gyrus | 43 | |
| 5.35 | 57 | 1 | 11 | Precentral Gyrus | 6 | |
| 5.66 | 26 | −42 | −16 | −5 | Insula | 13 |
| 5.33 | 34 | 50 | −73 | 9 | Middle Temporal Gyrus | 39 |
| 4.49 | 42 | −79 | 11 | Middle Occipital Gyrus | 19 | |
| 3.04 | 48 | −69 | 22 | Middle Temporal Gyrus | 39 | |
| 5.09 | 21 | 46 | −10 | −5 | Insula | 22 |
| 4.87 | 142 | −20 | −59 | −21 | Cerebellum | * |
| 4.42 | −20 | −73 | −15 | Cerebellum | * | |
| 4.33 | −12 | −62 | −26 | Cerebellum | * | |
| 4.32 | 19 | −51 | −29 | 12 | Superior Temporal Gyrus | 41 |
| 3.04 | −51 | −23 | 7 | Superior Temporal Gyrus | 41 | |
| 3.84 | 30 | 4 | 44 | −17 | Medial Frontal Gyrus | 11 |
| 2.60 | −4 | 46 | −13 | Medial Frontal Gyrus | 11 | |
| 3.84 | 13 | 57 | −35 | 29 | Inferior Parietal Lobule | 40 |
| 3.37 | 65 | −30 | 29 | Inferior Parietal Lobule | 40 | |
| 3.63 | 35 | 65 | −6 | 26 | Precentral Gyrus | 6 |
| 3.50 | 65 | −16 | 28 | Postcentral Gyrus | 1 | |
| 3.48 | 23 | −6 | −23 | 45 | Paracentral Lobule | 31 |
| 3.24 | 23 | −4 | −2 | 41 | Cingulate Gyrus | 24 |
z>2.68, p<.005
Figure 2.

Areas of increased activation with mental arithmetic stress in CAD patients with mental stress-induced myocardial ischemia (MSI) versus CAD patients without MSI. Increases are seen in anterior cingulate and inferior frontal gyrus (in addition to parietal cortex).
Figure 3.
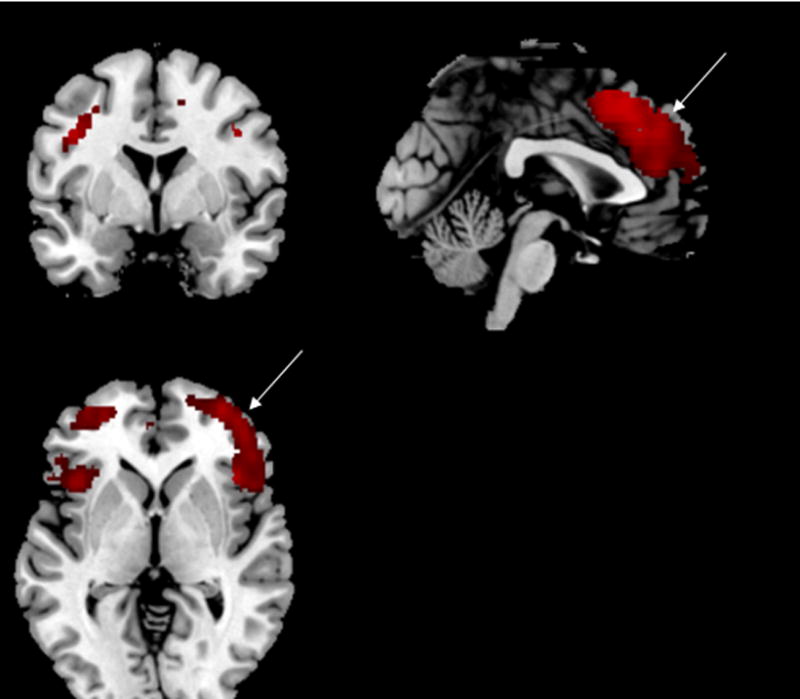
Areas of increased activation with public speaking stress in CAD patients with mental stress-induced myocardial ischemia (MSI) versus CAD patients without MSI. Increases are seen in in anterior cingulate and inferior frontal gyrus (and also parietal cortex).
Discussion
This study showed that CAD patients with mental stress-induced ischemia (MSI) compared to those without MSI had increased activation in the medial frontal gyrus, anterior cingulate, inferior frontal gyrus, and parietal cortex (including inferior parietal lobule and supramarginal gyrus), brain areas involved in memory, emotion, and perception of the self in time and space. Activation in these areas was seen with both mental arithmetic and public speaking stress. Mental arithmetic additionally activated the insula, an important output to regulation of peripheral cardiovascular responses to stress (61). Public speaking, on the other hand, further activated pre- and postcentral gyrus (motor function and sensation) and middle temporal gyrus (auditory function). MSI was also associated with decreased activation in cerebellum, cingulate and medial frontal gyrus. Consistent with prior reports of increased resting and mental stress-induced myocardial perfusion defects (74), and increased cardiovascular reactivity to mental stress (40) in MSI+ patients, in the current study CAD patients with MSI had an increase in both resting and stress-induced myocardial perfusion defects compared to CAD patients without MSI, and we have previously reported an increase in mental stress-induced heart rate and blood pressure in this sample in the MSI+ versus MSI- patients (75). There was no relationship, however, between baseline myocardial perfusion defects in MSI+ patients and brain activation with stress in any of these regions.
