Abstract
Oncogene-induced senescence (OIS) is an intrinsic tumor suppression mechanism that requires the p53 and RB pathways and post-translational activation of C/EBPβ through the RAS-ERK cascade. We previously reported that in transformed/proliferating cells, C/EBPβ activation is inhibited by G/U-rich elements (GREs) in its 3′UTR. This mechanism, termed “3′UTR regulation of protein activity” (UPA), maintains C/EBPβ in a low-activity state in tumor cells and thus facilitates senescence bypass. Here we show that C/EBPβ UPA is overridden by AMPK signaling. AMPK activators decrease cytoplasmic levels of the GRE binding protein HuR, which is a key UPA component. Reduced cytoplasmic HuR disrupts 3′UTR-mediated trafficking of Cebpb transcripts to the peripheral cytoplasm – a fundamental feature of UPA – thereby stimulating C/EBPβ activation and growth arrest. In primary cells, oncogenic RAS triggers a Ca++-CaMKKβ-AMPKα2-HuR pathway, independent of AMPKα1, that is essential for C/EBPβ activation and OIS. This axis is disrupted in cancer cells through down-regulation of AMPKα2 and CaMKKβ. Thus, CaMKKβ-AMPKα2 signaling constitutes a key tumor suppressor pathway that activates a novel UPA-cancelling mechanism to unmask the cytostatic and pro-senescence functions of C/EBPβ.
Keywords: oncogene-induced senescence, oncogenic RAS, C/EBPβ, AMPK, 3′UTR
INTRODUCTION
Cellular stresses such as metabolic imbalances, DNA damage, or activated oncogenes can induce senescence, a stable form of growth arrest that plays a key role in eliminating damaged cells and suppressing cancer1,2. Senescent cells frequently express a panel of pro-inflammatory cytokines and growth factors, their receptors, and matrix proteases collectively known as the “senescence-associated secretory phenotype” or SASP3. C/EBPβ, along with the tumor suppressors p53 and Rb, is required for oncogene-induced senescence (OIS) in primary fibroblasts. C/EBPβ is an auto-inhibited protein that becomes post-translationally activated by oncogenic RAS signaling via the RAF-MEK-ERK cascade, which induces phosphorylation on several C/EBPβ residues that stimulate its DNA-binding, homodimerization, and transactivation functions4,5. The de-repressed form of C/EBPβ has cytostatic activity and cooperates with NF-κB to regulate numerous SASP genes in senescent cells6–9.
Interestingly, C/EBPβ activity is suppressed in immortalized and transformed cells, but not in senescent primary cells, by the 3′ untranslated region (3′UTR) of its mRNA10. This novel mechanism, termed “3′UTR regulation of protein activity” (UPA), requires a 100 nt 3′UTR sequence encompassing several G/U-rich elements (GREs). The GRE inhibits C/EBPβ activity by localizing Cebpb transcripts to a peripheral region of the cytoplasm. In this location, newly-translated C/EBPβ is inaccessible to its activating kinase, p-ERK1/2, which is confined to a separate perinuclear cytoplasmic domain in cells expressing oncogenic RAS (Fig. 1a)10. By preventing C/EBPβ phosphorylation/activation, UPA contributes to senescence bypass in cancer cells. C/EBPβ UPA also requires the ARE/GRE binding protein, HuR (ELAVL1), which associates with the Cebpb GRE region. HuR is a ubiquitously-expressed factor that controls the stability or translation of many mRNAs and shuttles between the cytoplasm and nucleus in a regulated manner that governs its cytoplasmic availability11,12. Elevated cytoplasmic HuR is frequently observed in tumors and correlates with increased malignancy and poor prognosis13,14, consistent with HuR’s role in repressing the cytostatic activity of C/EBPβ10 as well as stabilizing mRNAs that encode mitogenic proteins such as cyclins15.
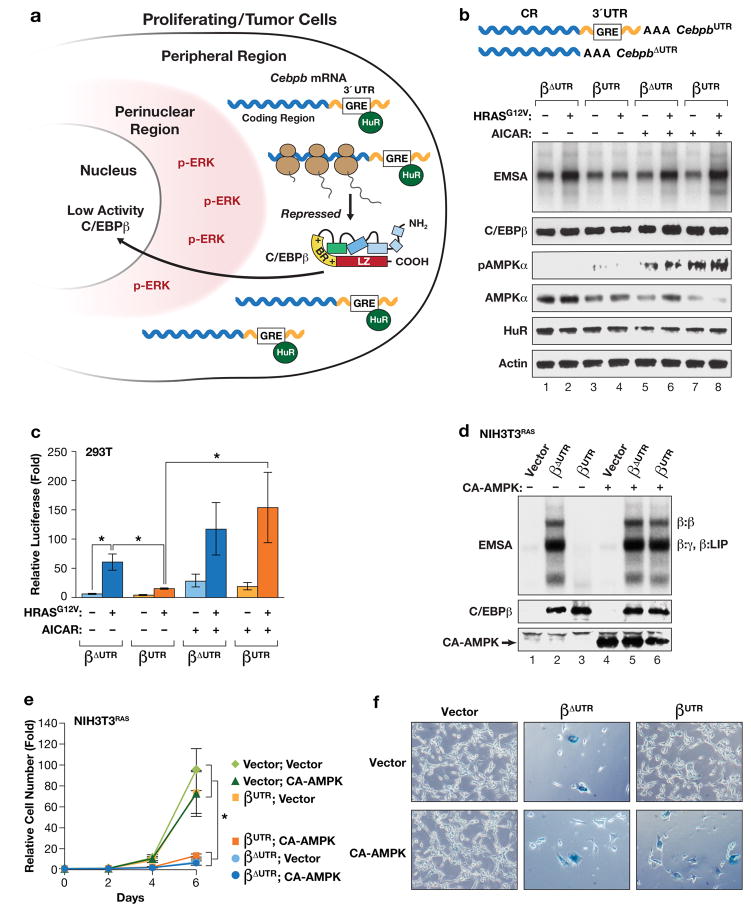
Figure 1.
AMPK signaling abrogates 3′UTR inhibition of RAS-induced C/EBPβ activation. (a) Model depicting “3′UTR regulation of protein activity” (UPA)10 in proliferating and transformed cells. The UPA mechanism involves mutually exclusive localization of Cebpb mRNAs (in the peripheral cytoplasm) and the C/EBPβ kinase, activated ERK1/2 (p-ERK) (in the perinuclear cytoplasm). (b, c) The AMPK agonist AICAR overrides UPA to activate C/EBPβ. The effect of AICAR on C/EBPβ DNA binding (b) and transactivation (c) was analyzed in HEK293 cells. Cells were transfected with C/EBPβ constructs containing or lacking the Cebpb 3′UTR (βUTR and βΔUTR, respectively), without or with HRASG12V, and treated with vehicle or 1 mM AICAR for 16 hr prior to harvest. In (b), nuclear extracts normalized for C/EBPβ levels were analyzed by EMSA using a consensus C/EBP probe. The image was cropped to remove the top and bottom (free probe) portions of the gel. In (c), transactivation assays were performed using a C/EBP reporter, 2XC/EBP-Luc. Luciferase activity, normalized to total protein in each lysate, is plotted as fold increase over the reporter alone. n=3; error bars represent S.E.M. Statistical differences between groups were determined by Student’s two-tailed t test; *p<0.05. (d) Expression of a constitutively active AMPKα1 catalytic subunit (CA-AMPK) reverses UPA inhibition of C/EBPβ DNA binding in RAS-transformed NIH3T3 cells. NIH3T3RAS cells, which express low levels of endogenous C/EBPβ23, were infected with retroviruses expressing βUTR or βΔUTR, without or with CA-AMPK, and assayed for C/EBPβ DNA binding by EMSA. The various C/EBPβ dimeric complexes are indicated. γ: C/EBPγ; LIP is a truncated translational isoform of C/EBPβ61. (e) The same cells were analyzed for proliferation over a 6-day time course. n=3; error bars represent S.E.M. Statistical differences between groups were determined by Student’s t test; *p<0.05. (f) The cells were also stained for the senescence marker, SA-β-Gal. The proportion of SA-β-Gal+ cells in each population is shown in Supplementary Fig. 1c.
AMP-activated kinase (AMPK) is a key cellular energy sensor whose activity is stimulated by elevated AMP/ATP ratios in response to metabolic stresses such as glucose deprivation, mitochondrial dysfunction, and hypoxia16. Activated AMPK promotes metabolic reprogramming by phosphorylating proteins that restore energy homeostasis16,17, but can also elicit cell cycle arrest, in part by inducing p53 and inhibiting mTOR signaling17. Accordingly, AMPK has anti-oncogenic functions18 that may also involve its upstream kinase, LKB1, a tumor suppressor that is lost in many cancers19. Since pharmacological AMPK activators such as metformin are under evaluation for cancer treatment and prevention, it is important to elucidate the effector pathways that mediate the anti-tumor effects of AMPK signaling.
AMPK has been linked to senescence of primary fibroblasts20 by reducing cytoplasmic HuR levels21,22. This occurs through AMPK-mediated phosphorylation and subsequent acetylation of the nuclear transporter, importin α1, increasing its affinity for HuR and facilitating nuclear translocation22. Therefore, we hypothesized that AMPK signaling might disrupt C/EBPβ 3′UTR inhibition by reducing HuR availability, allowing conversion of C/EBPβ to its activated, pro-senescent form. Here we show that C/EBPβ is activated by AMPK agonists that override negative regulation by its 3′UTR, leading to a cytostatic response. Moreover, establishment of OIS in primary cells requires signaling through a RAS-CaMKKβ-AMPKα2-HuR pathway that negates C/EBPβ UPA. Our findings reveal a novel pro-senescence pathway triggered by oncogenic stress that is frequently disrupted in tumor cells.
RESULTS
AMPK signaling overrides Cebpb 3′UTR inhibition to stimulate C/EBPβ DNA-binding and cytostatic activity
To investigate whether AMPK signaling reverses C/EBPβ 3′UTR inhibition (UPA), we used 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) to activate AMPK in 293T cells transfected with C/EBPβ constructs lacking or containing the 3′UTR (CebpbΔUTR or CebpbUTR, respectively). C/EBPβ proteins were expressed without or with HRASG12V and lysates were analyzed for DNA binding by EMSA using a C/EBP site probe (Fig. 1b). As expected10, the 3′UTR suppressed RAS-induced augmentation of C/EBPβ DNA binding (compare βUTR and βΔUTR; Fig. 1b). However, 3′UTR inhibition was abrogated in cells treated with AICAR, while βΔUTR DNA binding was largely unaffected by the drug. AICAR also stimulated AMPK phosphorylation (p-T172) and decreased the cytoplasmic levels of HuR21 (Fig. 1b). In addition, HRASG12V enhanced βΔUTR-mediated transactivation of a C/EBP-driven reporter (2xC/EBP-Luc), whereas the transcriptional activity of βUTR was only weakly activated by RAS (Fig. 1c). The 3′UTR inhibitory effect was again abolished by AICAR. Similarly, co-expression of a constitutively active form of the AMPKα2 catalytic subunit (CA-AMPK) reversed 3′UTR-mediated repression of C/EBPβ in DNA-binding and transactivation assays (Supplementary Fig. 1a,b).
