Abstract
Objective
To examine longitudinal associations between structural magnetic resonance imaging (MRI) and cognition in a diverse sample.
Method
Older adults (n=444; MAge=74.5 ± 7.0) − 121 African Americans, 212 Whites, and 111 Hispanics – underwent an average of 5.3 (SD=2.7) annual study visits, including brain MRI and cognitive assessment. Approximately half were cognitively normal at baseline (global Clinical Dementia Rating M=0.5, SD=1.2). Of the patients with dementia, most (79%) were diagnosed with Alzheimer’s disease (AD). MRI measures of gray matter volume (baseline and change), and hippocampal and white matter hyperintensity (WMH) volumes (baseline) were used to predict change in global cognitive functioning. Multilevel latent variable modeling was used to test the hypothesis that brain effects on cognitive change differed across ethnoracial groups.
Results
In a multivariable model, global gray matter change was the strongest predictor of cognitive decline in Whites and African Americans and specific temporal lobe change added incremental explanatory power in Whites. Baseline WMH volume was the strongest predictor of cognitive decline in Hispanics and made an incremental contribution in Whites.
Conclusions
We found ethnoracial group differences in associations of brain variables with cognitive decline. The unique patterns observed in Whites appeared to suggest a greater influence of AD in this group. In contrast, cognitive decline in African Americans and Hispanics was most uniquely attributable to global gray matter change and baseline WMH, respectively. Brain changes underlying cognitive decline in older adults are heterogeneous and depend on fixed and modifiable risk factors that differ based on ethnicity and race.
Keywords: Cross-cultural comparison, Aging, Cognition, Neuroimaging, Dementia
Some of the most salient risk factors for dementia – including apolipoprotein (APOE) genotype, cerebrovascular disease, and diabetes mellitus – have been shown to differ across ethnic and racial groups. Although cognitive decline and, eventually, dementia result from progressive brain injury and dysfunction, different pathogenetic pathways are likely to have different brain manifestations and different cognitive outcomes that reflect the specific patterns of brain impairment. We have previously demonstrated an association between brain structure changes and cognitive functioning changes in a diverse sample of older adults without explicitly considering ethnoracial differences (Fletcher et al., 2017). The goal of the current study is to expand on our previous work by investigating whether and how the associations between brain variables and cognitive change differ across ethnoracial groups. We will focus on three of the largest ethnoracial groups in the United States: African Americans, Whites, and people of Hispanic, Latino, or other Spanish origin ethnicity (which, for simplicity, will be referred to as Hispanic in the current paper).
There is evidence for ethnoracial differences in the pathogenesis of dementia, which implies that cognitive impairment might have different brain substrates across groups (Zahodne et al., 2015). For example, Hispanic ethnicity has been associated with less frequent possession of at least one APOE ε4 allele (Haan et al., 2003; O’Bryant et al., 2013a, 2013b). Some have interpreted the existing evidence to suggest that dementia in Hispanics may be largely driven by metabolic dysfunction (e.g., diabetes) and depression (Johnson et al., 2015; Mayeda, Haan, Kanaya, Yaffe, & Neuhaus, 2013; O’Bryant et al., 2011, 2013a). For African Americans and Whites, the pathogenetic mechanisms of dementia may be less dependent on metabolic factors and more dependent on variables such as inflammation (Goldstein, Zhao, Steenland, & Levey, 2015; Jordanova, Stewart, Davies, Sherwood, & Prince, 2007; O’Bryant et al., 2010, 2014; Windham et al., 2014). In particular, dementia in African Americans may be strongly related to an increased prevalence of cardiovascular risk factors that can be mediated by inflammatory mechanisms (Froehlich, Bogardus, & Inouye, 2001; Howard, 2013; Kurian & Cardarelli, 2007; Tang et al., 2001). In contrast, dementia in Whites may have a more pronounced genetic influence (Evans et al., 2003; Farrer et al., 1997).
Studies of ethnoracial differences in brain effects on cognition are limited and do not show consistent patterns of findings. In one cross-sectional study, for instance, hippocampal volumes and white matter hyperintensity (WMH) burden were found to be differentially associated with episodic memory test scores in White versus Hispanic individuals and language, processing speed, and executive functioning test scores in African American versus White individuals (Zahodne et al., 2015). In contrast, DeCarli et al., (2008) found that the relationship between hippocampal volumes and episodic memory ability did not differ across African Americans, Hispanics, and Whites. However, vascular risk factors were more pronounced in African Americans, and this group was more likely to have non-amnestic MCI (DeCarli et al., 2008). Similarly, Mungas, Reed, Farias, & DeCarli (2009) found stronger association between spatial difficulties and WMH in African Americans compared to Whites and Hispanics. These cross-sectional measures of cognitive function are broadly influenced by life history variables (e.g., education, early life experiences; Melrose et al., 2015) that often differ by ethnoracial group and contribute to dementia risk independent of brain injury pathways (Diaz-Venegas, Downer, Langa, & Wong, 2016; Liu, Glymour, Zahodne, Weiss, & Manly, 2015; Melrose et al., 2015; Sisco et al., 2015; Zahodne, Stern, & Manly, 2015). In many cases, such life history variables have stronger relations than brain variables on cross-sectional cognitive test scores (Mungas et al., 2009).
In contrast to the prominent influence of life history variables on cross-sectional cognitive test scores, there is evidence that longitudinal decline in cognitive functioning is a relatively specific marker for brain changes (Early et al., 2013; Fletcher et al., 2017; Mungas et al., 2010). There have been very few studies to examine the co-occurrence of changes in brain structure and cognitive functioning, especially in diverse samples, and those have largely relied upon basic cognitive screening instruments such as the Mini-Mental State Examination (Morra et al., 2009). The limitations of basic screening measures highlight the need for a more comprehensive battery of cognitive tests that is free from cultural and linguistic biases, floor and ceiling effects, and which has been validated for tracking longitudinal changes in cognition. The present study used the Spanish and English Neuropsychological Assessment Scales (SENAS) to measure changes in cognitive functioning over time. The SENAS provides psychometrically matched measures of episodic memory, semantic memory, spatial skills, and executive functioning and has been optimized for measuring cognitive change in ethnoracially and linguistically diverse older adults (Crane et al., 2008; Mungas, Reed, Marshall, & González, 2000; Mungas, Reed, Crane, Haan, & González, 2004; Mungas, Reed, Haan, & González, 2005a; Mungas, Reed, Tomaszewski Farias, & DeCarli, 2005b; Mungas, Widaman, Reed, & Tomaszewski Farias, 2011).