In patients with MSI, mental stress was associated with increased activation in the parietal lobe, including both supramarginal gyrus and inferior parietal lobule. The parietal lobe modulates perception of the self in space and time, perception of contextual cues, and visuospatial memory (76–80) in addition to modulation of peripheral cardiovascular responses to stress (81). This brain area plays a key role in increased awareness and hyper-vigilance during threat or attack (76, 82). Studies have also implicated this region in stress-related psychiatric disorders (83) and risk for cardiovascular disease.(84) Our findings suggest increased parietal cortical response to stress could underlie MSI.
The inferior frontal gyrus, which was also activated with stress in the MSI+ patients, is involved in processes that are relevant to stress, including regulation of attention and outcome expectancies (80). A recent meta-analysis of brain imaging studies in humans showed that the inferior frontal gyrus and anterior insula were the only two brain areas that consistently activate with both physiological and psychological stressors (85). The inferior frontal gyrus, which has connections to the parietal lobe, in conjunction with parietal lobule could mediate a heightening of activity in brain areas involved in perception and cognition during stress.
The medial prefrontal cortex (anterior cingulate) was activated with stress in MSI patients. This area is involved in the modulation of emotion, problem solving, selective attention and other higher cognitive functions (86–89). It also works in tandem with the insula to regulate peripheral autonomic activity (90, 91) and is responsible for activation of peripheral cortisol and sympathetic responses to stress (86, 92). Previous studies have implicated this area in symptoms of depression (93, 94) and posttraumatic stress disorder (61).
MSI patients showed increased activation of the left insula during mental arithmetic stress. The insula is a key brain area with output to peripheral cardiovascular systems that respond to stress (81, 95) including activation of sympathetic systems and deactivation of parasympathetic function. It also has important connections to brain areas involved in the stress response (96), and altered function and structure in this region has been linked to several psychiatric disorders related to stress, including PTSD (96–104) that have also been linked to increased morbidity and mortality related to CAD (105). Increased stress reactivity in susceptible CAD patients may lead to greater insula activation with stress, and this may represent a mechanism for increased MSI in vulnerable patients.
The Brain Heart Laterality Hypothesis states that asymmetric activation of sympathetic inputs to the heart during stress can be the cause of arrhythmias. The greatest risk of potentially life threatening ventricular arrhythmias is felt to occur from left lateral brain activation with ipsilateral activation of sympathetic pathways to the left side of the heart (106). Studies have shown that asymmetric brain responses to stress result in pro-arrhymic assymetric sympathetic inputs to the heart (107). Prior studies have shown a correlation between increased blood pressure and heart rate during mental stress and activation in the right insula and cerebellum and with heart rate in the anterior cingulate, while cardiovascular reactivity was associated with decreased prefrontal and temporal activation (108). Studies have also implicated insula and somatosensory cortex in representation of peripheral autonomic function (109). The current study found increased left insula and increased right somatosensory activation, and decreased medial prefrontal activation, with stress in MSI+ patients. Interpreted in conjunction with prior studies these results suggest that unique brain activation and deactivation patterns in MSI+ patients may be associated with increased risk for sudden cardiac death. This may explain in part the increase in morbidity and mortality in these patients compared to those with exercise-induced myocardial ischemia (28, 110, 111).
Several of the unique areas activated with speaking stress probably relate to increased demand on regions with specific functions. The precentral gyrus controls motor activity, and giving a speech under negative feedback pressure likely involves more demand on motor function. Similarly the middle temporal gyrus mediates primary auditory function, and greater demands with speech stress likely led to greater activation in this area as well. Both types of stressors resulted in decreases in function in cerebellum and parts of the posterior cingulate during MSI. Decreased function implies in the MSI+ group could be related to the fact that MSI- patients activate these areas to a greater extent, or that the control task is associated with greater activation than the stress task. These areas are involved spatial and motor processing tasks that play a key role in the stress response (92). Recently, there has been an increased appreciation for the role of the cerebellum in social and emotional processing and regulation in addition to its role in motor control (112–115). A failure in this brain region to mount a successful response to stress may contributes to maladaptive cardiovascular responses to stress.
The current study did not find specific predictors of mental stress ischemia, including medication or behavioral factors. We did not show a relationship between medications and mental stress induced ischemia in the current study. This is consistent with other studies from our group and others that did show that anti-ischemic medications affect ischemic responses to stress (31, 75, 116, 117). The association with depression and other psychosocial factors has also been variable. We have found associations with depression and anger in a separate sample of post-MI patients (24, 118), but not in this sample of overall older patients with broadly defined CAD. The current study was not specifically designed, however, to assess the relationship between depression or other behavioral factors like hostility, which limits our ability to make conclusions in this area.