We further examined the ability of AMPK to override UPA in NIH3T3RAS cells, which express low endogenous C/EBPβ levels due to its down-regulation by oncogenic RAS23,24. Analysis of 3T3RAS cells stably expressing CebpbΔUTR or CebpbUTR confirmed that the 3′UTR repressed C/EBPβ DNA binding, with no significant effect on protein levels (Fig. 1d, lanes 2 and 3). However, co-expression of CA-AMPK stimulated C/EBPβ binding activity in CebpbUTR cells, which was comparable to that of CebpbΔUTR without CA-AMPK (compare lanes 2 and 6). Cell proliferation assays showed that the presence of activated C/EBPβ, either through expression of CebpbΔUTR alone or CebpbUTR co-expressed with CA-AMPK, caused growth arrest (Fig. 1e) accompanied by an increase in senescent (SA-βGal+) cells (Fig. 1f; Supplementary Fig. 1c). CA-AMPK alone did not appreciably inhibit proliferation or induce senescence in 3T3RAS cells. Notably, CA-AMPK did not further enhance the cytostatic or DNA-binding functions of CebpbΔUTR, indicating that AMPK stimulates C/EBPβ activity predominantly by reversing 3′UTR inhibition. 3T3RAS-CebpbΔUTR cells potently up-regulated SASP genes (Il1a, Il6, and Cxcl1) compared to 3T3RAS cells, while this induction was not observed in 3T3RAS-CebpbUTR cells (Supplementary Fig. 1d). Interestingly, CA-AMPK failed to activate SASP genes in CebpbUTR–expressing cells despite stimulating C/EBPβ DNA binding and, in fact, reduced expression of these genes in CebpbΔUTR cells. CA-AMPK also inhibited RAS-induced activation of NF-κB (i.e., nuclear p65 levels; Supplementary Fig. 1e), supporting prior observations that AMPK can suppress inflammation by blocking NF-κB signaling25–27. Thus, NF-κB blockade by AMPK at least partly accounts for the failure to induce SASP genes.
To determine whether AMPK alters 3′UTR-dependent peripheral localization of Cebpb transcripts, we examined the cytoplasmic location of Cebpb mRNAs in NIH3T3 cells using the MS2-GFP-nls RNA tagging system10,28. Confocal microscopy revealed that 8xMs2b-tagged CebpbΔUTR transcripts were distributed uniformly in the cytoplasm, whereas 8xMs2b-CebpbUTR mRNAs were excluded from the perinuclear region and were more abundant in the cell periphery (Fig. 2a). However, co-expression of CA-AMPK disrupted the peripheral localization of CebpbUTR transcripts, generating a pan-cytoplasmic pattern similar to that of CebpbΔUTR. CA-AMPK also caused nuclear translocation of HuR and increased phosphorylation of endogenous C/EBPβ on the Thr188 ERK site, as determined by IF imaging using a phospho-C/EBPβ antibody (Supplementary Fig. 1f), consistent with reversal of UPA inhibition.
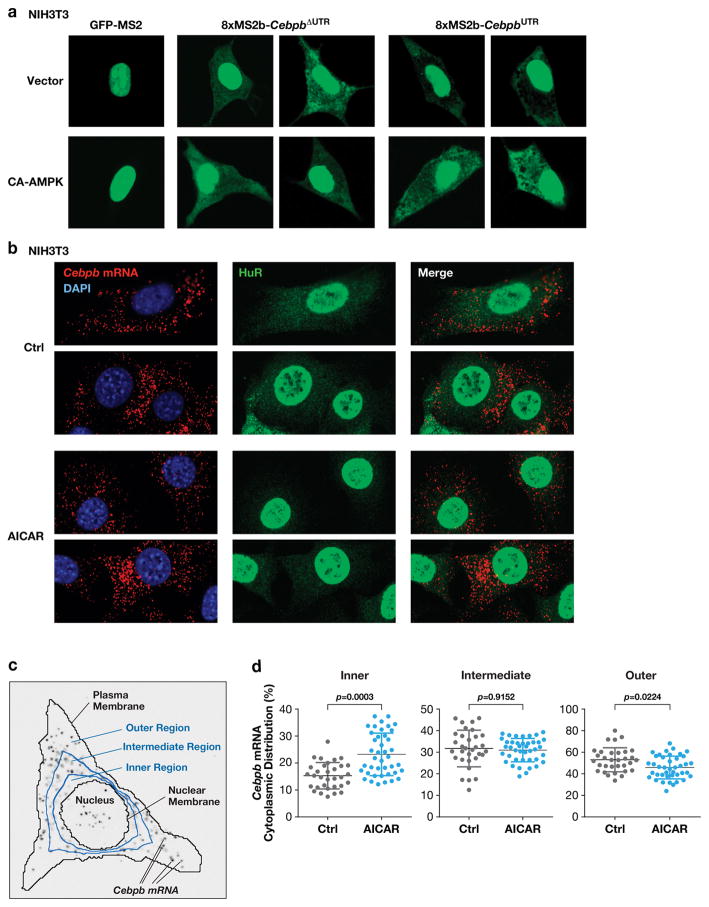
Figure 2.
AMPK signaling decreases cytoplasmic HuR levels and disrupts peripheral localization of Cebpb transcripts. (a) NIH3T3 cells were transfected with the MS2-GFP-nls reporter alone or together with MS2 binding site-tagged CebpbΔUTR or CebpbUTR vectors, ±CA-AMPK. After 42 hr, cells were analyzed by confocal fluorescence microscopy to visualize cytoplasmic distribution of the transcripts. (b) Localization of endogenous Cebpb mRNA in NIH3T3 cells using RNA FISH. Control and AICAR treated cells were analyzed by FISH using a Cebpb hybridization probe. The cells were also immunostained for HuR. (c) Scheme for quantification of Cebpb mRNA distribution. The cytoplasm was segmented into inner, intermediate, and outer regions by dividing the nuclear-plasma membrane distance radially into thirds. The proportion of the total RNA FISH signal in the three regions was determined for each cell analyzed. (d) Quantitative data from the experiment of panel (b) are shown in the scatter plots. n=32 control cells, 41 AICAR-treated cells. Statistical significance was calculated using Student’s two-tailed t test; *p<0.05.
We next used in situ hybridization (RNA FISH) to determine whether the location of endogenous Cebpb transcripts is affected by AMPK signaling. Appreciable cytoplasmic HuR was seen in control NIH3T3 cells, and Cebpb FISH signals were concentrated toward the cell periphery (Fig. 2b). However, AICAR treatment caused a strong reduction in cytoplasmic HuR, coinciding with more uniform Cebpb mRNA localization. To quantify these data in an unbiased manner, we developed an algorithm to assess Cebpb mRNA polarization. FISH signals were assigned to one of three cytoplasmic segments (inner, intermediate, outer), defined by boundaries placed at 1/3 and 2/3 of the radial distance between the nuclear and plasma membranes (Fig. 2c; see Methods). The distribution of mRNAs among the three segments was then determined. This method confirmed that AMPK activation decreased the proportion of Cebpb transcripts residing in the outer segment while increasing the nuclear-proximal fraction (Fig. 2d). Thus, AMPK signaling abrogates 3′UTR-mediated trafficking of Cebpb transcripts to the cell periphery, allowing RAS-induced post-translational activation of C/EBPβ translated from mRNAs located in the perinuclear region.
AMPK-induced cytostatic responses in tumor cells and primary MEFs involve activation of C/EBPβ
To extend our findings, we asked whether C/EBPβ activity is augmented by AMPK signaling in human cancer cells. CA-AMPK decreased the proliferation of A549 lung adenocarcinoma cells, and this effect was partially reversed by C/EBPβ depletion (Fig. 3a). Similar results were observed in MCF7 breast cancer cells (Supplementary Fig. 2a). In addition, CA-AMPK modestly increased C/EBPβ DNA binding and enhanced homodimerization (homodimers are the cytostatic form of C/EBPβ)9 (Fig. 3b). C/EBPβ was also activated by metformin, a commonly prescribed anti-diabetic that decreases cellular ATP levels by inhibiting complex I of the mitochondrial respiratory chain and thus indirectly activates AMPK29, and by salicylate, recently shown to be a direct AMPK agonist30. Both agents stimulated AMPK phosphorylation (p-Thr172) and increased C/EBPβ DNA binding in A549 cells (Supplementary Fig. 2b). The increase in C/EBPβ DNA binding occurred despite a decrease in ERK1/2 activity (Supplementary Fig. 2b), consistent with studies showing that RAS-ERK signaling can be suppressed by AMPK activation31. The ability of salicylate to augment C/EBPβ DNA binding required AMPK, as this response was lost when both AMPKα isoforms (1 and 2) were simultaneously depleted (Supplementary Fig. 2c). Metformin and salicylate decreased cytoplasmic HuR levels and induced phosphorylation on C/EBPβ Thr235 (the ERK site in human C/EBPβ), while the increase in p-C/EBPβ was blocked by the MEK1/2 inhibitor, U0126 (Fig. 3c).
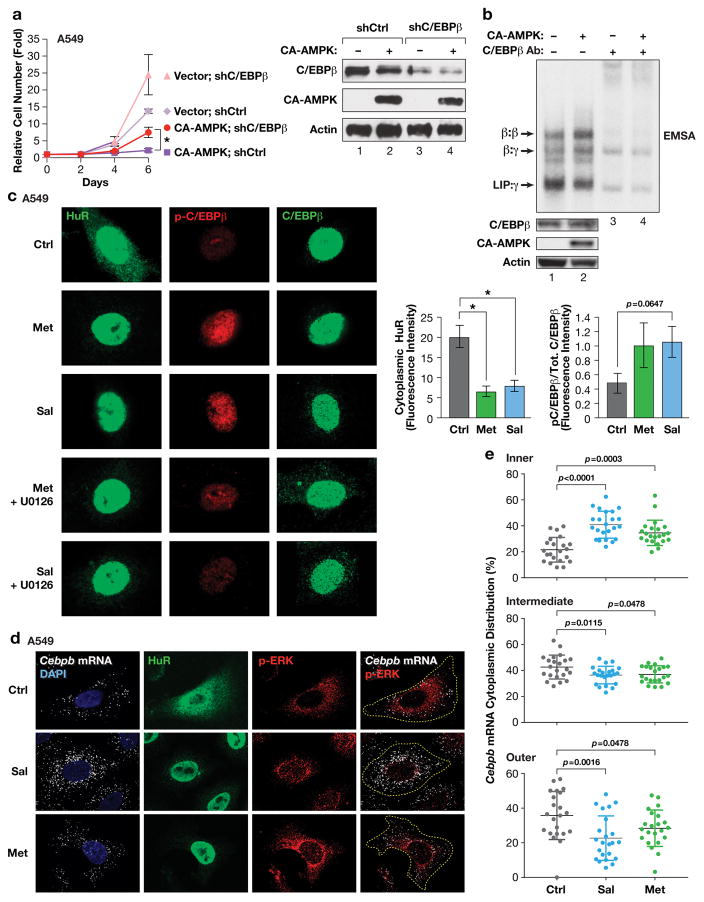
Figure 3.