Cognitive decline is the hallmark of dementia, is a relatively specific marker for brain disease and degeneration, and appears to be minimally affected by confounding variables associated with ethnoracial differences in cross-sectional scores (Early et al., 2013; Mungas et al., 2009). To help disentangle pathological brain changes from premorbid differences in cognitive abilities, it is important to understand the longitudinal associations between brain structure and cognitive function in diverse older adults. Identification of ethnoracial group differences in brain-cognition associations can be valuable for appropriate interventions and for better understanding the variables that create risk and protective factors for the various pathogenetic mechanisms that can cause cognitive decline and dementia. By accounting for important background variables (e.g., age, education, APOE ε4 genotype), and relations of baseline brain integrity with cognitive change, we can explore the hypothesis that the associations between cognitive changes and brain changes differ by ethnoracial group.
Method
The methods described below are nearly identical to those reported in our companion paper (Fletcher et al., 2017) and are reprinted here for reference.
Participants
The University of California Davis (UCD) Aging Diversity Cohort provided the study sample. This is a longitudinal study of cognitive aging in an educationally, ethnically, and cognitively diverse cohort of older adults. This cohort approximates the diverse racial, ethnic, and socioeconomic composition of a six-county catchment area in the central Sacramento/San Joaquin valley and east San Francisco Bay area of Northern California, is composed of Hispanics, African Americans, and non-Hispanic Whites, has wide variability in educational attainment, and spans a spectrum of cognitive function from normal to mildly impaired to demented. About two-thirds of this cohort were recruited through a community screening program that was designed to recruit community dwelling individuals without regard to their level of cognitive function, that is, to represent the range and distribution of cognitive function in the community. The other one-third were initially seen for clinical evaluation at a university memory/dementia clinic and referred for research. Cohort composition and recruitment methods are described in Hinton et al. (2010).
Participants were 444 persons who had received at least two cognitive evaluations and at least one MRI brain scan; 282 had two or more scans. There were 212 Whites, 111 Hispanics, and 121 African Americans; 64 Hispanics were tested in Spanish and all others were tested in English. Approximately two-thirds of the Hispanic participants reported Mexican ancestry. The community screening program identified 302 individuals (97 Whites, 98 Hispanics, 107 African Americans) while 142 (115 Whites, 13 Hispanics, 14 African Americans) were clinical referrals. Clinical diagnosis was made via consensus conference using standard diagnostic criteria (e.g., Albert, 2011; McKhann, 1984; McKhann, 2011; Winblad, 2004).
Participants were evaluated and followed within the research program of the UCD Alzheimer’s Disease Center (ADC). Enrollment began in 2001 and a rolling enrollment design was used to build the cohort with substantial enrollment continuing through 2010. All participants in this study had at least two evaluations but due to rolling enrollment there was variability in the number of evaluations completed by each participant. Inclusion criteria for the longitudinal cohort included age 60 or older at their first examination and ability to speak English or Spanish. Exclusion criteria included unstable major medical illness, major primary psychiatric disorder, and substance abuse or dependence in the last five years. Participants received clinical evaluations through the UCD ADC on a roughly annual basis that included diagnosis, based on standard diagnostic criteria, of normal cognition versus mild cognitive impairment (MCI) versus dementia as well as etiologic diagnosis. All participants signed informed consent, and all human subject involvement was overseen by institutional review boards at University of California at Davis, the Veterans Administration Northern California Health Care System, and San Joaquin General Hospital in Stockton, California.
Cognitive Assessment
The cognitive outcomes in this study were composite measures of episodic memory, semantic memory, executive function, and spatial ability derived from the SENAS. The SENAS has undergone extensive development as a battery of cognitive tests relevant to cognitive aging that allow for valid comparisons across race/ethnic groups (Mungas et al. 2004; Mungas et al., 2005a; Mungas et al., 2000; Mungas et al., 2005b). The episodic memory composite score is derived from a multi-trial word-list-learning test (Mungas et al., 2004). The semantic memory composite is derived from highly correlated verbal (object-naming) and nonverbal (picture association) tasks. The executive function composite is constructed from component tasks of category fluency, phonemic (letter) fluency, and working memory (digit-span backward, visual-span backward, list sorting). Spatial ability was measured using the SENAS Spatial Localization scale, which assess ability to perceive and reproduce two-dimensional spatial relationships that are increasingly complex. These measures were administered at all evaluations. Language of test administration was determined by an algorithm that combined information regarding each participant’s language preference in several specific contexts (e.g., conversing at home, listening to radio or television, conversing outside the home, preferred language for reading). Administration procedures, measure development, and psychometric characteristics of the SENAS battery are described in more detail elsewhere (Mungas et al., 2004).
MRI Measures
Cross-sectional baseline volumes
MRI baseline measurements were made as part of our in-house processing pipeline described previously (e.g. Fletcher, 2014; Lee et al., 2010). Briefly, structural MRI images were processed to remove the skull using an atlas-based method (Aljabar, Heckemann, Hammers, Hajnal, & Rueckert, 2009) followed by human quality control to provide generally minor cleanup if needed. Structural MRI brain images were then nonlinearly registered to a minimal deformation template (MDT) synthetic brain image (Kochunov et al., 2001) adapted for age range of 60 and above; the registration was performed by a cubic B-spline deformation (Rueckert, Aljabar, Heckemann, Hajnal, & Hammers, 2006). Gray, white, and CSF tissues segmentation was performed using automatic tissue class initialization followed by iterated alternating voxel class assignment and tissue class parameter estimation until convergence, in an algorithm designed to enhance accuracy at likely tissue boundaries (Fletcher, Singh, Harvey, Carmichael, & DeCarli, 2012). Finally, native lobar gray matter volumes were computed by reverse transforming MDT lobar regions of interest (ROIs) into native space using the B-spline registration parameters. Lobar ROIs included frontal, temporal, parietal and occipital lobes. The lobar ROIs used in our analyses were defined in MDT space by an experienced neurologist and have been used in a prior publication from our laboratory (Lee et al., 2010).