This research is subject to several limitations. First, our findings are not generalizable outside of populations of CAD patients (119, 120).. Nonetheless, this is an important population to study because of their high morbidity and mortality. The results may also not be generalizable to specific CAD groups. For instance, our sample had only slightly more white than African-American (AA) subjects. This is typical of the racial distribution of Georgia, but not many other areas of the country. Although AAs have an increase in hypertension (119) and oxidative stress (120), studies have not shown an increase in stress-induced blood pressure reactivity (120). Therefore the impact on race in this group is unclear. Another limitation of the current study is that, although laboratory studies can offer better control of stress exposure through a standardized experimental protocol, they may not reflect multiple and various naturally occurring real-life stressors. This limits our ability to generalize that the results are applicable to the variety of stresses existing in daily life. Future studies of ambulatory monitoring of cardiovascular function in naturalistic daily life settings will represent an important step in addressing this need. There was an increase in heart rate, blood pressure, and subjective distress on the cardiac compared to the brain imaging day. This is likely due to several factors, including the fact that the cardiac imaging day came first so there was some adjustment to the stress challenge on the brain imaging day. Additionally, participants were upright on the cardiac day versus prone on the imaging day. This allowed the testers to more fully engage with the participants, as evidenced by the fact that they reported more subjective distress on that day. We did not, however, find any differences in stress-induced peripheral vasconstriction between the two days, however, which would be consistent with the idea that stress exerted an equivalent effect on cardiac function. We have previously reported in this sample increased cardiovascular reactivity to stress in the MSI+ patients, which could have driven the differences in brain activation between the groups, although there was no relationship with myocardial function and brain activation. Additionally, there could have been differences in effort with mental stress tasks between groups, although we attempted to limit this by varying task difficulty according to ability to successfully perform the task. Another limitation is in our ability to conclude that mental stress acted through the brain to cause myocardial ischemia in susceptible participants. For one thing, cardiac and brain stress were performed on separate days (mainly due to the fact that the scanners were in different locations). Therefore the stress episode seen in conjunction with myocardial ischemia was not the same stress episode associated with the brain activations. Even if they did occur on the same day we would be limited in our ability to determine cause and effect. For instance, stress might affect brain regions which drive peripheral autonomic response leading to myocardial ischemia, which in turn leads to activation in other other brain regions. For those and other reasons we can only point to associations between patterns of cardiac and brain responses to mental stress which suggest potential models by which stress could act through the brain to induce myocardial ischemia. The fact that we did not find a relationship between myocardial perfusion and stress-induced brain activation in the MSI+ patients, however, suggest that the current findings are not entirely driven by deficiencies in myocardial function.
The study also had several strengths. We have found our methods of measurement of stress-induced myocardial ischemia and vasoconstriction to be highly reproducible in our laboratory (65). A sample of 22 participants underwent repeated measures of PAT, systolic (SBP) and diastolic blood pressure (DBP) and heart rate (HR) at two time points within 8 weeks using the same mental stress protocol. Bland-Altman plots showed excellent reproducibility of PAT measurements, with only one data point falling outside the 95% limits of agreement. For SBP, DBP and HR, Bland-Altman plots showed that >95% of data points were within the 95% limits of agreement (65). These findings indicate a high level of reliability of our mental stress protocol. Other strengths of the current study include the fact that this is the largest study to date to examine brain correlates of MSI, and used state-of-the-art imaging instrumentation and methodology.
In conclusion, we found that MSI in CAD patients was associated with increased activation in several brain areas, including the parietal lobe, inferior frontal lobe, anterior cingulate, and insula. The findings suggest that brain areas involved in memory, fear inhibition, and visuospatial processing of threat may mediate stress-related myocardial dysfunction.
Acknowledgments
This study was supported by NIH research grants P01 HL101398, HL088726, MH076955, MH067547–01, MH56120, RR016917, HL077506, HL068630, HL109413, and HL125246. We wish to acknowledge Delicia Votaw, C.N.M.T., and Margie Jones, C.N.M.T., for their assistance with imaging and analysis procedures and Nancy Murrah, R.N., Janice Parrott, R.N., Karen Sykes, and Steve Rhodes, R.N., for assistance with patient assessments and clinical research.
Abbreviations
- AA
African American
- ACE
angiotensin converting enzyme
- ACS
Acute Coronary Syndrome
- BDI
Beck Depression Inventory
- BMI
body mass index
- CAD
coronary artery disease
- DSM
Diagnostic and Statistical Manual
- F
female
- HRPET
High Resolution PET
- HRRT
High Resolution Research Tomograph
- IRB
Investigational Review Board
- M
male
- MI
myocardial infarction
- MIPS
Mental Stress Ischemia and Prognosis Study
- MSI
Mental Stress Ischemia
- NA
Native American
- PAT
Peripheral Arterial Tonometry
- PET
Positron Emission Tomography
- PTSD
posttraumatic stress disorder
- SCID
Structured Clinical Interview for DSM
- SPECT
Single Photon Emission Tomography
- spm
statistical parametric mapping
- SUDS
Subjective Units of Distress Scale
- VA
Veterans Administration
Footnotes
The authors have no conflicts of interest to declare in reference to this research.