AMPK signaling activates C/EBPβ in human tumor cells by negatively regulating the HuR-UPA pathway. (a) CA-AMPK induces proliferation arrest in A549 lung adenocarcinoma cells that is partially dependent on C/EBPβ. CA-AMPK was expressed with control or C/EBPβ knockdown vectors and cell proliferation was analyzed over a time course. Levels of CA-AMPK and C/EBPβ were determined by immunoblotting (right panel). n=2 experiments, assayed in triplicate; error bars represent S.E.M. Statistical differences between groups were determined by Student’s t test; *p<0.05. (b) Activated AMPK stimulates C/EBPβ DNA binding and homodimerization in A549 cells. Nuclear extracts from control and CA-AMPK-expressing cells were analyzed by EMSA. C/EBPβ protein levels were normalized in the EMSA reactions so that changes in intrinsic binding activity could be assessed. C/EBPβ-containing complexes were identified by antibody supershift assays (lanes 3–4). (c) The AMPK activators metformin and salicylate induce HuR nuclear translocation and increased phosphorylation on C/EBPβ Thr235 in A549 cells. Right panels show quantification of HuR cytoplasmic intensity and p-C/EBPβ:total C/EBPβ levels in control and drug-treated cells. n=5 cells; statistical significance was calculated using Student’s two-tailed t test; *p<0.05. (d) CEBPB transcripts are excluded from the perinuclear region in A549 cells but become more uniformly distributed following metformin or salicylate treatment. mRNAs were visualized by RNA FISH; the cells were also immunostained for p-ERK and HuR. (e) Cytoplasmic CEBPB mRNA distribution in cells described in panel (d) was quantified as detailed in Fig. 2. n=22 control cells, 23 salicylate-treated cells, 22 metformin-treated cells. Statistical significance was calculated using Student’s two-tailed t test; *p<0.05.
RNA FISH analysis showed that endogenous CEBPB transcripts are largely excluded from the perinuclear cytoplasm in A549 cells, while phospho-ERK1/2 is concentrated in this region (Fig. 3d). Both metformin and salicylate altered CEBPB mRNA polarization, increasing the proportion of transcripts located in the nuclear-proximal region. Quantitative analysis supported a statistically significant relocation of CEBPB mRNA to the juxtanuclear region upon AMPK activation (Fig. 3e), replicating the effect seen in NIH3T3 cells. Thus, in tumor cells AMPK agonists disrupt peripheral CEBPB mRNA localization and stimulate C/EBPβ activity, which partially mediates the cytostatic effects of these agents.
We next used an HuR mutant to confirm that HuR re-localization is critical for AMPK-induced activation of C/EBPβ. Phosphorylation on HuR Ser202 by Cdk1 stimulates nuclear translocation of HuR, and substitution of this residue with Ala (HuRS202A) increases its cytoplasmic retention32. Although we found that stable expression of HuRS202A is cytotoxic, it can be transiently expressed to test the role of HuR translocation in AMPK-induced activation of C/EBPβ. WT and mutant HuR proteins appended with a tandem affinity purification (TAP) tag were expressed in A549 cells and imaged using a TAP antibody. In unstimulated cells, both proteins showed comparable cytoplasmic abundance as well as a prominent nuclear pool (Supplementary Fig. 3a,b). However, salicylate significantly reduced the cytoplasmic levels of WT HuR, whereas HuRS202A remained largely unaffected. Moreover, drug-treated cells expressing WT HuR showed increased phosphorylation on the C/EBPβ ERK site (Thr235 in human C/EBPβ), while HuRS202A blocked this phosphorylation. HuRS202A but not WT HuR also suppressed salicylate-induced activation of C/EBPβ DNA binding (Supplementary Fig. 3c). These data strongly support the notion that HuR eviction from the cytoplasm is a critical step in C/EBPβ activation by AMPK signaling.
We next addressed whether C/EBPβ is an effector of AMPK-induced growth arrest/senescence in primary cells. Expression of CA-AMPK inhibited proliferation of WT MEFs, whereas this response was significantly diminished in Cebpb−/− cells (Supplementary Fig. 4a). AMPK also enhanced C/EBPβ DNA binding in these cells and increased homodimer levels (Supplementary Fig. 4b). CA-AMPK expression coincided with elevated numbers of SA-β-Gal+ cells in WT MEFs but not in Cebpb−/− MEFs (Supplementary Fig. 4c), and reduced cytoplasmic HuR levels in both WT and Cebpb−/− cells (Supplementary Fig. 4d). Similar results were obtained using salicylate to activate AMPK (Supplementary Fig. 5a–d). Conversely, treatment of MEFs with the AMPK inhibitor, compound C, increased cytoplasmic HuR levels and inhibited C/EBPβ DNA binding (Supplementary Fig. 5e).
AMPK signaling opposes cell proliferation in part by inhibiting mTORC1 activity33,34. To determine whether the cytostatic effects of AMPK involve activation of C/EBPβ downstream of mTORC1 blockade, we treated MEFs with the mTOR inhibitor, rapamycin. Rapamycin elicited similar cytostatic responses in WT and Cebpb−/− MEFs, showing that the anti-proliferative effect is independent of C/EBPβ (Supplementary Fig. 5f). Moreover, the drug failed to enhance C/EBPβ DNA binding despite inhibiting phosphorylation of the mTORC1 target, S6K (Supplementary Fig. 5g). Thus, AMPK signaling activates C/EBPβ and inhibits mTORC1 through independent pathways.
AMPKα2 is required for RAS-induced activation of C/EBPβ and OIS in MEFs
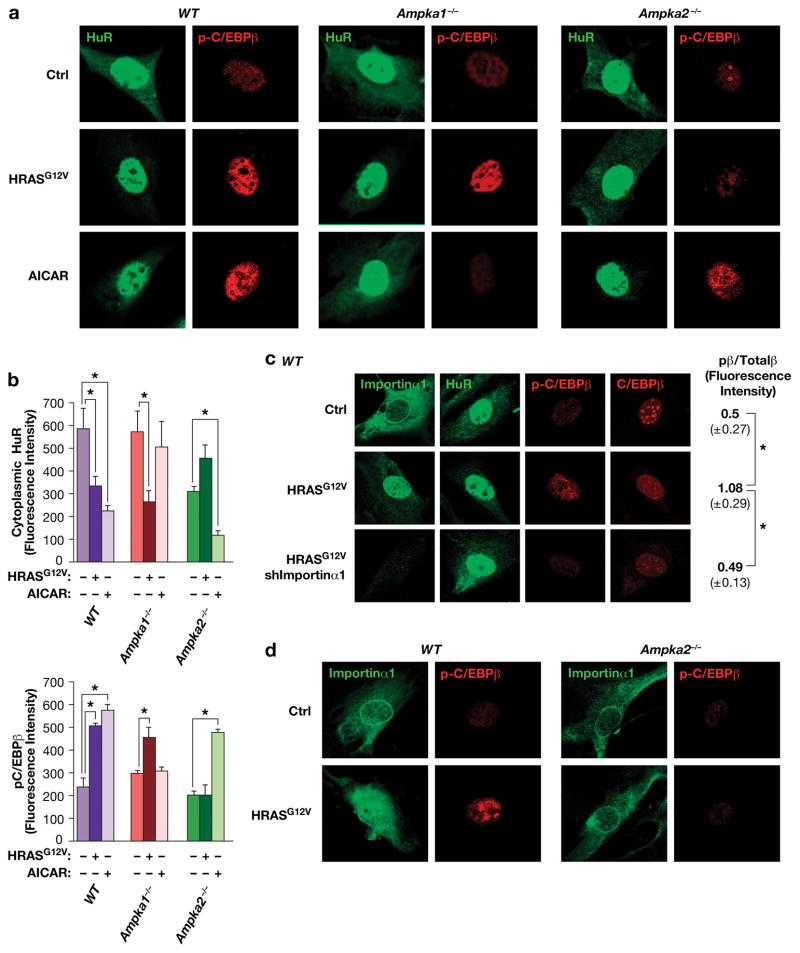
Our observation that C/EBPβ is an important effector of AMPK-driven senescence raised the possibility that AMPK-C/EBPβ signaling may also be involved in OIS. We initially sought to analyze MEFs lacking both AMPKα1 and 2 (DKO cells)35, as these cells are devoid of all AMPK activity. However, low passage DKO cells proliferated very poorly, precluding their use in OIS studies (Supplementary Fig. 6a). MEFs lacking AMPKα1 alone also showed impaired growth in culture which was not further decreased by expression of HRASG12V, and these cells were highly senescent even in the absence of RAS (~60% SA-βGal+) (Fig. 4a). By contrast, Ampka2−/− cells proliferated much more rapidly than WT MEFs and were largely refractory to RAS-induced growth arrest and senescence (Fig. 4b), consistent with previous observations36. Furthermore, C/EBPβ DNA binding was stimulated by HRASG12V in AMPKα1-depleted cells, like WT MEFs (Fig. 4c), but showed almost no induction in Ampka2−/− cells (Fig. 4d). Levels of activated ERK were similar in RAS-expressing WT and Ampka2−/− cells (Fig. 4d), indicating that lack of C/EBPβ activation is not due to reduced RAS-ERK signaling. Thus, AMPKα1 is dispensable for RAS-induced senescence and C/EBPβ activation despite being the major AMPKα subunit in MEFs37, while the minor AMPKα2 isoform plays a critical role in OIS and C/EBPβ activation.
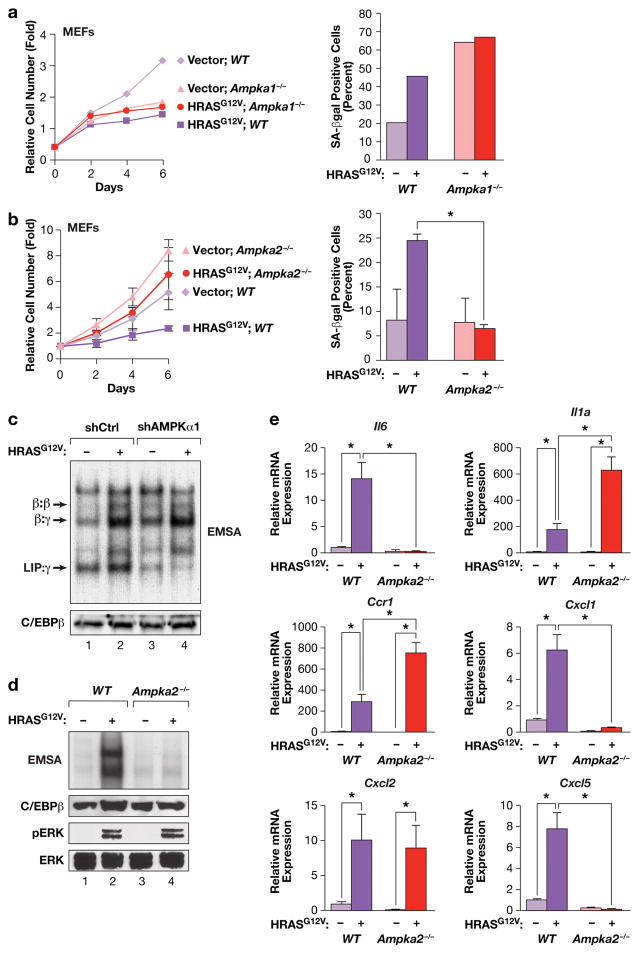
Figure 4.
HRASG12V-induced senescence and C/EBPβ activation in MEFs is dependent on AMPKα2. (a) RAS-induced growth arrest and senescence is independent of AMPKα1 but requires AMPKα2. WT and Ampka1−/− MEFs were infected with control or HRASG12V-expressing retroviruses, and cell proliferation (left panel) and senescence (SA-β-Gal staining, right panel) were analyzed. n=1 experiment. b) RAS-induced growth arrest and senescence requires AMPKα2. WT and Ampka2−/− MEFs were infected with control or HRASG12V-expressing retroviruses, and cell proliferation (left panel) and senescence (SA-β-Gal staining, right panel) were analyzed. n=3 experiments for growth curves (assayed in triplicate), n=2 for SA-β-Gal assays; error bars represent S.E.M. Statistical differences were determined by Student’s t test; *p<0.05. (c) C/EBPβ DNA binding is not affected by loss of AMPKα1. AMPKα1 was depleted in WT MEFs by shRNA knockdown, without or with expression of HRASG12V. Nuclear extracts were analyzed by EMSA using a canonical C/EBP site probe. (d) RAS-induced activation of C/EBPβ DNA binding is disrupted in Ampka2−/− MEFs. Nuclear extracts were analyzed by EMSA using a canonical C/EBP site probe. Levels of C/EBPβ, p-ERK and total ERK are also shown. (e) Analysis of SASP gene expression in WT and Ampka2−/− MEFs by qRT-PCR. n=2 biological replicates, each sample assayed in triplicate. Values are averages and error bars represent S.D. Statistical significance was calculated using Student’s two-tailed t test; *p<0.05.