WMH quantification
White matter hyperintensities were quantified at baseline time scans using an automated segmentation algorithm using T1 and fluid-attenuated inversion recovery (FLAIR) images in a method of our laboratory described previously (DeCarli et al., 2005). Briefly, the technique involves segmenting voxels of the FLAIR image with intensities exceeding 2.5 standard deviations above the FLAIR mean, after this image has been normalized so that the intensity mode is at a standard value. Refinements include mapping WMH probability priors from a pre-determined atlas onto native images in order to better account for likelihoods of WMH occurrences.
Longitudinal volume change
For participants having at least two longitudinal structural MRI scan acquisitions, we computed longitudinal structural change between the most widely separated time points. We used a tensor-based morphometry (TBM) method designed to enhance sensitivity and specificity for biological change by incorporating estimates of likely tissue boundaries (Fletcher, 2014; Fletcher et al., 2013). TBM generates deformation fields registering brain scans at differing time points and uses these to estimate local volume changes between the scans (Ashburner & Friston, 2000). This processing was done via an in-house processing pipeline that has been previously described (Fletcher et al., 2016). Briefly, we linearly aligned images at time 1 and time 2 to a “halfway space” to avoid interpolation biases when only one image is transformed. Each brain scan was then corrected for field inhomogeneities using an atlas-based technique and finally tissue-segmented using an algorithm sensitive to edge presence. The log-transformed determinant of the 3×3 Jacobian matrix of the TBM deformation at each voxel (i.e. log-Jacobian) quantifies local brain change.
To perform voxel-wise longitudinal change analysis across subjects in a common space, we transformed subject native-space log-Jacobian images onto MDT template space as described above for baseline volumes. Statistical analysis of longitudinal change in native space was performed using ROIs transformed to native space also as described above, then calculating the mean log-Jacobian for each subject in segmented GM on each native ROI.
APOE Genotyping
Apolipoprotein E (APOE) genotyping was carried out using the LightCycler ApoE mutation detection kit (Roche Diagnostics, Indianapolis, IN).
Data Analysis
Measures and data processing
SENAS measures of Episodic Memory, Semantic Memory, Executive Function, and Spatial Ability were the primary dependent variables. Baseline lobar MRI volumes and change in these volumes were the primary independent variables (prefrontal, temporal, parietal minus post-central gyrus, occipital). Cognitive and baseline MRI variables were reasonably normally distributed. MRI change variable were symmetrical but had high kurtosis. The Blom inverse normal rank order transformation was applied to all cognitive and MRI variables to establish a common scale (M = 0, SD = 1) and normalize the variables. Age (centered at 70) and education (centered at 12) were continuous covariates. Gender, ethnoracial group, language of test administration, and APOE ε4 status were categorical covariates with female, White, English test administration, and APOE ε4 negative serving as reference categories.
Analysis of variance and the χ2 test were used to compare baseline characteristics of study participants across ethnoracial groups. Mixed effects regression analyses were used to estimate parallel process growth models to characterize cognitive trajectories and to assess the impact of covariates and MRI variables on baseline cognitive scores and rate of change.
MRI modeling
MRI measures tend to be highly correlated and cross-sectional MRI volumes are strongly related to size of the intracranial vault independent of brain atrophy. We used latent variable methods to adjust baseline volumes for intracranial volume (ICV) and to decompose correlated groups of MRI measures into global components and lobe-specific deviations from the global averages. Figure 1 shows a schematic model of this approach. For baseline, MRI volumes were regressed on ICV and a global factor (brain bl) was fitted using the four regional ROIs as indicators. The ROI-specific residuals were captured as latent variables. ICV and the global volume factor were constrained to be uncorrelated to identify the model and residuals were uncorrelated with ICV and brain bl. This process yielded 6 variables for subsequent analyses: ICV, brain bl, and four specific lobar volumes not fully explained by ICV or brain bl. For change, the four ROI change measures were indicators for a global change factor (brain ch) and latent variables were used to capture ROI specific change not fully explained by the brain ch. This yielded five latent variables that were used as independent variables in subsequent models: brain ch and the four lobe-specific change variables.
Figure 1.
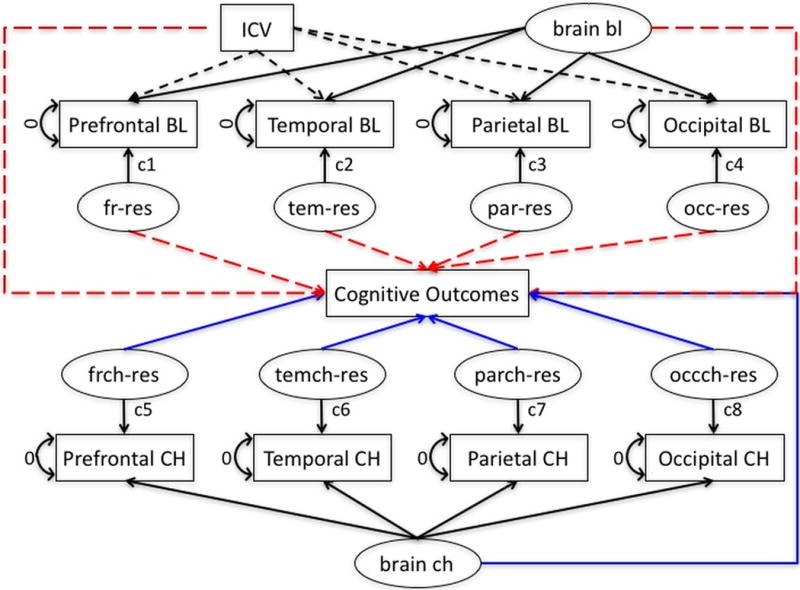
Analytic model for decomposing MRI baseline and change. ICV = intracranial volume; bl = baseline; fr = prefrontal lobe gray matter; res – residual; tem = temporal lobe gray matter; par = parietal lobe gray matter; occ = occipital lobe gray matter; frch = prefrontal lobe gray matter change; temch = temporal lobe gray matter change; parch = parietal lobe gray matter change; occch = occipital lobe gray matter change; ch = change.