References
- 1.World Health Organization. Cardiovascular diseases (CVDs) http://www.who.int/mediacentre/factsheets/fs317/en/2016 [cited 2016 March 16]
- 2.Strike PC, Steptoe A. Behavioral and emotional triggers of acute coronary syndromes: a systematic review and critique. Psychosom Med. 2005;67:179–86. doi: 10.1097/01.psy.0000155663.93160.d2. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarino V, Bremner JD. Traumatic stress is heartbreaking. Biol Psychiatry. 2013;74:790–2. doi: 10.1016/j.biopsych.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaccarino V, Bremner JD. Psychiatric and behavioral aspects of cardiovascular disease. In: Bonow RO, Mann DL, Zipes OP, Libby P, editors. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th. Philadelphia, PA: Saunders; 2014. [Google Scholar]
- 5.Zatzick DF, Marmar CR, Weiss DS, Browner WS, Metzler TJ, Golding JM, Stewart A, Schlenger WE, Wells KB. Posttraumatic stress disorder and functioning and quality of life outcomes in a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1997;154:1690–5. doi: 10.1176/ajp.154.12.1690. [DOI] [PubMed] [Google Scholar]
- 6.Turner JH, Neylan TC, Schiller NB, Li Y, Cohen BE. Objective Evidence of Myocardial Ischemia in Patients with Posttraumatic Stress Disorder. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003;37:268–77. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 8.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 9.Batten SV, Aslan M, Maciejewski PK, Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. J Clin Psychiatry. 2004;65:249–54. doi: 10.4088/jcp.v65n0217. [DOI] [PubMed] [Google Scholar]
- 10.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–6. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 11.Boltwood MD, Taylor CB, Boutte Burke M, Grogin H, Giacomini J. Anger report predicts coronary artery vasomotor response to mental stress in atherosclerotic segments. Am J Cardiol. 1993;72:1361–5. doi: 10.1016/0002-9149(93)90180-k. [DOI] [PubMed] [Google Scholar]
- 12.Gabbay FH, Krantz DS, Kop WJ, Hedges SM, Klein J, Gottdiener JS, Rozanski A. Triggers of myocardial ischemia during daily life in patients with coronary artery disease: Physical and mental activities, anger and smoking. J Am Coll Cardiol. 1996;27:585–92. doi: 10.1016/0735-1097(95)00510-2. [DOI] [PubMed] [Google Scholar]
- 13.Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H, Muller JE. Triggering of acute myocardial infarction onset by episodes of anger. Circulation. 1995;92:1720–5. doi: 10.1161/01.cir.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 14.Mostofsky E, Maclure M, Tofler GH, Muller JE, Mittleman MA. Relation of outbursts of anger and risk of acute myocardial infarction. Am J Cardiol. 2013;112:343–8. doi: 10.1016/j.amjcard.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burg MM, Lampert R, Joska T, Batsford W, Jain D. Psychological traits and emotion-triggering of ICD shock-terminated arrhythmias. Psychosom Med. 2004;66:896–902. doi: 10.1097/01.psy.0000145822.15967.15. [DOI] [PubMed] [Google Scholar]
- 16.Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D. Emotional and physical precipitants of ventricular arrhythmia. Circulation. 2002;106:1800–5. doi: 10.1161/01.cir.0000031733.51374.c1. [DOI] [PubMed] [Google Scholar]
- 17.Vaccarino V, Bremner JD. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev. 2017;74:297–309. doi: 10.1016/j.neubiorev.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gianaros PJ, Derbyshire SWG, May JC, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42:627–35. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burg MM, Vashist A, Soufer R. Mental stress ischemia: present status and future goals. J Nucl Cardiol. 2005;12:523–9. doi: 10.1016/j.nuclcard.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 20.Arri SS, Ryan M, Redwood SR, Marber MS. Mental stress-induced myocardial ischaemia. Heart. 2016;102:472–80. doi: 10.1136/heartjnl-2014-307306. [DOI] [PubMed] [Google Scholar]
- 21.Bremner JD, Cheema FA, Ashraf A, Afzal N, Fani N, Reed J, Musselman DL, Ritchie JC, Faber T, Votaw JR, Nemeroff CB, Vaccarino V. Effects of a cognitive stress challenge on myocardial perfusion and plasma cortisol in coronary heart disease patients with depression. Stress Health. 2009;25:267–78. doi: 10.1002/smi.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaccarino V. Mental Stress-Induced Myocardial Ischemia. In: Baune BT, Tully PJ, editors. Cardiovascular Diseases and Depression - Treatment and Prevention in Psychocardiology. Springer; 2016. [Google Scholar]
- 23.Vaccarino V, Bremner JD. Posttraumatic Stress Disorder and Risk of Cardiovascular Disease. In: Alvarenga M, Byrne D, editors. Handbook of Psychocardiology. Singapore: Springer; 2015. [Google Scholar]