Since activated C/EBPβ promotes transcription of SASP genes, we asked whether their induction by HRASG12V is affected in Ampka2−/− cells. The six SASP genes tested were efficiently induced in WT MEFs; by contrast, three (Il6, Cxcl1 and Cxcl5) showed severely diminished expression in AMPKα2 null cells (Fig. 4e). Levels of Il1a and Ccr1 were increased in Ampka2−/− cells relative to WT MEFs, while Cxcl2 was unaffected. Thus, AMPKα2 deficiency abrogates expression of a subset of SASP genes. As IL-6 has been shown to reinforce OIS through autocrine signaling7, its impaired expression may contribute to the senescence bypass in Ampka2−/− MEFs. In MEFs depleted for AMPKα1, five of the SASP genes, except for Ccr1, showed moderately decreased expression (~50% of WT levels) (Supplementary Fig. 6b). However, Il6, Cxcl1 and Cxcl5 levels were reduced to a much greater extent in cells lacking AMPKα2 than AMPKα1.
The residual level of C/EBPβ DNA binding in Ampka2−/− cells may be adequate to activate transcription of SASP genes such as Il1a, whose induction is also strongly dependent on NF-κB. To address the possible basis for increased Il1a expression in Ampka2−/− MEFs, we asked whether NF-κB activation was affected by loss of AMPKα2. IF assays of p65 subcellular distribution showed more efficient HRASG12V-induced nuclear translocation of p65 in Ampka2−/− cells than in WT MEFs (Supplementary Fig. 6c). Quantitative analysis confirmed that this difference was significant (Supplementary Fig. 6d). Thus, AMPKα2 may partially suppress NF-κB induction in senescent MEFs, although not sufficiently to block the SASP. Increased NF-κB activity may potentiate transcription of certain SASP genes such as Il1a in Ampka2−/− cells.
Oncogenic RAS and AMPK agonists act through different pathways to induce HuR translocation and C/EBPβ activation
We next investigated whether AMPKα2 regulates HuR nuclear translocation in senescing cells. Immunostaining revealed appreciable levels of cytoplasmic HuR in low passage WT MEFs, as expected for cells that retain the ability to proliferate (Fig. 5a). By contrast, expression of HRASG12V caused a marked decrease in cytoplasmic HuR, which was associated with enhanced phosphorylation on C/EBPβ Thr188. Comparable responses were seen in Ampka1−/− cells. In contrast, Ampka2−/− MEFs did not display RAS-induced HuR translocation or increased p-C/EBPβ levels (Fig. 5a,b; Supplementary Fig. 6e). Interestingly, the opposite results were observed in cells treated with AICAR. AICAR provoked nuclear translocation of HuR and C/EBPβ phosphorylation in WT and Ampka2−/− MEFs but not in Ampka1−/− cells. These data suggest that AMPKα2 mediates oncogenic RAS-induced HuR re-localization, C/EBPβ activation and senescence, whereas AMPKα1 is the critical sensor of metabolic stress and activates growth arrest/senescence pathways in response to increased AMP.
Figure 5.
AMPKα2 is required for HuR translocation and C/EBPβ phosphorylation induced by HRASG12V but not AICAR. (a) Control, HRASG12V-expressing and AICAR-treated MEFs (WT, Ampka1−/− and Ampka2−/−) were analyzed by IF staining to assess HuR localization and p-C/EBPβ (Thr188) levels. (b) Cytoplasmic HuR levels and p-C/EBPβ:total C/EBPβ ratios were determined by quantitating images from the experiment of panel (a). n=4 cells; error bars represent S.E.M. Statistical differences were determined by Student’s t test; *p<0.05. (c) The nuclear transport chaperone, Importin α1, undergoes HRASG12V-induced nuclear translocation and is required for HuR shuttling and C/EBPβ activation. WT MEFs were infected with control or HRASG12V-expressing retroviruses, without or with Importin α1 knockdown (shImportin α1). Cells were immunostained for Importin α1, HuR, p-C/EBPβ, and total C/EBPβ. p-C/EBPβ:total C/EBPβ ratios are shown at the right. n=4 cells; error bars represent S.E.M. Statistical differences were determined by Student’s t test; *p<0.05. (d) AMPKα2 is required for nuclear translocation of Importin α1. WT and Ampka2−/− MEFs were analyzed for HRASG12V-induced Importin α1 nuclear re-localization and C/EBPβ phosphorylation (Thr188).
Since Importinα1 has been implicated in HuR nucleocytoplasmic shuttling21,22, we examined its role in RAS-induced HuR translocation. In control MEFs, Impα1 displayed cytoplasmic staining along with a prominent ring at the nuclear envelope (Fig. 5c). Upon HRASG12V expression, Impα1 became primarily nuclear and the nuclear ring disappeared, coinciding with HuR nuclear uptake and increased C/EBPβ phosphorylation on Thr188. Depletion of Impα1 in RAS-expressing MEFs prevented HuR re-localization and blocked C/EBPβ phosphorylation. Moreover, RAS was unable to trigger Impα1 nuclear translocation in Ampka2−/− cells (Fig. 5d). Therefore, Impα1 is a critical component of the RAS-AMPKα2-HuR-C/EBPβ pathway that promotes cell cycle arrest and senescence.
The AMPK kinase CaMKKβ links oncogenic RAS to the AMPKα2-HuR-C/EBPβ pro-senescence pathway
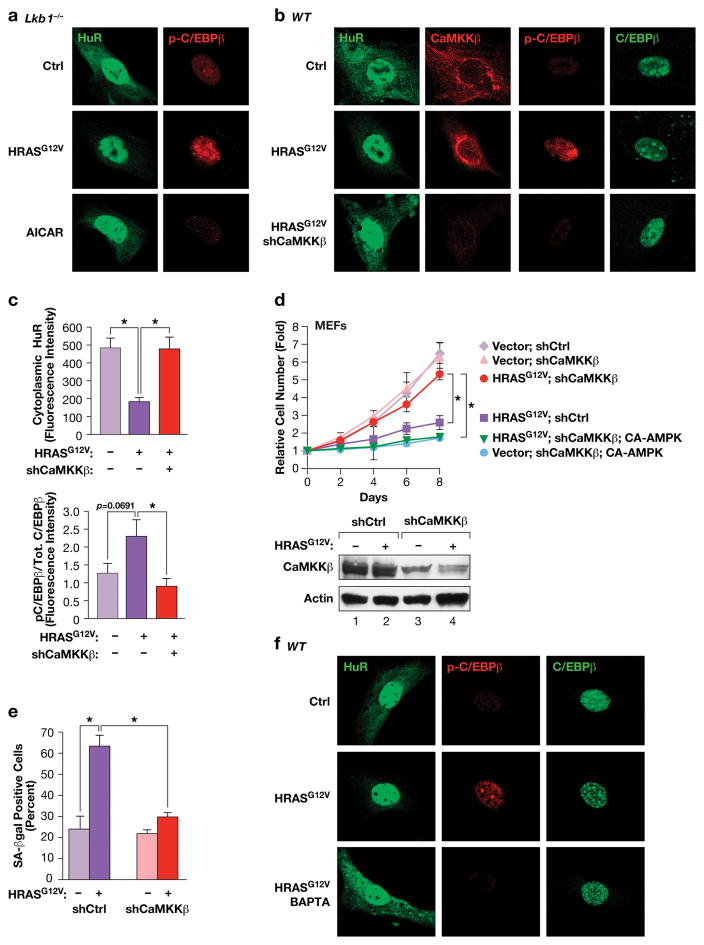
We next sought to identify the upstream kinase that relays RAS signals to AMPKα2 to activate OIS. LKB1 is a known tumor suppressor and a major AMPK kinase38,39. We found that Lkb1−/− MEFs display normal RAS-induced growth arrest, as reported previously40, as well as activation of C/EBPβ DNA binding, Thr188 phosphorylation, and HuR re-localization (Fig. 6a; Supplementary Fig.7a,b). However, like Ampka1−/− cells, Lkb1−/− MEFs were refractory to AICAR-induced HuR nuclear translocation and C/EBPβ phosphorylation (Fig. 7a). Thus, LKB1 is dispensable for C/EBPβ activation and OIS, indicating that another AMPK kinase acts downstream of RAS. A possible candidate is CaMKKβ (CaMKK2), which can phosphorylate and activate AMPK in response to increased intracellular calcium41,42. Indeed, depletion of CaMKKβ in MEFs prevented RAS-induced HuR translocation and suppressed C/EBPβ phosphorylation (Fig. 6b,c). CaMKKβ-depleted cells also showed a substantial reversal of RAS-induced growth arrest and senescence (Fig. 6d,e). However, over-expression of CA-AMPKα2, which contains a phosphomimetic substitution at Thr172, restored growth arrest in CaMKKβ-depleted MEFs (Fig. 6d), suggesting that AMPKα2 is a key target of CaMKKβ in cells undergoing OIS. Treatment of cells with the CaMKK inhibitor, STO-609, also blocked RAS-induced C/EBPβ phosphorylation and senescence (Supplementary Fig. 7c,d). Since CaMKKβ is a Ca++-dependent kinase, we examined the effects of a cell-permeant calcium chelator, BAPTA, on HRASG12V-induced responses. BAPTA treatment for 2 hr blocked HuR translocation and C/EBPβ phosphorylation in RAS-expressing MEFs (Fig. 6f). Collectively, these results demonstrate that HRASG12V-induced activation of the pro-senescence AMPKα2-HuR-C/EBPβ pathway requires CaMKKβ and mobilization of intracellular Ca++.
Figure 6.
The AMPK kinase CaMKKβ, but not LKB1, controls HRASG12V-induced nuclear translocation of HuR and C/EBPβ activation through a Ca++-dependent pathway. (a) IF staining of HuR and p-C/EBPβ in control and HRASG12V-expressing or AICAR-treated Lkb1−/− MEFs. (b) CaMKKβ depletion prevents HRASG12V-induced activation of the HuR-C/EBPβ pathway. WT MEFs expressing non-specific or shCaMKKβ hairpin RNAs, ± HRASG12V, were analyzed by IF staining for HuR, CaMKKβ, p-C/EBPβ (Thr188) and total C/EBPβ. (c) Quantification of cytoplasmic HuR and p-C/EBPβ:total C/EBPβ ratios from the experiment of panel (b). n=7 cells; error bars represent S.E.M. Statistical differences were determined by Student’s t test; *p<0.05. (d) Proliferation assays were performed using the cells described in panel (b) without or with expression of CA-AMPKα2. n=3; error bars represent S.E.M. Statistical differences at day 8 were determined by Student’s t test; *p<0.05. Lower panel: immunoblot confirming CaMKKβ depletion. (e) Effect of CaMKKβ depletion on RAS-induced senescence (SA-β-Gal positive cells). n=130 cells scored from two independent experiments; error bars represent S.E.M. Statistical differences were determined by Student’s t test; *p<0.05. (f) The Ca++ chelator, BAPTA, blocks HRASG12V-induced HuR nuclear translocation and C/EBPβ phosphorylation. Control and HRASG12V-expressing MEFs were treated with vehicle or 10 μM BAPTA for 2 hr prior to fixation. The cells were immunostained for the indicated proteins.