Longitudinal modeling of cognitive trajectories
Mixed effects, parallel process longitudinal analyses were performed using Mplus version 7.3 multilevel modeling (Muthén & Muthén, 1998–2015). This modeling platform simultaneously estimates “within” and “between” level parameters. Figure 2 shows a schematic of the basic modeling approach. In the “within” part of the model, each of the four cognitive outcomes was regressed on time in study. This generated person-specific intercept and linear slope random effects for each outcome. These random effects were dependent variables in the “between” part of the model. Mixed effects models estimate the baseline value and rate of change of outcomes of interest (Within model), and also estimate how differences in these components relate to variables of interest (fixed effects) that differ between subjects (e.g., covariates, MRI variables; Between model). The inclusion of random effects accounts for individual variation not measured by the variables included in the model. Mixed effects models allow for heterogeneity in the number of assessment time points and in the lags between assessments across persons. This approach replicates that reported in a previous paper.
Figure 2.
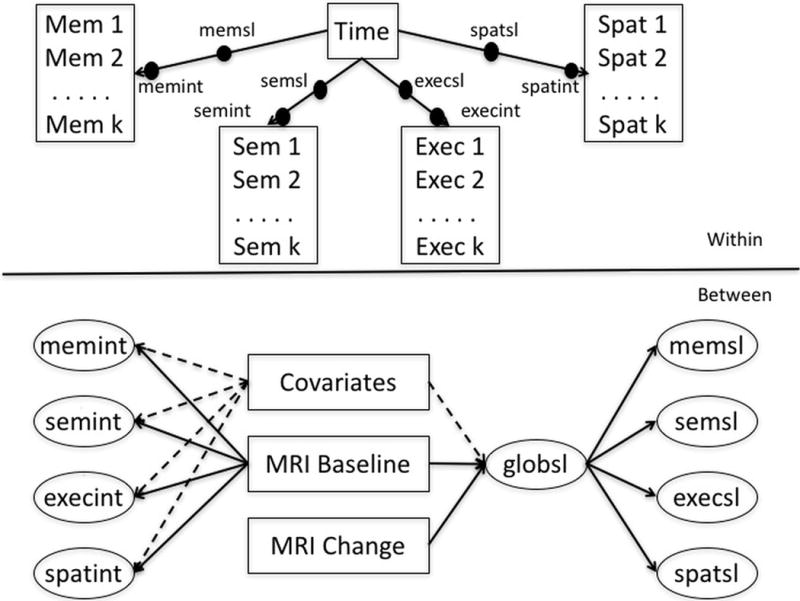
Analytic model for cognitive trajectory components, MRI variables, and covariates. Mem = Spanish and English Neuropsychological Assessment System (SENAS) Episodic Memory Index; Sem = SENAS Semantic Memory Index; Exec = SENAS Executive Functioning Index; Spat = SENAS Spatial Index; memsl = slope of change in SENAS Episodic Memory Index over time; memint = intercept (baseline) of SENAS Episodic Memory Index; semsl = slope of change in SENAS Semantic Memory Index over time; semint = intercept (baseline) of SENAS Semantic Memory Index; execsl = slope of change in SENAS Executive Functioning Index over time; execint = intercept (baseline) of SENAS Executive Functioning Index; spatsl = slope of change in SENAS Spatial Index over time; spatint = intercept (baseline) of SENAS Spatial Index; MRI = magnetic resonance imaging; global = slope of change in global cognition.
We utilized multiple group models to evaluate ethnoracial group similarities and differences in effects of brain variables and covariates on cognitive baseline and change components. In effect, the model depicted in Figure 2 is estimated for each group. Multiple group analysis allow parameters to be estimated independently in different groups. Alternatively, specific model parameters can be constrained to be the same across groups. Less constrained models can be compared to nested, more constrained models to determine if the fit is significantly better when the parameters of interest are allowed to differ across groups. Ethnoracial group (African American, White, Hispanic) was the grouping variable for these analyses. The likelihood ratio test for nested models (Satorra & Bentler, 2001) was used to determine if freely estimating specific parameters across groups resulted in significantly better model fit to the data.
Model building proceeded in steps. Step 1 developed a base model to estimate intercept and slope random effects for all four outcomes, and included a within-subjects term to account for practice effects. For each of the four cognitive outcome measures, a variable coding for previous exposure vs. no exposure was created and included as a time-varying fixed effect. The initial model allowed the eight random effects latent variables (intercept and slope random effects for each of the four outcomes) to freely correlate, but we then estimated second order latent variables (one with intercepts as indicators, one with slopes) that explained the correlations among the random effects. This step was taken to determine whether baseline cognition and cognitive change in our sample was best characterized by the four SENAS composite measures of Episodic Memory, Semantic Memory, Executive Function, and Spatial separately, or by second-order latent variables that estimate “global cognition” reflected in covariance among the four SENAS measures. We compared fit of models with 0, 1, and 2 second order factors using comparative fit indices including the Akaike Information Criterion (AIC; Akaike, 1987), the Bayesian Information Criterion (BIC; Schwarz, 1978), and the sample size-adjusted Bayesian Information Criterion (aBIC; Sclove, 1987). These indices differ in the relative weighting of model fit and model parsimony, with AIC valuing parsimony the least and BIC the most. Lower values on all indices indicate better model fit.
In Step 2, we added APOE genotype and age, gender, education, and ethnoracial group as fixed effect covariates to explain cognition baseline and change. We examined interaction effects involving ethnoracial group and other covariates in preliminary analyses and retained any significant interactions in subsequent analyses.
In Step 3 we examined univariable effects of baseline MRI volumes and MRI volume change on cognition. First, we added specific MRI baseline volumes one at a time to the best model from Step 2 to examine simple associations of baseline MRI variables with cognitive intercepts and slopes adjusted for covariates. These volumes included ICV, hippocampus (adjusted for ICV), total WMH, brain bl (the global baseline volume factor), and each lobar ROI adjusted for ICV - but not for brain bl. We then fit four separate models that jointly included brain bl and ICV as well as each individual, specific lobar measurement as presented in Figure 1. Finally, we generated a multivariate model that jointly included significant MRI variables from previous steps. We then followed a similar process for MRI change variables. We first examined simple associations with cognition, adjusted for covariates, then examined each lobe specific residual entered jointly with brain ch, and finally, fit a multivariate model incorporating significant effects from previous analyses in this step. In Step 4 we generated a final multivariable model that jointly included significant effects from previous steps.