- 24.Pimple P, Shah A, Rooks C, Bremner JD, Nye J, Ibeanu I, Murrah N, Shallenberger L, Kelley M, Raggi P, Vaccarino V. Association between anger and mental stress-induced myocardial ischemia. Am Heart J. 2015;169:115–21. doi: 10.1016/j.ahj.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pimple P, Shah AJ, Rooks C, Bremner JD, Nye J, Ibeanu I, Raggi P, Vaccarino V. Angina and mental stress-induced myocardial ischemia. J Psychosom Res. 2015;78:433–7. doi: 10.1016/j.jpsychores.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramadan R, Sheps D, Esteves F, Zafari AM, Bremner JD, Vaccarino V, Quyyumi AA. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. JAHA. 2013;2:e000321. doi: 10.1161/JAHA.113.000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soufer R, Bremner JD, Arrighi JA, Cohen I, Zaret BL, Burg MM, Goldman-Rakic P. Cerebral cortical hyperactivation in response to mental stress in patients with coronary artery disease. Proc Natl Acad Sci U S A. 1998;95:6454–9. doi: 10.1073/pnas.95.11.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaccarino V, Shah AJ, Rooks C, Ibeanu I, Nye JA, Pimple P, Salerno A, D’Marco L, Karohl C, Bremner JD, Raggi P. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med. 2014;76:171–80. doi: 10.1097/PSY.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaccarino V, Wilmot K, Al Mheid I, Ramadan R, Pimple P, Shah AJ, Garcia EV, Nye J, Ward L, Hammadah M, Kutner M, Long Q, Bremner JD, Esteves F, Raggi P, Quyyumi AA. Sex Differences in Mental Stress-Induced Myocardial Ischemia in Patients With Coronary Heart Disease. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei J, Rooks C, Ramadan R, Shah AJ, Bremner JD, Quyyumi AA, Kutner M, Vaccarino V. Meta-analysis of mental sress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am J Cardiol. 2014;114:187–92. doi: 10.1016/j.amjcard.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arrighi JA, Burg M, Cohen IS, Kao AH, Pfau S, Caulin-Glaser T, Zaret BL, Soufer R. Myocardial blood-flow response during mental stress in patients with coronary artery disease. Lancet. 2000;356:310–1. doi: 10.1016/S0140-6736(00)02510-1. [DOI] [PubMed] [Google Scholar]
- 32.Arrighi JA, Burg M, Cohen IS, Soufer R. Simultaneous assessment of myocardial perfusion and function during mental stress in patients with chronic coronary artery disease. J Nucl Cardiol. 2003;10:267–74. doi: 10.1016/s1071-3581(02)43235-7. [DOI] [PubMed] [Google Scholar]
- 33.Deanfield JD, Shea M, Kensett M, Horlock P, Wilson RA, deLandsheere CM, Selwyn AP. Silent myocardial ischaemia due to mental stress. Lancet. 1984;2:1001–5. doi: 10.1016/s0140-6736(84)91106-1. [DOI] [PubMed] [Google Scholar]
- 34.Lacy CR, Contrada RJ, Robbins ML, Tannenbaum AK, Moreyra AE, Chelton S, Kostis JB. Coronary vasoconstriction induced by mental stress (simulated public speaking) Am J Cardiol. 1995;75:503–5. doi: 10.1016/s0002-9149(99)80590-6. [DOI] [PubMed] [Google Scholar]
- 35.Vaccarino V, Votaw J, Faber T, Veledar E, Murrah NV, Jones LR, Zhao J, Su S, Goldberg J, Raggi JP, Quyyumi AA, Sheps DS, Bremner JD. Major depression and coronary flow reserve detected by positron emission tomography. Arch Intern Med. 2009;169:1668–76. doi: 10.1001/archinternmed.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carney RM, Blumenthal JA, Catellier D, Freedland KE, Berkman LF, Watkins LL, Czajkowski SM, Hayano J, Jaffe AS. Depression as a risk factor for mortality following acute myocardial infarction. Am J Cardiol. 2003;62:212–9. doi: 10.1016/j.amjcard.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1049–53. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 38.Vaccarino V, Goldberg J, Rooks C, Shah AJ, Veledar E, Faber TL, Votaw JR, Forsberg CW, Bremner JD. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol. 2013;62:97–978. doi: 10.1016/j.jacc.2013.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stébenne P, Bacon SL, Austin A, Paine NJ, Arsenault A, Laurin C, Meloche B, Gordon J, Dupuis J, Lavoie KL. Positive and negative affect Is related to experiencing chest pain during exercise-induced myocardial ischemia. Psychosom Med. 2017;79:395–403. doi: 10.1097/PSY.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 40.Blumenthal JA, Jiang W, Waugh RA, Frid DJ, Morris JJ, Coleman E, Hanson M, Babyak M, Thyrum ET, Krantz DS, O’Connor C. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life. Circulation. 1995;92:2102–8. doi: 10.1161/01.cir.92.8.2102. [DOI] [PubMed] [Google Scholar]
- 41.Stone PH, Krantz DS, McMahon RP, Goldberg AD, Becker LC, Chaitman BR, Taylor HA, Cohen JD, Freedland KE, Bertolet BD, Coughlin C, Pepine CJ, Kaufmann PG, Sheps DS, for the PIMI Study Group Relationship among mental stress-induced ischemia and ischemia during daily life and during exercise: The Psychophysiologic Investigations of Myocardial Ischemia (PIMI) study. J Am Coll Cardiol. 1999;33:1476–84. doi: 10.1016/s0735-1097(99)00075-3. [DOI] [PubMed] [Google Scholar]
- 42.Deanfield JE, Maseri A, Selwyn AP, Ribeiro P, Chierchia S, Krikler S, Morgan M. Myocardial ischaemia during daily life in patients with stable angina: Its relation to symptoms and heart rate changes. Lancet. 1983;2:753–8. doi: 10.1016/s0140-6736(83)92295-x. [DOI] [PubMed] [Google Scholar]
- 43.Schang SJ, Pepine CJ. Transient asymptomatic S-T segment depression during daily activity. Am J Cardiol. 1977;39:396–402. doi: 10.1016/s0002-9149(77)80095-7. [DOI] [PubMed] [Google Scholar]
- 44.Schiffer F, Hartley LH, Schulman CL, Abelmann WH. Evidence for emotionally-induced coronary arterial spasm in patients with angina pectoris. Br Heart J. 1980;44:62–6. doi: 10.1136/hrt.44.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, Hilton-Chalfen S, Hestrin L, Bietendorf J, Berman DS. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318:1005–12. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- 46.LaVeau PJ, Rozanski A, Krantz DS, Cornell CE, Cattanach L, Zaret BL. Transient left ventricular dysfunction during provocative mental stress in patients with coronary artery disease. Am Heart J. 1989;118:1–8. doi: 10.1016/0002-8703(89)90064-1. [DOI] [PubMed] [Google Scholar]