Figure 7.
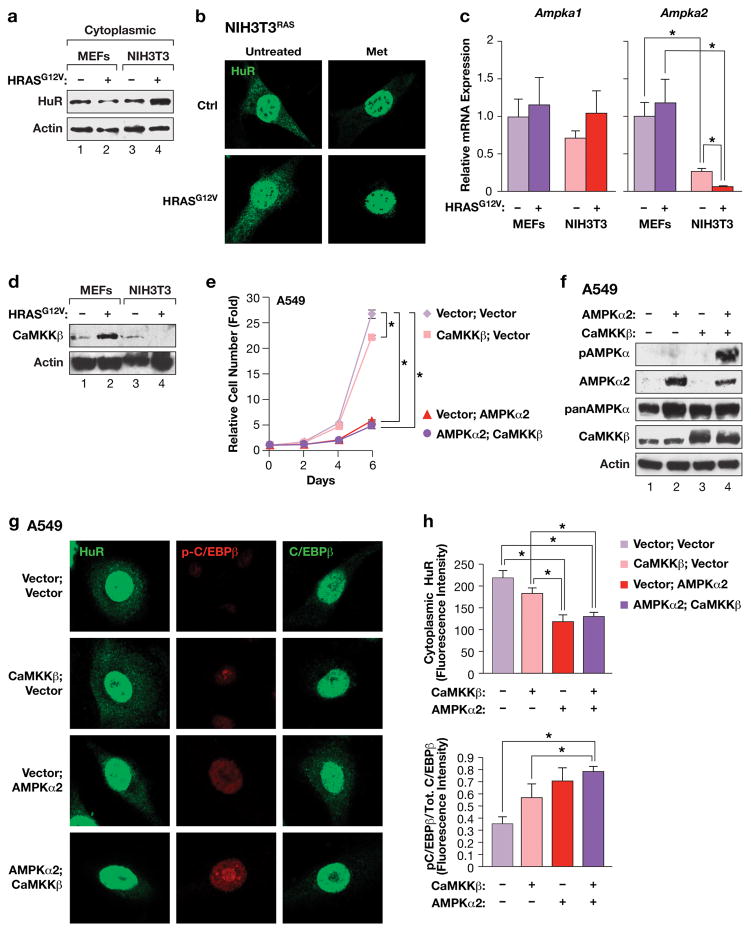
Proliferation of RAS-transformed cells is associated with elevated cytoplasmic HuR and requires down-regulation of AMPKα2 and CaMKKβ. (a) Immunoblot of cytoplasmic HuR levels in MEFs and NIH3T3 cells, without or with expression of HRASG12V. (b) Immunostaining of HuR in control or HRASG12V-transformed NIH3T3 cells, ± metformin treatment. (c) Ampka1 and Ampka2 mRNA levels in MEFs and NIH3T3 cells, ± HRASG12V. Transcript levels were determined by qRT-PCR. n=3 independent biological replicates, each sample assayed in triplicate; error bars represent S.D. Statistics were calculated using Student’s two tailed t test.; *p<0.05. (d) Immunoblot comparing CaMKKβ levels in MEFs and NIH3T3 cells, without or with expression of HRASG12V. (e) Proliferation of A549 cells over-expressing AMPKα2 and/or CaMKKβ. n=2 independent biological replicates, each time point assayed in triplicate; error bars represent S.E.M. Statistics were determined for day 6 using Student’s two tailed t test.; *p<0.05. (f) Immunoblot showing over-expression of AMPKα2 and CaMKKβ and CaMKKβ-dependent phosphorylation of AMPKα2 in A549 cells. Lysates from the cell populations described in panel (e) were analyzed on blots probed with the indicated antibodies. The anti-p-Thr172 (AMPKα) antibody recognizes modified forms of both AMPKα isoforms. (g) IF imaging of A549 cells expressing AMPKα2 and/or CaMKKβ. Cells were immunostained for HuR, or p-C/EBPβ and total C/EBPβ. (h) Quantitation of cytoplasmic HuR levels and p-C/EBPβ:total C/EBPβ ratios in the cells described in panel g. n=9 cells; error bars represent S.E.M. Statistical differences were determined by Student’s t test; *p<0.05.
Down-regulation of AMPKα2 and CaMKKβ is critical for oncogenic RAS-driven transformation and cancer
The essential role of the CaMKKβ-AMPKα2-HuR axis in RAS-induced senescence suggested that this pathway must be bypassed in tumor cells. Therefore, we analyzed the effect of oncogenic RAS on cytoplasmic HuR levels in MEFs and NIH3T3 cells to compare senescent and transformed fibroblasts, respectively. Immunoblotting confirmed that RAS decreased cytoplasmic HuR in MEFs (Fig. 7a), supporting the IF data. By contrast, cytoplasmic HuR was modestly increased in NIH3T3 cells, which was also seen by IF staining (Fig. 7b). However, NIH3T3RAS cells remained responsive to metformin-induced HuR nuclear translocation. Thus, RAS-induced HuR re-localization is abrogated in immortalized NIH3T3 cells. qRT-PCR analysis showed that Ampka1 mRNA levels were unaffected by HRASG12V in both MEFs and NIH3T3 cells and were comparable in all four cell populations (Fig. 7c). However, Ampka2 transcripts were nearly 5-fold lower in control NIH3T3 cells compared to MEFs, and HRASG12V further decreased them to nearly undetectable levels. Furthermore, CaMKKβ protein levels were decreased upon expression of HRASG12V in NIH3T3 cells, but were modestly up-regulated by RAS in MEFs (Fig. 7d). Thus, low CaMKKβ and AMPKα2 levels in immortalized cells correlate with RAS-induced transformation and maintenance of cytoplasmic HuR.
To determine whether CaMKKβ and AMPKα2 down-regulation in NIH3T3RAS is causally related to bypass of RAS-induced senescence and instatement of C/EBPβ UPA, we expressed AMPKα2 ± CaMKKβ, without or with CebpbUTR. AMPKα2 alone reduced cell proliferation by ~30% (6-day time point), and CebpbUTR decreased cell growth by a similar amount (Supplementary Fig. 8a). However, both proteins together reduced proliferation by nearly 75%, and a further decrease was observed when CaMKKβ was also co-expressed. The two proteins also cooperated to induce senescence and increased expression of SASP genes (Supplementary Fig. 8b,c). Furthermore, AMPKα2 triggered HuR eviction from the cytoplasm and induced C/EBPβ phosphorylation on Thr188, which was most apparent in CebpbUTR-expressing cells (Supplementary Fig. 8e). Thus, AMPKα2 converts NIH3T3RAS cells to a phenotype resembling that of senescent MEFs, especially when C/EBPβ is also expressed.
We also tested the consequences of expressing AMPKα2 and/or CaMKKβ in A549 cells. AMPKα2 alone had a potent cytostatic effect that was not further increased by co-expression of CaMKKβ (Fig. 7e). These cells contain detectable levels of endogenous CaMKKβ, which may explain the relatively minor effects of its over-expression, whereas they express very low levels of AMPKα2 (Fig. 7f). AMPK phosphorylation (p-Thr172) was not seen in control cells and was weakly detected in cells expressing AMPKα2 alone, while much higher levels were observed in cells over-expressing both CaMKKβ and AMPKα2. Abundant AMPK levels (detected by a pan-AMPK antibody) were apparent in all cells, indicating that A549 cells express a non-phosphorylated pool of AMPKα1. Thus, the AMPKα1 isoform cannot be phosphorylated by CaMKKβ, whereas this upstream kinase selectively modifies AMPKα2. Accordingly, over-expression of AMPKα2 in A549 cells decreased cytoplasmic HuR levels and induced C/EBPβ phosphorylation, and both effects were enhanced by co-expression of CaMKKβ (Fig. 7g,h).
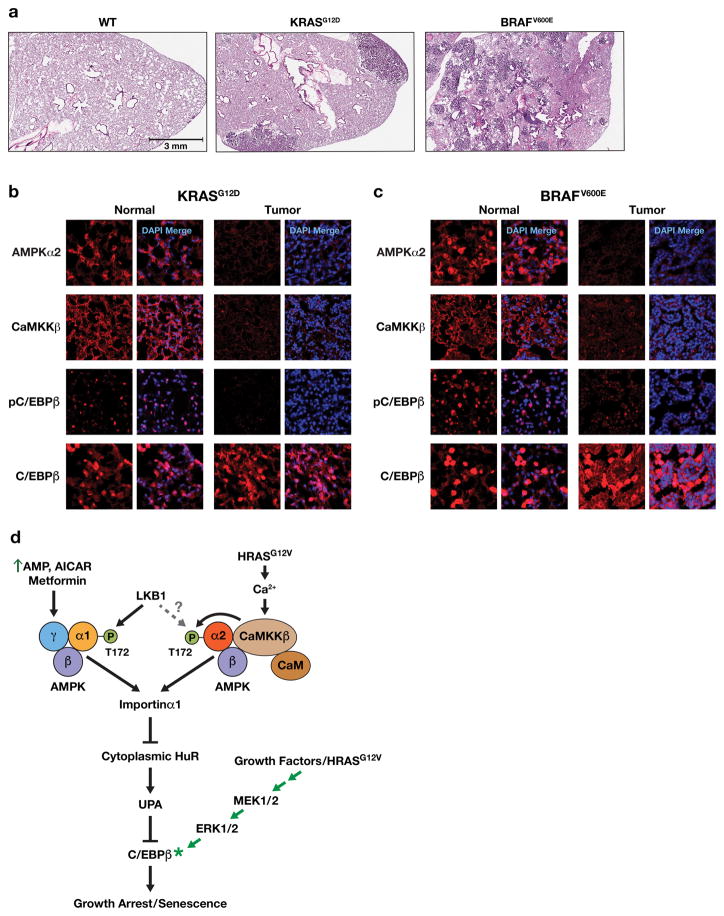
Down-regulation of AMPKα2 has been reported in breast tumors and other cancers43,44, suggesting that it has tumor suppressor functions in vivo. Therefore, we used a KrasG12D-induced mouse lung adenocarcinoma (ADC) model45 to investigate whether the CaMKKβ-AMPKα2-C/EBPβ pathway is disrupted in these tumors (Fig. 8a). IF staining showed detectable AMPKα2 levels in normal areas of the lung but much lower expression in ADCs (Fig. 8b). CaMKKβ was also significantly reduced in tumor areas. Moreover, p-C/EBPβ (Thr188) was decreased in ADCs relative to normal lung, while total C/EBPβ levels were comparable in both regions, indicating that the activated form of C/EBPβ is diminished in neoplastic cells. We also analyzed benign lung adenomas induced in LSL-BRAFV600E/+ mice (Fig. 8a) through intratracheal instillation of Ad.Cre virus46. These BRAFV600E-dependent tumors displayed reduced levels of AMPKα2, CaMKKβ and p-C/EBPβ compared to normal tissue (Fig. 8c). Thus, both Kras- and BRAF-driven lung tumorigenesis is associated with disruption of the CaMKKβ-AMPKα2 axis, preventing RAS signaling from activating this pro-senescence pathway. In line with these findings, analysis of AMPKα1 (PRKAA1) and AMPKα2 (PRKAA2) mRNA expression across a range of human cancers (Oncomine) showed decreased levels of PRKAA2 in neoplasms such as brain/CNS, gastric, head and neck, and particularly colorectal cancers (Supplementary Fig. 9a). PRKAA1 was also down-regulated in specific cancers such as breast, but in colorectal cancers PRKAA2 consistently displayed greater reduction (Supplementary Fig. 9b).