All parameters were simultaneously estimated within each model. Latent variables for MRI variables, latent random effects for cognitive variables, and regressions of cognitive random effects on MRI variables and covariates were simultaneously estimated. While we estimated intercept random effects and evaluated the effects of MRI variables on intercepts, the focus of this report is on cognitive change so intercept results are not reported. Complete data was not available on all variables, and the missing data analysis option of Mplus was used. Mplus uses full information maximum likelihood estimation, which provides unbiased parameter estimates in the context of missing at random (Newman, 2003). Missing data was primarily missing by design, which meets requirements for missing at random (Bollen & Curran, 2006). All individuals with longitudinal cognitive assessments were used to estimate baseline and change random effects for the four cognitive outcomes and how demographic covariates and APOE ε4 influenced cognitive baseline and change. MRI change data was not available for the full sample but was missing by design. Statistical power for MRI effects is reduced by missing data, but parameter estimates should be unbiased.
Mixed model regression analyses are sensitive to assumptions of linearity, normality, and constant variance. These assumptions were examined using graphical and statistical diagnostics. Residuals and random effects were examined to assure that they were normally distributed, and plots of residuals against predicted values and effects were examined to verify that non-linear trends in the data or non-constant variances were not present. Additional diagnostics included evaluation of variance components related to random effects and within subject error variance to address adequacy of statistical estimation procedures associated with the random effects modeling.
Voxelwise analysis of longitudinal change
We performed voxel based analyses to characterize the associations of brain regions with cognitive change. Voxelwise log-Jacobian images in template space enable cross-sectional averaging and regression analysis of longitudinal change, to identify patterns of atrophy and associations with cognition independent of prior hypotheses about locations where these might occur. In voxel-based regressions, the log-Jacobian change value at a voxel was an independent variable with covariates of age, gender, education and interscan interval between baseline and follow-up scans. Outcomes or dependent variables were cognitive baseline (intercept) and change (slope) measurements estimated as described above from the Step 1 unconditional model that included a global slope. We focused on global cognitive slope as the outcome of interest. Significance testing for regression coefficients of log-Jacobians predicting cognitive outcomes was performed by non-parametric super-threshold cluster testing (Nichols & Holmes, 2002) with 1000 iterations of random permutations. Clusters of a determined threshold with size in the top 5% of the distribution generated by these iterations were deemed significant. We selected a range of thresholds for the coefficient t-values, generating significant contiguous clusters for each threshold. To generate images of average estimated change over equivalent intervals, we further scaled each subject log-jacobian image in MDT space by 2 /Δ where Δ is the interscan interval. These normalized log-Jacobians represented change of each subject over two years.
Results
Sample Characteristics
Sample characteristics are presented in Table 1. Participants completed an average of 5.3 annual study visits, and this did not differ by group (F [2,441] = 2.196, p = .112). About 59% were females. Gender differed across ethnoracial groups (χ2 [2] = 19.74, p = .001); African Americans and Hispanics were more likely to be female but White cases were evenly divided among males and females. About 51% were Normal, 36% had a diagnosis of MCI, and 13% were Demented. Diagnosis differed by group (χ2 [4] = 38.77, p = .001) with Whites more likely to have a diagnosis of MCI and Hispanics more likely to be Normal. There was no significant difference in suspected dementia etiology across ethnoracial groups (χ2 [12] = 10.89, p = .538). Approximately two thirds of the sample was recruited from the community (68%). Recruitment source differed by group (χ2 [2] = 92.45, p = .001), with clinic referrals more likely to be Whites. Average age was about 75 years and this differed across groups (F [2, 441] = 6.63, p = .001) with Hispanics younger on average than African Americans and Whites. Average education was 12.8 and differed across groups (F [2, 441] = 95.59, p = .001), with highest education in Whites, slightly lower average education in African Americans, and much lower levels in Hispanics. APOE ε4 differed across groups (χ2 [2] = 18.72, p = .001) with approximately 50% lower prevalence of ε4 positivity in Hispanics compared to the other two groups. Groups did not differ in terms of the frequency of baseline MRI availability (χ2 [2] = 1.22, p = .545), but did differ in terms of the frequency of follow-up MRI availability (χ2 [2] = 8.67, p = .013), with the least data available for African Americans and the most available for Hispanics.
Table 1.
Sample characteristics.