- 47.Krantz DS, Helmers KF, Bairey CN, Nebel LE, Hedges SM, Rozanski A. Cardiovascular reactivity and mental stress-induced myocardial ischemia in patients with coronary artery disease. Psychosom Med. 1991;53:1–12. doi: 10.1097/00006842-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Ramachandruni S, Fillingim RB, McGorray SP, Schmalfuss CM, Cooper GR, Schofield RS, Sheps DS. Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. J Am Coll Cardiol. 2006;47:987–91. doi: 10.1016/j.jacc.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 49.Boyle SH, Samad Z, Becker RC, Williams R, Kuhn C, Ortel TL, Kuchibhatla M, Prybol K, Rogers J, O’Connor C, Velazquez EJ, Jiang W. Depressive symptoms and mental stress-induced myocardial ischemia in patients with coronary heart disease. Psychosom Med. 2013;75:822–31. doi: 10.1097/PSY.0b013e3182a893ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldberg AD, Becker LC, Bonsall R, Cohen JD, Ketterer MW, Kaugman PG, Krantz DS, Light KC, McMahon RP, Noreuil T, Pepine CJ, Raczynski JM, Stone PH, Strother D, Taylor H, Sheps DS. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress: Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI) Circulation. 1996;94:2402–9. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- 51.Sheps DS, McMahon RP, Becker L, Carney RM, Freedlan KE, Cohen JD, Sheffield D, Goldberg AD, Ketterer MW, Pepine CJ, Raczynski JM, Light K, Krantz DS, Stone PH, Knatterud GL, Kaufmann PG. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia Study. Circulation. 2002;105:1780–4. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- 52.Jain D, Burg M, Soufer R, Zaret BL. Prognostic implications of mental stress-induced silent left ventricular dysfunction in patients with stable angina pectoris. Am J Cardiol. 1995;76:31–5. doi: 10.1016/s0002-9149(99)80796-6. [DOI] [PubMed] [Google Scholar]
- 53.Legault SE, Langer A, Armstong P. Usefulness of ischemic response to mental stress in predicting silent myocardial iscemia during ambulatory monitoring. Am J Cardiol. 1995;75:1007–11. doi: 10.1016/s0002-9149(99)80713-9. [DOI] [PubMed] [Google Scholar]
- 54.Jiang W, Babyak M, Krantz DS, Waugh RA, Coleman E, Hanson MM, Frid DJ, McNutty S, Morris JJ, O’Connor CM, Blumenthal JA. Mental stress-induced myocardial ischemia and cardiac events. J Am Med Assoc. 1996;275:1651–6. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 55.Krantz DS, Santiago HT, Kop WJ, Bairey Merz CN, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999;84:1292–7. doi: 10.1016/s0002-9149(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 56.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V, Quyyumi AA. The Mental Stress Ischemia Prognosis Study (MIPS): Objectives, study design, and prevalence of inducible ischemia. Psychosom Med. 2016 doi: 10.1097/PSY.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kounis NG, Tsigkas G, Hahalis G, Soufras GD. The brain, the coronary arteries, and the Kounis syndrome. Psychosom Med. 2015;77:101–2. doi: 10.1097/PSY.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 58.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–16. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 59.Soufer R. Neurocardiac interaction during stress-induced myocardial ischemia: How does the brain cope? Circulation. 2004;110:1710–3. doi: 10.1161/01.CIR.0000144841.84987.50. [DOI] [PubMed] [Google Scholar]
- 60.Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37:141–53. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- 61.Campanella C, Bremner JD. Neuroimaging of PTSD. In: Bremner JD, editor. Posttraumatic Stress Disorder: From Neurobiology to Treatment Hoboken. New Jersey: Wiley-Blackwell; 2016. pp. 291–320. [Google Scholar]
- 62.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 63.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSMIV-Patient Edition (SCID-P) Washington, D.C: American Psychiatric Press; 1995. [Google Scholar]
- 64.Hassan M, York KM, Li H, Li Q, Lucey DG, Fillingim RB, Sheps DS. Usefulness of peripheral arterial tonometry in the detection of mental stress-induced myocardial ischemia. Clin Cardiol. 2009;32:E1–6. doi: 10.1002/clc.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan S, Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, Isakadze N, Shah A, Levantsevych O, Pimple PM, Kutner M, Ward L, Garcia EV, Nye J, Mehta PK, Lewis TT, Bremner JD, Raggi P, Quyyumi AA, Vaccarino V. Sex differences in hemodynamic and microvascular mechanisms of myocardial ischemia induced by mental stress. Arterioscler Thromb Vasc Biol. 2018;38:473–80. doi: 10.1161/ATVBAHA.117.309535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmand M, Weinhard K, Casey ME, Eriksson L, Jones WF, Reed JH, Treffert J, Lenox M, Luk P, Bao J, Young JW, Baker K, Miller SD, Knoess C, Vollmar S, Richerzhagen N, Flugge G, Heiss WD, Nutt R. Performance evaluation of a new LSO High Resolution Research Tomograph-HRRT. IEEE Trans Med Imaging. 1999;2:1067–71. [Google Scholar]
- 67.Weinhard K, Schmand M, Casey ME, Baker K, Bao J, Eriksson L, Jones WF, Knoess C, Lenox M, Lercher M, Luk P, Michel C, Reed JH, Richerzhagen N, Treffert J, Vollmar S, Young JW, Heiss WD, Nutt R. The ECAT HRRT: Performance and first clinical application of the new high resolution research tomograph. IEEE Trans Med Imaging. 2000;3 17/2-/6. [Google Scholar]
- 68.Holly TA, Abbott BG, Al-Mallah M, Calnon DA, Cohen MC, DiFilippo FP, Ficaro EP, Freeman MR, Hendel RC, Jain D, Leonard SM, Nichols KJ, Polk DM, Soman P, Cardiology aASoN Single photon-emission computed tomography. J Nucl Cardiol. 2010;17:941–73. doi: 10.1007/s12350-010-9246-y. [DOI] [PubMed] [Google Scholar]
- 69.Friston K. Introduction to Experimental Design and Statistical Parametric Mapping. 2004 http://www.fil.ion.ucl.ac.uk/spm.doc/intro.