Figure 8.
AMPKα2, CaMKKβ and p-C/EBPβ levels are markedly decreased in KRASG12D- and BRAFV600E-driven mouse lung tumors. (a) H&E stained lung areas from a normal WT mouse, a KrasLA2/+ mouse45 containing adenocarcinomas (ADC) and a LSL-BRAFV600E/+ animal46 bearing multiple adenomas. (b) Normal and lung tumor areas from a 165 day-old KrasLA2/+ mouse; sections were immunostained for AMPKα2, CaMKKβ, p-C/EBPβ (Thr188) and total C/EBPβ. (c) Normal and lung tumor areas from a 190 day-old LSL-BRAFV600E/+ animal (103 days after intratracheal instillation of Ad.Cre virus); sections were immunostained for AMPKα2, CaMKKβ, p-C/EBPβ (Thr188) and total C/EBPβ. (d) Model depicting AMPK-dependent pathways that mediate C/EBPβ activation and senescence in response to energy stress/AMPK agonists or oncogenic RAS. AMPK signaling suppresses the UPA mechanism that inhibits C/EBPβ activation and thus licenses C/EBPβ activation. To become activated, C/EBPβ also requires signaling through the RAS-ERK cascade to induce phosphorylation on Thr188 as well as other modifications5,62.
DISCUSSION
Here we identify a novel OIS pathway that involves AMPK as a critical intermediate. We show that AMPKα2, but not AMPKα1, functions as a kinase downstream of oncogenic RAS that elicits nuclear translocation of HuR in primary fibroblasts. Activation of this pathway decreases the cytoplasmic availability of HuR, thereby dampening the 3′UTR mechanism that suppresses C/EBPβ activity. When active, UPA targets Cebpb transcripts to the peripheral cytoplasm, which is physically separate from the perinuclear location of p-ERK and other kinases such as CK210,47. This prevents C/EBPβ phosphorylation, possibly because translation and phosphorylation are tightly coupled. However, AMPK activators such as RAS or metformin reduce cytoplasmic HuR and disrupt peripheral trafficking of Cebpb transcripts, causing the mRNA to become pan-cytoplasmic as is seen for transcripts lacking the 3′UTR10. Thus, relocation of Cebpb transcripts to the perinuclear region allows locally translated C/EBPβ to access p-ERK1/2 and CK247, facilitating phosphorylation and functional activation of C/EBPβ.
In primary cells, RAS signals through the AMPKα2-HuR-C/EBPβ axis to elicit growth arrest and senescence. The canonical AMPK pathway triggered by elevated AMP:ATP ratios can also activate C/EBPβ and promote senescence, but this response primarily involves AMPKα1 and remains intact in AMPKα2-deficient cells. As depicted in Fig. 8d, the two AMPKα isoforms have non-overlapping functions, at least with respect to the stimuli investigated here. Our findings support and extend previous studies showing that AMPKα2, but not AMPKα1, suppresses RAS-driven transformation of mouse fibroblasts36. Furthermore, it was recently reported that the ubiquitin-conjugating enzyme, UBE2O, is an oncoprotein that targets AMPKα2 but not AMPKα1 for proteasomal degradation in certain cancers44. Therefore, AMPKα2 down-regulation achieved through decreased mRNA expression and/or enhanced protein turnover appears to be a key mechanism of senescence bypass in tumor cells.
We found that RAS signaling to AMPK does not involve LKB1 but instead requires the alternative kinase, CaMKKβ. CaMKKβ and calmodulin (CaM) can form a complex with AMPKα41, although no selectivity for AMPKα isoforms has been reported. This complex also contains the regulatory subunit AMPKβ, but not the γ subunit that binds AMP. As a result, CaMKKβ-AMPK assemblies are unresponsive to AMP and instead are activated by Ca++. Ca++ release from the ER is triggered by the lipid second messenger, IP3, and this response can be elicited by RAS activation. Hence, increased Ca++ flux could stimulate CaMKKβ-AMPKα2 in cells expressing oncogenic RAS. Accordingly, we found that a Ca++ chelator blocks RAS-induced HuR translocation and C/EBPβ activation. The fact that CaMKKβ and AMPKα2 are both down-regulated in cancers underscores their important anti-oncogenic and pro-senescence functions. To our knowledge, this is the first evidence of a tumor suppressor role ascribed to CaMKKβ.
Although our work highlights the anti-oncogenic functions of AMPKα2, Faubert et al.18 reported that AMPKα1 also has intrinsic tumor suppressor activity in a Myc-driven model of lymphomagenesis. This occurs primarily through the ability of AMPKα1 to inhibit the Warburg effect (a switch to aerobic glycolysis). These findings, together with the fact that LKB1 is frequently lost in RAS tumors, indicate that LKB1-AMPKα1 signaling can negatively regulate tumor initiation or progression. Nonetheless, AMPKα2 levels are down-regulated in many human malignancies and mouse lung tumors. Therefore, we suggest that the anti-oncogenic activity of AMPKα1 primarily involves a response to metabolic stress, most likely involving LKB1 as an upstream regulator. LKB1 loss would facilitate continued proliferation of tumor cells under conditions of metabolic imbalance. By contrast, AMPKα2 is a specific mediator of RAS signaling and induces a pro-senescence program via targets such as HuR and its effectors, including C/EBPβ. Along with these anti-oncogenic functions, at least low levels of AMPK activity are required for tumor cells to adapt to nutrient-poor and oxygen-deficient environments, and pro-oncogenic roles for AMPK have been reported48–50. Thus, tumor suppression by AMPKs and their upstream kinases is complex and is influenced by cellular contexts. Further studies are needed to fully understand how components of the AMPK axis can positively and negatively affect tumorigenesis.
Another difference between the RAS-AMPKα2 and AMP-AMPKα1 axes involves their ability to regulate NF-κB. Canonical AMPK activators such as metformin are known to suppress inflammation by inhibiting one or more nodes in the NF-κB pathway25–27. However, oncogenic RAS induces transcription of NF-κB-dependent SASP genes in senescent MEFs despite signaling through AMPKα2, and activates NF-κB in senescent cells51. AMPKα2 appears to partially inhibit NF-κB in MEFs undergoing OIS (Supplementary Fig. 6c,d). However, NF-κB activation does occur in these cells and causes SASP induction. It is possible that the low levels of AMPKα2 in MEFs are insufficient to efficiently suppress NF-κB signaling in response to RAS signaling. In any case, our findings underscore the beneficial effects of using AMPK agonists that inhibit NF-κB and suppress inflammation to prevent or treat cancer. We suggest that stimuli that increase cellular AMP levels, such as metformin, exercise and calorie restriction, act primarily through AMPKα1 and not via the CaMKKβ-AMPKα2 axis.
Our data show that reversal of C/EBPβ 3′UTR inhibition (UPA) is an important target of the RAS-CaMKKβ-AMPKα2-HuR pathway. This pathway licenses post-translational activation of C/EBPβ and promotes OIS. Pre-senescent, proliferating MEFs display appreciable cytoplasmic HuR and reduced C/EBPβ activity, conditions that are acutely reversed by oncogenic RAS signaling. Thus, oncogenic RAS activates C/EBPβ and senescence through two separate but convergent effector pathways: CaMKKβ-AMPKα2 overrides C/EBPβ UPA, while the RAF-ERK cascade provides a strong C/EBPβ activating signal (Fig. 8d). In contrast, immortalized cells are resistant to the UPA-cancelling effects of RAS by as yet unknown pathways, possibly linked to loss of tumor suppressors, leading to down-regulation of CaMKKβ and/or AMPKα2.
To date, UPA regulation has only been demonstrated for C/EBPβ, although the Cebpa 3′UTR can suppress RAS-induced activation of C/EBPβ when appended to the Cebpb coding region10. However, it seems unlikely that UPA regulation is unique to C/EBPβ. Conceivably, similar mechanisms could control other pro-senescence regulators, which together may form a tumor suppressor network activated by RAS-CaMKKβ-AMPKα2 signaling. Our findings warrant further studies to identify additional proteins whose anti-oncogenic activities, rather than their expression, are constrained in tumor cells by 3′UTR-dependent mechanisms.
MATERIALS AND METHODS
Animal procedures and preparation of MEFs
Mice were maintained in accordance with National Institutes of Health animal guidelines following protocols approved by the NCI-Frederick Animal Care and Use Committee. Mouse embryonic fibroblasts (MEFs) were prepared from WT and Cebpb−/− embryos (E13.5)52; Ampka1−/−, Ampka2−/− and DKO mice and MEFs have been described35. Lkb1−/− MEFs40 were kindly provided by Dr. Nabeel Bardeesy. Primary MEFs were maintained at low passage and were cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Gibco) and 100 U/ml penicillin–streptomycin (Gibco) and Primocin (Invivogen). Two mouse lung tumor models were used: KrasLA2/+ mice45, which spontaneously develop lung tumors that progress to ADCs, and LSL-BRAFV600E/+ mice46, which develop benign adenomas following intratracheal instillation of Ad.Cre virus (7.5×106 Pfu/animal; Viral Vector Core Facility, University of Iowa College of Medicine).
Antibodies and reagents
Rabbit antibodies to C/EBPβ (C-19), ERK1 (C-19), NF-κB p65 (C-20), CaMKKβ (H-95) and actin (I-19-R), mouse antibodies to HuR (3A2), C/EBPβ (47A1), C/EBPβ (H7), p-ERK (E-4), Importin α1(B-9), and goat antibody to AMPKα2 (A-20) were purchased from Santa Cruz Biotechnology. Rabbit antibodies to AMPKα (#2532), pAMPKα (#2531), and p-ERK (#4377) were purchased from Cell Signaling Technologies. Rabbit antibody to p-C/EBPβ (T188) was from Abcam (pT235). Rabbit antibody to AMPKα2 (#21494) was from ThermoFisher. Anti-mouse (W4028) and anti-rabbit (W4018) HRP conjugated secondary antibodies were from Promega. Anti-Mouse Alexa® Fluor 488 and anti-rabbit Alexa® Fluor 594 conjugated secondary antibody was from ThermoFisher. Anti-rabbit Alexa® Fluor 647 conjugated secondary antibody was from Abcam. X-tremeGENE HP DNA transfection reagent was from Roche Diagnostics. AICAR was obtained from Cell Signaling Technology, metformin was from Calbiochem, sodium salicylate was from ThermoFisher, STO-609 was from Sigma, and BAPTA was from Abcam.
Cells and cell culture
NIH3T3 cells (American Type Culture Collection) were maintained in DMEM supplemented with 10% calf serum (Colorado Serum Company). A549, MCF7 and HEK-293T cell lines and 293GP2 packaging cells (American Type Culture Collection) were cultured in DMEM supplemented with 10% FBS (Gibco). All media were supplemented with Normocin (InvitroGen) to prevent Mycoplasma contamination. Cell lines were not authenticated. Where appropriate, cells were treated with the indicated doses of AMPK activators AICAR, metformin or sodium salicylate for the specified durations.