| African American | Hispanic | White | Total | |
|---|---|---|---|---|
| n | 121 | 111 | 212 | 444 |
| Number of Visits – M (SD) | 5.1 (2.4) | 5.8 (2.8) | 5.1 (2.7) | 5.3 (2.7) |
| Baseline MRI Available; n (%) | 110 (90.9%) | 96 (86.5%) | 186 (87.7%) | 392 (88.3%) |
| Change MRI Available; n (%) | 64 (52.9%) | 78 (70.3%) | 140 (66.0%) | 282 (63.5%) |
| Gender - Female; n (%) | 87 (71.9%) | 71 (64.0%) | 102 (48.1%) | 260 (58.6%) |
| Age - M (SD) | 75.2 (6.9) | 72.5 (6.6) | 75.2 (7.1) | 74.5 (7.0) |
| Education - M (SD) | 13.3 (3.3) | 8.6 (5.3) | 14.8 (3.3) | 12.8 (4.6) |
| Recruitment Source – Clinic; n (%) | 14 (11.6%) | 13 (11.7%) | 115 (54.2%) | 142 (32.0%) |
| Global CDR – M (SD) | 0.5 (1.3) | 0.4 (1.1) | 0.5 (1.2) | 0.5 (1.2) |
| Diagnosis - Normal; n (%) | 68 (56.7%) | 72 (66.7%) | 82 (39.0%) | 222 (50.7%) |
| Diagnosis - MCI; n (%) | 37 (30.8%) | 17 (15.7%) | 105 (50.0%) | 159 (36.3%) |
| Diagnosis - Demented; n (%) | 15 (12.5%) | 19 (17.6%) | 23 (11.0%) | 57 (13.0%) |
| Possible AD; n (%) | 1 (6.7%) | 2 (10.5%) | 3 (20.0%) | 6 (10.5%) |
| Probable AD; n (%) | 10 (66.7%) | 13 (68.4%) | 16 (69.6%) | 39 (68.4%) |
| Probable VaD; n (%) | 1 (6.7%) | 1 (5.3%) | 0 (0.0%) | 2 (3.5%) |
| Possible DLB; n (%) | 0 (0.0%) | 2 (10.5%) | 1 (4.3%) | 3 (5.3%) |
| FTD; n (%) | 0 (0.0%) | 0 (0.0%) | 1 (4.3%) | 1 (1.8%) |
| Mixed; n (%) | 3 (20.0%) | 0 (0.0%) | 2 (8.7%) | 5 (8.8%) |
| Missing; n (%) | 0 (0.0%) | 1 (5.3%) | 0 (0.0%) | 1 (1.8%) |
| APOE ε4 Positive; n (%) | 54 (44.6%) | 26 (23.4%) | 101 (47.6%) | 181 (40.8%) |
Note. CDR = Clinical Dementia Rating (baseline score); MCI = mild cognitive impairment; AD = Alzheimer’s disease; VaD = vascular dementia; DLB = dementia with Lewy bodies; FTD = frontotemporal dementia; APOE = apolipoprotein E.
Model for Cognitive Intercepts and Slopes
Table 2 shows fit indices for different models for explaining covariance of cognitive intercepts and slopes. The best fitting model by all three criteria had separate intercepts for the four cognitive outcomes, but a global slope factor that accounted for covariance among the four slope random effects. Separate analyses within ethnoracial groups confirmed that the model with a global slope and individual intercepts provided optimal fit in all three groups.
Table 2.
Fit indices of alternate models to characterized covariance among cognitive intercepts and slopes.
| Model | AIC | BIC | aBIC |
|---|---|---|---|
| Separate Intercepts - Separate Slopes | 16890 | 17254 | 17057 |
| Global Intercept - Separate Slopes | 17024 | 17305 | 17153 |
| Separate Intercepts - Global Slope | 16888 | 17170 | 17017 |
| Global Intercept - Global Slope | 17026 | 17261 | 17134 |
Note. AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion; aBIC = Sample size adjusted Bayesian Information Criterion. The bolded row indicates the model with the best relative fit indices.
Covariate Effects
We next tested for differences in covariate effects on cognitive trajectory components, separately evaluating each covariate and its effect on intercepts and global slope. A model in which all covariate effects on all intercepts and global slope were freely estimated served as a common basis for comparison. Group differences in individual covariate effects on intercepts were evaluated by comparing the base freely estimated model with a model in which the effects of the covariate on all four intercepts were constrained to be equal across groups. None of these groupwise tests were significant for any of the covariates, so tests of differences for intercepts of individual cognitive variables were not performed. Group differences in the effect of each covariate on global slope were evaluated by comparing the freely estimated model with a model in which the covariate effect on the global slope was constrained to equality across groups. Again, significant differences were not observed in covariate effects. Subsequent models constrained covariate effects to be the same across ethnoracial groups. Results of tests for differential covariate effects are presented in Table 3.
Table 3.
Likelihood Ratio Tests for Significance of Ethnoracial Group Differences in Covariate Effects on Cognitive Intercepts and Global Cognitive Slope.
| Covariate | Component | χ2 difference | df | p |
|---|---|---|---|---|
| Gender | Intercepts | 5.83 | 8 | .666 |
| Education | Intercepts | 12.79 | 8 | .119 |
| Age (Baseline) | Intercepts | 5.59 | 8 | .693 |
| Recruitment Source | Intercepts | 11.62 | 8 | .169 |
| APOE ε4 | Intercepts | 9.31 | 8 | .317 |
| Gender | Global Slope | 1.02 | 2 | .601 |
| Education | Global Slope | 1.04 | 2 | .594 |
| Age (Baseline) | Global Slope | 3.18 | 2 | .204 |
| Recruitment Source | Global Slope | 1.62 | 2 | .444 |
| APOE ε4 | Global Slope | 3.82 | 2 | .148 |
Note. APOE = apolipoprotein E.
Two of the five covariates in the model exerted a significant influence on global cognitive decline. Clinic recruitment source was associated with faster cognitive decline (b = − 0.06, SE = 0.01) as was APOE ε4 genotype (b = −0.03, SE = 0.01). To ensure the robustness of the across-group equality constraints on covariate effects, we also ran the models with these parameters freely estimated and found no meaningful differences in the results (data not shown).
Brain Volume Effects on Global Cognitive Change - Single Variable Models
Table 4 shows the effects of brain baseline and change variables on global cognitive slope. These models included one brain variable at a time and covariates. Global gray baseline and temporal lobe gray baseline were associated with global cognitive slope in all three groups, as were all gray matter change variables, except occipital gray change, which was related only in Whites. Prefrontal gray baseline was related to cognitive change in African Americans and Whites, parietal gray baseline in African Americans and Hispanics, and hippocampus baseline in Hispanics and Whites.
Table 4.