- 70.Bremner JD, Staib L, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biol Psychiatry. 1999;45:806–16. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, Yun L-S, Chen K. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry. 1997;154:918–25. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- 72.Lane RD, Reiman EM, Ahern CE, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997;154:926–33. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- 73.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System, An Approach to Cerebral Imaging. Stuttgart, Germany: Georg Thieme; 1988. [Google Scholar]
- 74.Akinboboye O, Krantz DS, Kop WJ, Schwartz SD, Levine J, Del Negro A, Karasik P, Berman DS, O’Callahan M, Ngai K, Gottdiener JS. Comparison of mental stress-induced myocardial ischemia in coronary artery disease patients with versus without left ventricular dysfunction. Am J Cardiol. 2005;95:322–6. doi: 10.1016/j.amjcard.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 75.Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, Chou D, Obideen M, O’Neal WT, Sullivan S, Samman Tahhan A, Kelli HM, Ramadan R, Pimple P, Sandesara P, Shah AJ, Ward L, Ko Y-A, Sun Y, Uphoff I, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Sheps DS, Raggi P, Vaccarino V, Quyyumi AA. Hemodynamic, catecholamine, vasomotor and vascular responses: Determinants of myocardial ischemia during mental stress. Int J Cardiol. 2017;243:47–53. doi: 10.1016/j.ijcard.2017.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bremner JD, Krystal JH, Southwick SM, Charney DS. Functional neuroanatomical correlates of the effects of stress on memory. J Trauma Stress. 1995;8:527–54. doi: 10.1007/BF02102888. [DOI] [PubMed] [Google Scholar]
- 77.Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–4. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- 78.Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Spatial working memory in humans as revealed by PET. Nature. 1993;363:623–5. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 79.Petersen SE, Fox PT, Posner MI, Mintun MA, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single word processing. Nature. 1988;331:585–9. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- 80.Zandbelt BB, Bloemendaal M, Neggers SFW, Kahn RS, Vink M. Expectations and violations: delineating the neural network of proactive inhibitory control. Hum Brain Mapp. 2013;34:2015–24. doi: 10.1002/hbm.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Morree HM, Szabo BM, Rutten G-J, Kop WJ. Central nervous system involvement in the autonomic responses to psychological distress. Neth Heart J. 2013;21:64–9. doi: 10.1007/s12471-012-0351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bremner JD. Functional neuroanatomical correlates of traumatic stress revisited 7 years later, this time with data. Psychopharmacol Bull. 2003;37:6–25. [PubMed] [Google Scholar]
- 83.van Rooij SJH, Geuze E, Kennis M, Rademaker AR, Vink M. Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacology. 2015;40:667–75. doi: 10.1038/npp.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chuang Y-F, Eldreth D, Erickson KI, Varma V, Harris G, Fried LP, REbok GW, Tanner EK, Carlson MC. Cardiovascular risks and brain function: a functional magnetic resonance imaging study of executive function in older adults. Neurobiol Aging. 2014;35:1396–403. doi: 10.1016/j.neurobiolaging.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kogler L, Mueller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, Dernti B. Psychosocial versusl physiological stress–meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage. 2015;119:235–51. doi: 10.1016/j.neuroimage.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 87.Morgan CA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–8. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 88.Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Memory. 2002;9:402–7. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 90.Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage. 2008;42:169–77. doi: 10.1016/j.neuroimage.2008.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagai M, Hoshide S, Kario K. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J Am Soc Hypertens. 2010;4:174–82. doi: 10.1016/j.jash.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 92.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–43. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 93.Drevets WC, Price JL, Simpson JRJ, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 94.George MS, Ketter TA, Parekh PI, Rosinsky N, Ring HA, Pazzaglia PJ, Marangell LB, Callahan AM, Post RM. Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop) J Neuropsychiatry Clin Neurosci. 1997;9:55–63. doi: 10.1176/jnp.9.1.55. [DOI] [PubMed] [Google Scholar]
- 95.Nagai K, Hoshide S, Kario K. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J Am Soc Hypertens. 2010;4:174–82. doi: 10.1016/j.jash.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 96.Nagai M, Kishi K, Kato S. Insular cortex and neuropsychiatric disorders: a review of recent literature. Eur Psychiatry. 2007;22:387–94. doi: 10.1016/j.eurpsy.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 97.Javanmard M, Shlik J, Kennedy SH, Vaccarino FJ, Houle S, Bradwejn J. Neuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time points. Biol Psychiatry. 1999;45:872–82. doi: 10.1016/s0006-3223(98)00348-5. [DOI] [PubMed] [Google Scholar]