Plasmids
An expression plasmid for CA-AMPK53 was kindly provided by Dr. Rusty Jones (McGill University) and the insert was transferred to pBabe-puro and pWZL-blast. Expression plasmids for mouse C/EBPβ coding region (C/EBPβΔUTR) and C/EBPβ coding region plus 3′UTR (C/EBPβUTR) have been described previously10; the genes were transferred to pBabe-puro and pWZL-blast retroviral vectors. Lentiviral expression vectors for AMPKα2 and CaMKKβ were provided by Dr. Dominic Esposito (FNLCR, Frederick, MD). Lentiviral vectors containing an shRNA construct simultaneously targeting AMPKα1/2 (human and mouse)54 and a control shRNA vector were kindly provided by Dr. Thomas Brown (Wright State University). Lentiviral shRNA vectors targeting mouse CaMKKβ (#TRCN0000276649), AMPKα1 (#TRCN0000360770) and Importin α1 (#TRCN0000093514) were purchased from Sigma-Aldrich. Human C/EBPβ shRNA and a non-targeting shRNA control in pSuperRetro-neo were described previously10. Human HRASG12V was expressed from pWZL-hygro6. Plasmids for retroviral packaging have been described9; lentiviral packaging/envelope plasmids pMD2.G (#12259), pMDLg/pRRE (#12251), and pRSV/Rev (#12253) were obtained from Addgene.
Retroviral and lentiviral infections
Retroviral and lentiviral plasmids were transfected into 293GP2 and 293T packaging cell lines, respectively, using standard CaPO4 precipitation. For lentiviral transfection, 20 μg of lentiviral vector, 6 μg of pMD2.G, 10 μg of pMDLg/pRRE and 5 μg of pRSV/Rev were co-transfected into 293T cells. For retroviral transfection, 20 μg of retroviral vector and 4 μg of VSVG plasmids were co-transfected into 293GP2 cells in 10cm dishes. 48–72 h after transfection, viral supernatants were collected every 12 h, pooled, filtered (0.45 mm), supplemented with 8 mg/ml polybrene, and used to infect target cells. Three infections were performed and cells were subjected to the appropriate antibiotic selection. Multiple genes were introduced by sequential infection and drug selection.
Transient transfection and luciferase assays
HEK293T cells were transfected with 200 ng C/EBPβ vector and 100 ng of pcDNA3-HRASG12V in 60 mm dishes. After transfection, cells were cultured in complete media for 24h and then serum starved overnight prior to harvesting. For transactivation assays, 293T cells were transfected with 100 ng 2× C/EBP-luc reporter and 5 ng C/EBPβ vector with 10 ng pcDNA3-HRASG12V plasmid. Cells were lysed in 1× passive lysis buffer (Promega) and luciferase activity was measured using a GLOMAX 20/20 luminometer (Promega). Luciferase values were normalized to total protein concentration of the lysate. Data are presented as fold activation of the reporter alone and represent means ±SD of at least three independent experiments.
Growth curves
Retrovirally or lentivirally infected cells were seeded at 2.5×104 cells/well in 6-well plates. At the indicated times, cells were washed with phosphate-buffered saline (PBS), fixed in 10% formalin, rinsed with water, stained with 0.1% crystal violet (Sigma) for 30 min, rinsed extensively, and dried. The dye was extracted with 10% acetic acid and absorbance measured at 590 nm. All values were normalized to day 0 (the first day after plating). For proliferation assays in the presence of AMPK activators, cells were treated with metformin (10 mM for MEFs, 1 mM for A549 cells) or sodium salicylate (0.3 mM for MEFs, 3 mM for A549 cells) for the duration of the growth assay. Proliferation was assayed as described above. All growth assays were conducted in triplicate wells.
Colony assays
Cells (MEFs) were seeded at 2.5×104 cells/dish in 10 cm dishes. Media was replaced weekly. After two weeks, cells were washed with phosphate-buffered saline (PBS), fixed in 10% acetic acid for 30 min, rinsed with water, stained with 0.4% crystal violet (Sigma) for 30 min, rinsed extensively, and dried. Plates were scanned and colonies counted on the images. The assays were performed in duplicate.
Senescence-associated β-galactosidase (SA-β-gal) assay
WT and Cebpb−/− MEFs (passage 2–4) were plated at 5×104 cells per well in 6-well plates and cultured for 3–4 days. Cells were fixed and stained (Senescence Detection Kit; Calbiochem) according to the manufacturer’s instructions with the following modification: the staining solution was titrated with 2M HCl to reduce the pH below 6.0 to optimize SA-βgal staining in mouse cells. SA-β-gal stained cells were counted by acquiring at least four fields using 10× bright field microscopy, and are expressed as a percentage of the total cells scored.
mRNA localization
The GFP-MS2-nls reporter system has been described previously55. pcDNA-CebpbUTR-Ms2b and pcDNA-CebpbΔUTR-Ms2b vectors were generated by inserting the array of MS2 binding sites from the RSV-lacZ-MS2b vector55 immediately downstream of the Cebpb coding region, as described10. NIH3T3 cells were plated at 5×104 cells/35mm dish and transfected with 200 ng GFP-MS2-nls reporter plasmid and 2.3 μg of the Cebpb-Ms2b vector using Polyfect (Qiagen) following the manufacturer’s instructions. After 36 hr, the cells were stained with 1 μg/ml DAPI (Sigma). Fluorescence images of unfixed/live cells were taken with a Zeiss LCI-510 confocal microscope.
RNA FISH
Cells were seeded on μ-Slides VI0.4 (Ibidi). Forty-eight hours after plating, the cells were washed in Cytoskeleton Buffer (CB)56 (10 mM MES pH 6.1, 150 mM NaCl, 5 mM MgCl2, 5 mM EGTA, 5 mM glucose) and permeabilized in ice-cold Pre-Fixative mix57 (2% paraformaldehyde, 0.01% glutaraldehyde, 0.05% saponin, in CB) for 15 min at 4°C. Cells were then fixed with ice-cold Fixative mix (2% paraformaldehyde, 0.01% glutaraldehyde, in CB) for 100 min at 4°C. The cells were washed with CB twice and fixative was quenched by addition of 50 mM NH4Cl for 5 min at 20°C. For the RNA fluorescence in situ hybridization (FISH) procedure, probe hybridization, signal amplification and post-hybridization washes were performed using the QuantiGene ViewRNA ISH Cell Assay (Affymetrix) according to the manufacturer’s protocol. A human CEBPB probe (VA1-18129) or mouse Cebpb probe (VB1-10094) was used.
Cell segmentation and RNA FISH data analysis
The nucleus and cell membrane boundary were segmented and identified using Graphcut algorithm developed previously58. Briefly, a point on the cell was first selected that performs a polar transform of a circular area surrounding the cell. Next, the algorithm calculates a gradient image of the transform and creates a source-sink directed graph of the image using the pixels as nodes and their gradient values as edges. A max-flow mincut then segments the cartesian image. Finally, the algorithm reconverts the image to cartesian coordinates. In addition, the user can modify any local segmentation that was inaccurate; the addition of this point increases the edge strength of that pixel on the polar graph and corrects the segmentation. The localization of mRNA single molecule image was determined using two dimensional Gaussian-fitting by ThunderSTORM59. The number and percentage of localizations were quantified in three distinct regions of the cell using a home-built algorithm in Matlab (Split_region.m). The script utilizes a euclidean distance transform (EDT) to generate the distance from each pixel to the boundaries of the cell and nucleus. The “inner region” was defined as the area within the cell whose pixels are at least twice as close to the nucleus as they are to the cell boundary (plasma membrane), excluding the nucleus. The “intermediate region” encompasses everything excluding the inner and out regions and the nucleus. The “outer region” contains pixels that are at least twice as close to the cell boundaries than the nuclear boundaries. The proportion of localizations in each region was then calculated, with any localizations within the nucleus removed from the total.
Code availability
Graphcut and Split_region.m software is freely available upon request.
Immunofluorescence
After RNA FISH, immunofluorescence was performed by incubating cells with HuR (1:400) and p-ERK (1:100) primary antibodies for 16 hrs at 4°C in 5% albumin in PBS-S (PBS containing 0.05% Saponin). Cells were washed three times for 5 min at 20°C with PBS-S and incubated for 1 hr at 20°C with Alexa 488- and Alexa 647-conjugated secondary antibodies (1:1000) in 5% albumin in PBS-S. Cells were washed four times with PBS-S for 5 min at 20°C and co-stained with 0.1 μg/mL DAPI (in PBS) for 1 min at 20°C. Cells were washed twice with PBS, and images were acquired using a Zeiss LSM-780 confocal microscope. For other immunofluorescence experiments, 2.5×104 cells were plated in glass-bottomed chambers (LabTek) and fixed with ice-cold methanol for 20 min at -20°C. Cells were incubated with respective primary antibodies (1:100) overnight at 4°C in PBS with 5% Normal Goat Serum (Cell Signaling) and 0.1% TritonX100 (Sigma). Following four washes with PBS containing 1% BSA and 0.1% TritonX100, cells were incubated with Alexa 488 and Alexa 594 conjugated secondary antibodies (1:1000) for 1 hr at RT. After five washes, cells were stained with 0.1 μg/mL DAPI in PBS for 15 min at RT followed by two washes with PBS. Fluorescence images were taken with a Zeiss LSM-710 confocal microscope.
Quantitative image analysis
Fluorescence image analysis was performed using Image J software. To quantify cytoplasmic HuR levels, the nuclear fluorescence intensity was subtracted from the total fluorescence for each cell. The mean cytoplasmic intensity was determined and used for further statistical analysis. For C/EBPβ, only nuclear fluorescence intensities were measured. p-C/EBPβ:total C/EBPβ ratios were calculated for individual cells and then averaged.
Immunoblotting
Cells were harvested after washing twice with cold (4°C) PBS. The cells were lysed with low salt buffer (20 mM HEPES pH 7.9, 0.1 mM EDTA, 10 mM NaCl, 0.1% NP-40) on ice for 10 min. Nuclei were harvested by centrifugation at 3500 rpm for 10 min, and the supernatant was used as cytoplasmic fraction. Nuclei were lysed with high salt buffer (20 mM HEPES pH 7.9, 0.2 mM EDTA, 420 mM NaCl, 25% glycerol) at 4°C for 30 min with vigorous shaking. Nuclear debris was pelleted by centrifugation at 14,000 rpm for 5 min, and the supernatant was used for further experiments or stored at −70°C. For whole cell extracts, cells were lysed in medium salt buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate) on ice for 10 min with intermittent vortexing. Cellular debris was pelleted by centrifugation at 14,000 rpm for 10 min and supernatant was collected for further analysis. All buffers were supplemented with a protease and phosphatase inhibitor cocktail (Calbiochem). Protein samples (20–50μg) were resolved by electrophoresis on 10% or 12% Mini-PROTEAN TGX Precast Gels (Biorad) and electrophoretically transferred to PVDF membranes. The blots were probed with the appropriate primary antibodies, followed by goat anti-rabbit IgG or goat anti-mouse IgG conjugated to horseradish peroxidase and visualized using the enhanced chemiluminescence method (ThermoFisher).
Electrophoretic mobility shift assays (EMSA)
EMSA was performed essentially as described60. Briefly, a dsDNA probe containing a consensus C/EBP site was end-labeled with [32P]dATP (Amersham) and polynucleotidylkinase (Roche). DNA-binding assays were carried out in a 25 μl reaction containing 5–10 μg nuclear extract, 20 mM HEPES (pH 7.9), 200 mM NaCl, 5% Ficoll, 1 mM EDTA, 50 mM DTT, 0.01% Nonidet P-40, 1.75 μg poly(dI-dC), and 2×104 cpm probe. After incubation at room temperature for 20 min, 10–15 μl of the binding reaction was loaded onto a 6% polyacrylamide gel in TBE (90 mM Tris base, 90 mM boric acid, 0.5 mM EDTA) buffer and electrophoresed at 160 V for 2 h. Supershift assays were carried out by pre-incubating the nuclear extract with 1 μl of the appropriate antibody at 4°C for 30 min before adding the binding reaction mixture.