Brain Effects (SE) on Global Cognitive Change by Ethnoracial Group (univariable).
| Variable | African American | Hispanic | White |
|---|---|---|---|
| Global Gray BL | 0.034 (0.016)* | 0.041 (0.019)* | 0.057 (0.028)* |
| Prefrontal Gray BL | 0.020 (0.008)* | 0.008 (0.006) | 0.025 (0.011)* |
| Temporal Gray BL | 0.024 (0.011)* | 0.020 (0.006)* | 0.038 (0.011)* |
| Parietal Gray BL | 0.017 (0.007)* | 0.015 (0.007)* | 0.017 (0.010) |
| Occipital Gray BL | 0.005 (0.008) | 0.011 (0.006) | 0.000 (0.011) |
| Hippocampus BL | 0.023 (0.012) | 0.016 (0.007)* | 0.042 (0.009)* |
| WM Hyperintensity BL | −0.002 (0.006) | −0.022 (0.007)* | −0.024 (0.009)* |
| Intracranial Volume BL | −0.004 (0.008) | −0.002 (0.007) | −0.014 (0.013) |
| Global CH | 0.067 (0.017)* | 0.030 (0.011)* | 0.096 (0.016)* |
| Prefrontal CH | 0.035 (0.010)* | 0.021 (0.009)* | 0.067 (0.014)* |
| Temporal CH | 0.046 (0.011)* | 0.034 (0.011)* | 0.093 (0.013)* |
| Parietal CH | 0.045 (0.011)* | 0.019 (0.009)* | 0.087 (0.014)* |
| Occipital CH | 0.020 (0.012) | 0.001 (0.008) | 0.053 (0.014)* |
Note. BL = baseline, WM = white matter; CH = change.
p < 0.05
Specific Brain Volume Effects on Global Cognitive Change
The next step of analysis examined specific effects of lobar gray matter ROIs not explained by global gray matter. The primary independent variables in these analyses were the global gray matter volumes (baseline and change) and lobar ROIs residualized for the corresponding gray matter volume, and for ICV for baseline volumes. Separate models were fitted for each baseline gray matter ROI and each gray matter change ROI. Multiple group models freely estimated effects of these variables on Global Cognitive Slope and intercepts in the three ethnoracial groups. Baseline lobar specific gray matter volumes – that is, volumes residualized for global volume – were not related to cognitive change in any group. Specific temporal gray change was incrementally related to cognitive change beyond global gray change in Whites (estimate [SE] = 0.042 [0.010], p = .001); in Hispanics, this effect was of similar magnitude, but was estimated with less precision (estimate [SE] = 0.036 [0.020], p = .062).
Multivariable Model Including Baseline Brain Volumes and Brain Volume Change
The final analytic model included brain baseline and change variables that were significantly associated with global cognitive change in the previous steps, along with covariates. Brain volumes included as independent variables in the analysis were: baseline global gray matter, hippocampus, and WMH; and change in global gray matter and specific temporal lobe gray matter. These effects were freely estimated in the three groups in a multiple group model. Group differences in the effects of these variables were evaluated by estimating models in which specific effects were constrained to equality and comparing fit with the freely estimated base model using the modified likelihood ratio test.
Table 5 presents the results from the freely estimated multivariable model. Global gray change was significantly related to global cognitive slope in Whites and African Americans, but not Hispanics, after accounting for all other variables in the model. Specific temporal gray change made an incremental contribution in Whites. WMH was related to cognitive change in Hispanics and Whites. Summarized by ethnoracial group, global gray change, specific temporal change, and WMH were incrementally associated with cognitive change in Whites. Global gray change was the only variable that was related to cognitive change in African Americans, controlling for all other variables in the model, and WMH was the only significant incremental association in Hispanics.
Table 5.
Brain Effects (SE) on Global Cognitive Change by Ethnoracial Group (multivariable).
| Variable | African American | Hispanic | White |
|---|---|---|---|
| Global Gray Change | 0.063 (0.018)* | 0.017 (0.012) | 0.079 (0.013)* |
| Temporal Gray Change Residual | 0.008 (0.011) | 0.034 (0.023) | 0.039 (0.011)* |
| Global Gray Baseline | −0.003 (0.010) | 0.007 (0.010) | 0.007 (0.013) |
| Hippocampus Baseline | 0.006 (0.010) | 0.002 (0.010) | 0.011 (0.010) |
| WMH Baseline | −0.004 (0.005) | −0.022 (0.007)* | −0.022 (0.009)* |
| Intracranial Volume | −0.002 (0.007) | 0.005 (0.006) | −0.009 (0.011) |
Note. WMH = white matter hyperintensity.
p < .05
Table 6 shows the results of tests for group differences in specific effects in the multivariable model. Global gray change and WMH effects significantly differed across groups. None of the other variables had differential effects across group, but these variables were not independently associated with cognitive change in any group.
Table 6.
Likelihood Ratio Tests for Significance of Ethnoracial Group Differences in Brain Variable Effects on Global Cognitive Slope.
| Variable | χ2 difference | df | p |
|---|---|---|---|
| Global Gray Change | 36.85* | 2 | .001 |
| Temporal Gray Change | 4.89 | 2 | .087 |
| Global Gray Baseline | 0.67 | 2 | .715 |
| Hippocampus Baseline | 0.36 | 2 | .835 |
| WMH Baseline | 8.63* | 2 | .013 |
| Intracranial Volume | 1.45 | 2 | .484 |
Note. WMH = white matter hyperintensity.
p < .05
Discussion
A companion study based on this diverse sample but not stratified by ethnoracial group showed that cognitive decline was best explained by global gray matter atrophy and, incrementally, by specific temporal gray matter atrophy (Fletcher et al., 2017). Baseline WMH and hippocampal volume also made incremental contributions to cognitive decline. The current study examined MRI-based brain measures as predictors of cognitive decline within specific ethnoracial groups and compared results across groups. When MRI variables were entered individually as predictors of cognitive decline, baseline global gray matter and temporal lobe gray matter volume – along with volume changes in global gray matter and frontal, temporal, and parietal gray matter – were associated with cognitive decline in all three groups (Table 4). The effect sizes of gray matter change on cognitive change tended to be largest in Whites, smallest in Hispanics, and intermediate in African Americans. When significant simple effects were combined in a multivariable model, different patterns of results emerged as salient for the different ethnoracial groups (Table 5). Global gray matter change was the strongest predictor of cognitive decline in Whites and African Americans. Its effect significantly differed across groups (Table 6), and this variable was not incrementally associated with cognitive decline in Hispanics. Specific temporal lobe change effects did not significantly differ across groups, but this effect had a significant effect on cognitive decline only in Whites. Baseline WMH volume was the strongest predictor of cognitive decline in Hispanics and also made an incremental contribution in Whites, but not African Americans.