- 98.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 99.Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53:380–7. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 100.Teicher MH, Anderson CM, Ohashi K, Polcari A. Childhood maltreatment: Altered network centrality of cingulate, precuneus, temporal pole and insula. Biol Psychiatry. 2014;76:297–305. doi: 10.1016/j.biopsych.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lanius R, Frewen PA, Tursich M, Jetly R, McKinnon MC. Restoring large-scale brain networks in PTSD and related disorders: A proposal for neuroscientifically-informed treatment interventions. Eur J Psychotraumatol. 2015;6:27313. doi: 10.3402/ejpt.v6.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Simmons A, Strigo IA, Matthews SC, Paulus MP, Stein MB. Initial evidence of a failure to activate right anterior insula during affective set shifting in posttraumatic stress disorder. Psychosom Med. 2009;71:373–7. doi: 10.1097/PSY.0b013e3181a56ed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen S, Li L, Xu B, Liu J. Insular cortex involvement in declarative memory deficits in patients with post-traumatic stress disorder. BMC Psychiatry. 2009;9:39. doi: 10.1186/1471-244X-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen S, Xia W, Li L, Liu J, He Z, Zhang Z, Yan L, Zhang J, Hu D. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Res. 2006;146:65–72. doi: 10.1016/j.pscychresns.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 105.Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J. 2013;166:806–14. doi: 10.1016/j.ahj.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lane RD, Jennings JR. Hemispheric asymmetry, autonomic asymmetry and the problem of sudden cardiac death. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. Cambridge, MA: MIT Press; 1995. pp. 271–304. [Google Scholar]
- 107.Critchley HD, Taggart P, Sutton PM, Holdright DR, Batchvarov V, Hnatkova K, Malik M, Dolan RJ. Mental stress and sudden cardiac death: asymmetric midbrain activity as a linking mechanism. Brain. 2005;128:75–85. doi: 10.1093/brain/awh324. [DOI] [PubMed] [Google Scholar]
- 108.Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol (Lond) 2000;523:259–70. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Critchley HD, Mathias CJ, Dolan RJ. Neural correlates of first and second-order representation of bodily states. Nat Neurosci. 2001;4:207–12. doi: 10.1038/84048. [DOI] [PubMed] [Google Scholar]
- 110.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–25. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 111.Vaccarino V, Parsons L, Peterson ED, Rogers WJ, Kiefe CI, Canto J. Sex differences in mortality after acute myocardial infarction: changes from 1994 to 2006. Arch Intern Med. 2009;169:1767–74. doi: 10.1001/archinternmed.2009.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, Leggio M, Mariën P, Molinari M, Moulton E, Orsi L, Van Overwalle F, Papadelis C, Priori A, Sacchetti B, Schutter DJ, Styliadis C, Verhoeven J. Consensus Paper: Cerebellum and Emotion. Cerebellum. 2017;16:552–76. doi: 10.1007/s12311-016-0815-8. [DOI] [PubMed] [Google Scholar]
- 113.Leiner HC. Reappraising the cerebellum: What does the hindbrain contribute to the forebrain? Behav Neurosci. 1989;103:998–1008. doi: 10.1037//0735-7044.103.5.998. [DOI] [PubMed] [Google Scholar]
- 114.Hoche F, Guell X, Sherman JC, Vangel MG, Schmahmann JD. Cerebellar contribution to social cognition. Cerebellum. 2016;15:732–43. doi: 10.1007/s12311-015-0746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schutter DJLG, van Honk J. The cerebellum in emotion regulation: A repetitive transcranial magnetic stimulation study. Cerebellum. 2009;8:28–34. doi: 10.1007/s12311-008-0056-6. [DOI] [PubMed] [Google Scholar]
- 116.Kim CK, Bartholomew BA, Mastin ST, Taasan VC, Carson KM, Sheps DS. Detection and reproducibility of mental stress-induced myocardial ischemia with Tc-99m sestamibi SPECT in normal and coronary artery disease populations. J Nucl Cardiol. 2003;10:56–62. doi: 10.1067/mnc.2003.26. [DOI] [PubMed] [Google Scholar]
- 117.Jain D, Shaker SM, Burg M, Wackers FJT, Soufer R, Zaret BL. Effects of mental stress on left ventricular and peripheral vascular performance in patients with coronary artery disease. J Am Coll Cardiol. 1998;31:1314–22. doi: 10.1016/s0735-1097(98)00092-8. [DOI] [PubMed] [Google Scholar]
- 118.Wei J, Pimple P, Shah AJ, Rooks C, Bremner JD, Nye JA, Ibeanu I, Murrah N, Shallenberger L, Raggi P, Vaccarino V. Depressive symptoms are associated with mental stress-induced myocardial ischemia after acute myocardial infarction. PLoS One. 2014;9:e102986. doi: 10.1371/journal.pone.0102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 120.Kapuku G, Treiber F, Raouane F, Halbert J, Davis H, Youg-Mayes S, Robinson V, Harshfield G. Race/ethnicity determines the relationship between oxidative stress markers and blood pressure in individuals with cardiovascular disease risk. J Hum Hypertens. 2017;31:70–5. doi: 10.1038/jhh.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]