RNA isolation and RT-qPCR
Total RNA was prepared using the GeneJet RNA Purification kit (ThermoFisher). Reverse transcription followed by quantitative PCR (RT-qPCR) was used to measure mRNA expression levels. 100–1000 ng of total RNA was reverse transcribed using Maxima First Strand cDNA Synthesis kit (ThermoFisher) according to the manufacturer’s protocol. The resulting cDNA was analyzed by quantitative PCR using SsoAdvanced SYBR Green Supermix (Bio-Rad) in the CFX96 Real Time PCR System (Bio-Rad). The primers for Il1a, Il1b, Il6, Cxcl1, Cxcl2, Ccr1 and Ppia were obtained from Qiagen. Primers for Prkaa1 and Prkaa2 were obtained from Bio-Rad. All samples were assayed in triplicate and normalized to control values in the same samples. The housekeeping gene Ppia was used as an internal standard.
Statistical analysis
Statistical significance was calculated using Student’s t test and ANOVA. ANOVA was performed using SPSS and Student’s t test was performed using GraphPad Prism 7. p values less than 0.05 were considered to be significant. For growth assays in MEFs, individual experiments were excluded from the study if RAS-induced growth arrest was not observed in the WT group. For SASP gene expression studies, individual experiments were excluded from the analysis if induction by RAS was not statistically significant in the WT MEF group.
Supplementary Material
Acknowledgments
We thank R. Jones for the CA-AMPK vector, N. Bardeesy for Lkb1−/− MEFs, T. Brown for a pan-AMPKα knockdown vector, K. Saylor and N. Martin for animal husbandry and genotyping, and A. Kane (Scientific Publications, Graphics & Media, Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research) for preparation of figures. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppe J-P, Desprez P-Y, Krtolica A, Campisi J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annual Review of Pathology: Mechanisms of Disease. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakajima T, Kinoshita S, Sasagawa T, Sasaki K, Naruto M, Kishimoto T, et al. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc Natl Acad Sci USA. 1993;90:2207–2211. doi: 10.1073/pnas.90.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Shuman JD, Guszczynski T, Sakchaisri K, Sebastian T, Copeland TD, et al. RSK-mediated phosphorylation in the C/EBP{beta} leucine zipper regulates DNA binding, dimerization, and growth arrest activity. Mol Cell Biol. 2010;30:2621–2635. doi: 10.1128/MCB.00782-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sebastian T, Malik R, Thomas S, Sage J, Johnson PF. C/EBPbeta cooperates with RB:E2F to implement Ras(V12)-induced cellular senescence. EMBO J. 2005;24:3301–3312. doi: 10.1038/sj.emboj.7600789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 8.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huggins CJ, Malik R, Lee S, Salotti J, Thomas S, Martin N, et al. C/EBPgamma suppresses senescence and inflammatory gene expression by heterodimerizing with C/EBPbeta. Mol Cell Biol. 2013;33:3242–3258. doi: 10.1128/MCB.01674-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu SK, Malik R, Huggins CJ, Lee S, Sebastian T, Sakchaisri K, et al. 3′UTR elements inhibit Ras-induced C/EBPbeta post-translational activation and senescence in tumour cells. EMBO J. 2011;30:3714–3728. doi: 10.1038/emboj.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HH, Gorospe M. Phosphorylated HuR shuttles in cycles. Cell Cycle. 2008;7:3124–3126. doi: 10.4161/cc.7.20.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal. 2008;20:2165–2173. doi: 10.1016/j.cellsig.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Lopez de Silanes I, Lal A, Gorospe M. HuR: post-transcriptional paths to malignancy. RNA Biol. 2005;2:11–13. doi: 10.4161/rna.2.1.1552. [DOI] [PubMed] [Google Scholar]
- 14.Yoo PS, Sullivan CA, Kiang S, Gao W, Uchio EM, Chung GG, et al. Tissue microarray analysis of 560 patients with colorectal adenocarcinoma: high expression of HuR predicts poor survival. Ann Surg Oncol. 2009;16:200–207. doi: 10.1245/s10434-008-0209-3. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Caldwell MC, Lin S, Furneaux H, Gorospe M. HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J. 2000;19:2340–2350. doi: 10.1093/emboj/19.10.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Yang X, Lopez de Silanes I, Carling D, Gorospe M. Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J Biol Chem. 2003;278:27016–27023. doi: 10.1074/jbc.M300318200. [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Fan J, Yang X, Furer-Galban S, Lopez de Silanes I, von Kobbe C, et al. AMP-activated kinase regulates cytoplasmic HuR. Mol Cell Biol. 2002;22:3425–3436. doi: 10.1128/MCB.22.10.3425-3436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Yang X, Kawai T, Lopez de Silanes I, Mazan-Mamczarz K, Chen P, et al. AMP-activated protein kinase-regulated phosphorylation and acetylation of importin alpha1: involvement in the nuclear import of RNA-binding protein HuR. J Biol Chem. 2004;279:48376–48388. doi: 10.1074/jbc.M409014200. [DOI] [PubMed] [Google Scholar]
- 23.Sebastian T, Johnson PF. RasV12-mediated down-regulation of CCAAT/Enhancer Binding Protein {beta} in immortalized fibroblasts requires loss of p19Arf and facilitates bypass of oncogene-induced senescence. Cancer Research. 2009;69:2588–2598. doi: 10.1158/0008-5472.CAN-08-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salotti J, Sakchaisri K, Tourtellotte WG, Johnson PF. An Arf-Egr-C/EBPbeta pathway linked to ras-induced senescence and cancer. Mol Cell Biol. 2015;35:866–883. doi: 10.1128/MCB.01489-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: impact on healthspan and lifespan. Journal of molecular medicine. 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 27.Moiseeva O, Deschenes-Simard X, St-Germain E, Igelmann S, Huot G, Cadar AE, et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell. 2013;12:489–498. doi: 10.1111/acel.12075. [DOI] [PubMed] [Google Scholar]
- 28.Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ASH1 mRNA particles in living yeast. Molecular cell. 1998;2:437–445. doi: 10.1016/s1097-2765(00)80143-4. [DOI] [PubMed] [Google Scholar]
- 29.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen CH, Yuan P, Perez-Lorenzo R, Zhang Y, Lee SX, Ou Y, et al. Phosphorylation of BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation. Mol Cell. 2013;52:161–172. doi: 10.1016/j.molcel.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HH, Abdelmohsen K, Lal A, Pullmann R, Jr, Yang X, Galban S, et al. Nuclear HuR accumulation through phosphorylation by Cdk1. Genes Dev. 2008;22:1804–1815. doi: 10.1101/gad.1645808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 35.Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phoenix KN, Devarakonda CV, Fox MM, Stevens LE, Claffey KP. AMPKalpha2 Suppresses Murine Embryonic Fibroblast Transformation and Tumorigenesis. Genes & cancer. 2012;3:51–62. doi: 10.1177/1947601912452883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Song P, Zou MH. Inhibition of AMP-activated protein kinase alpha (AMPKalpha) by doxorubicin accentuates genotoxic stress and cell death in mouse embryonic fibroblasts and cardiomyocytes: role of p53 and SIRT1. J Biol Chem. 2012;287:8001–8012. doi: 10.1074/jbc.M111.315812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 39.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. Journal of biology. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- 41.Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008;7:377–388. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Fox MM, Phoenix KN, Kopsiaftis SG, Claffey KP. AMP-Activated Protein Kinase alpha 2 Isoform Suppression in Primary Breast Cancer Alters AMPK Growth Control and Apoptotic Signaling. Genes & cancer. 2013;4:3–14. doi: 10.1177/1947601913486346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vila IK, Yao Y, Kim G, Xia W, Kim H, Kim SJ, et al. A UBE2O-AMPKalpha2 Axis that Promotes Tumor Initiation and Progression Offers Opportunities for Therapy. Cancer Cell. 2017;31:208–224. doi: 10.1016/j.ccell.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 46.Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379–384. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basu SK, Lee S, Salotti J, Basu S, Sakchaisri K, Xiao Z, et al. Oncogenic RAS-Induced Perinuclear Signaling Complexes Requiring KSR1 Regulate Signal Transmission to Downstream Targets. Cancer Research. 2017 doi: 10.1158/0008-5472.CAN-17-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon SM, Hay N. The dark face of AMPK as an essential tumor promoter. Cellular logistics. 2012;2:197–202. doi: 10.4161/cl.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rios M, Foretz M, Viollet B, Prieto A, Fraga M, Costoya JA, et al. AMPK activation by oncogenesis is required to maintain cancer cell proliferation in astrocytic tumors. Cancer Research. 2013;73:2628–2638. doi: 10.1158/0008-5472.CAN-12-0861. [DOI] [PubMed] [Google Scholar]
- 51.Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, et al. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev. 2011;25:2125–2136. doi: 10.1101/gad.17276711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sterneck E, Tessarollo L, Johnson PF. An essential role for C/EBPβ in female reproduction. Genes Dev. 1997;11:2153–2162. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 54.Tangeman L, Wyatt CN, Brown TL. Knockdown of AMP-activated protein kinase alpha 1 and alpha 2 catalytic subunits. J RNAi Gene Silencing. 2012;8:470–478. [PMC free article] [PubMed] [Google Scholar]
- 55.Rook MS, Lu M, Kosik KS. CaMKIIalpha 3′ untranslated region-directed mRNA translocation in living neurons: visualization by GFP linkage. J Neurosci. 2000;20:6385–6393. doi: 10.1523/JNEUROSCI.20-17-06385.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheffler JM, Schiefermeier N, Huber LA. Mild fixation and permeabilization protocol for preserving structures of endosomes, focal adhesions, and actin filaments during immunofluorescence analysis. Methods Enzymol. 2014;535:93–102. doi: 10.1016/B978-0-12-397925-4.00006-7. [DOI] [PubMed] [Google Scholar]
- 57.Whelan DR, Bell TD. Image artifacts in single molecule localization microscopy: why optimization of sample preparation protocols matters. Scientific reports. 2015;5:7924. doi: 10.1038/srep07924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nandy K, Chellappa R, Kumar A, Lockett SJ. Segmentation of Nuclei From 3D Microscopy Images of Tissue via Graphcut Optimization. IEEE Journal of Selected Topics in Signal Processing. 2016;10:140–150. [Google Scholar]
- 59.Ovesny M, Krizek P, Borkovec J, Svindrych Z, Hagen GM. ThunderSTORM: a comprehensive ImageJ plug-in for PALM and STORM data analysis and super-resolution imaging. Bioinformatics. 2014;30:2389–2390. doi: 10.1093/bioinformatics/btu202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parkin SE, Baer M, Copeland TD, Schwartz RC, Johnson PF. Regulation of CCAAT/enhancer-binding protein (C/EBP) activator proteins by heterodimerization with C/EBPgamma (Ig/EBP) J Biol Chem. 2002;277:23563–23572. doi: 10.1074/jbc.M202184200. [DOI] [PubMed] [Google Scholar]
- 61.Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- 62.Nakajima K, Kusafuka T, Takeda T, Fujitani Y, Nakae K, Hirano T. Identification of a novel interleukin-6 response element containing an Ets-binding site and a CRE-like site in the junB promoter. Mol Cell Biol. 1993;13:3027–3041. doi: 10.1128/mcb.13.5.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.