These results show unique brain-behavior associations that differ based on ethnoracial group. Gray matter atrophy and, specifically, temporal lobe atrophy are commonly associated with AD pathology, whereas WMH is regarded as a marker of microvascular disease associated with non-AD processes like type 2 diabetes and cerebrovascular disease. These associations of brain changes with diseases are not entirely specific, however. Brain atrophy can also be associated with cerebrovascular disease (Schuff et al., 2009) and WMH can be a manifestation of AD brain injury (Brickman, 2013). But the overall pattern of results in the current study suggests that, despite the fact that mixed AD and WMH pathology is common (Chui & Ramirez-Gomez, 2015), different groups may be affected by a differential mix of these pathologies. Specifically, our results suggest that AD is a more important contributor to cognitive decline in Whites than in Hispanics, and this conclusion appears to be supported by the nearly twofold difference in APOE ε4 prevalence across these two groups (Table 1).
Although global WMH significantly contributed to cognitive decline in both Whites and Hispanics (Table 5), it is possible that the mechanisms of WMH contribution to cognitive decline differ between the groups, with WMH in Whites being more strongly related to AD pathology and in Hispanics being more strongly related to metabolic or cerebrovascular causes. Such an outcome would be consistent with evidence suggesting that rates of diabetes tend to be higher in Hispanic populations (Schneiderman et al., 2014), and that disrupted glucose metabolism is a prominent contributor to dementia in this population (Mayeda et al., 2013). Previous work from our group has shown differential regional distributions of WMH in AD and cerebrovascular disease (Yoshita et al., 2006; but also see Holland et al., 2008 and Gootjes et al., 2004), so it would also be relevant to examine whether regional WMH differentially predicts cognitive decline in Whites and Hispanics.
Global gray matter volume atrophy was the strongest contributor to cognitive decline in African Americans. One possible explanation for this finding is that it may reflect the effects of AD in this sample, since African Americans also had relatively high APOE ε4 prevalence. However, given the higher rate of cerebrovascular disease often reported in African Americans (Howard, 2013), it is somewhat surprising that WMH was not an independent predictor of cognitive decline in the current cohort.
In contrast to the significant effects described above, there were many ways in which the three groups did not differ. For example, in univariable analyses, atrophy in most areas of cortical gray matter was an important predictor of cognitive decline (Table 4). Similarly, the demographic (age, education, and gender), clinical (recruitment source) and genetic (APOE genotype) covariates did not exert differential effects on cognitive intercepts or slopes across the three ethnoracial groups (Table 3).
Two variables were found to exert a differential influence on cognitive change across groups using univariable analyses, but not using multivariable analyses: baseline global gray matter volume and hippocampal volume. In the univariable analyses (Table 4), baseline global gray matter volume was a significant predictor of cognitive decline in each group, with the largest effect observed in the White group and the smallest effect in the African American group. For baseline hippocampal volumes, the effect on cognitive decline was largest in the White group and smallest in the Hispanic group. Although this effect was numerically larger in the African American group compared to the Hispanic group, it was significant in the Hispanic group but not the African American group.
Only one baseline measure, WMH, was found to differentially affect subsequent cognitive decline when multivariable results were considered; this variable influenced Hispanics and Whites to a much greater degree than African Americans. None of the baseline regional cortical volume measurements were found in multivariable analyses to differentially affect rate of change in cognitive functioning over time, despite the fact that a few of these variables were differentially associated with change in univariable analyses. This pattern of results suggests that although baseline regional cortical gray matter effects are observable in univariable models, these effects on cognitive decline are largely consistent across ethnoracial group. This suggests that any differences that may exist between African Americans, Hispanics, and Whites in terms of the pathogenesis of anterograde cognitive decline are related to subsequent cortical volume changes and not gray matter integrity at a static baseline.
Although the current results are useful for determining similarities and differences in the brain-behavior relationships underlying cognitive decline in diverse populations, conclusions about underlying pathophysiological mechanisms for brain changes must be considered tentative. Ethnoracial groups can differ in many ways – such as genetics (e.g., APOE ε4 allele frequency), quality of education, early life experiences, occupational history, diet and nutritional status, medical risk factors, access to health care, socioeconomic status, smoking, and leisure activities. We did not directly measure the presence of neurodegenerative disease processes such as CSF AD biomarkers or PET imaging of brain amyloid. Consequently, the current data are limited with respect to identifying the exact reasons for the observed ethnoracial differences in how brain structure changes affect cognitive decline.
Another limitation of this study is that the sample size limits statistical power for detecting ethnoracial group differences in brain-cognition effects. This, for example, might account for the seeming contradiction that specific temporal lobe change did not differ across groups, but was significantly different from zero only in Whites. Another complication in the results is that variables that were significant predictors of cognitive change when entered separately were not independently related when entered in a multivariable model. However, while the variables that did emerge as significant incremental predictors in the final multivariable model may not be the only predictors of cognitive decline, they are likely the most salient variables. These results should also be interpreted with the understanding that availability of follow-up MRI scans differed across the three ethnoracial groups. MRI measures of brain change were available for 53% of African Americans, 66% of Whites, and 70% of Hispanics, which may introduce some bias into the longitudinal brain measurement data. Specifically, statistical power would likely be less for detecting effects in the African American group. Finally, the results presented in Table 2 show that a global slope provided a better fit for the data than separate slopes. This approach did not allow us to examine associations between brain changes and differential patterns of cognitive decline across the four cognitive domains measured by the SENAS.
The above caveats notwithstanding, the current results do help to shed light on important ethnoracial differences that can serve as catalysts for future research. For instance, the effects of WMH on cognitive decline may represent a modifiable risk factor that could be targeted through interventions aimed at reducing rates of type 2 diabetes and cerebrovascular disease. This would be especially important if future research is able to clarify group-specific differences in the pathogenetic mechanisms for WMH, such as blood glucose dysregulation, hypertension, inflammation, or AD pathology. These results also suggest that clinical trials of AD pharmacotherapies should be especially mindful of diversity when enrolling participants and monitoring outcomes. When enrolling individuals of Hispanic ethnicity into clinical trials targeting AD pathologies, there may be an increased likelihood of including participants whose cognitive decline is mediated by non-AD pathologies that cause WMH. Finally, these results also highlight the need for future research to investigate the upstream mechanisms responsible for the group differences reported here.
These results support previous research that dissociates the effects of brain-based and non-brain-based variables on baseline cognitive status and rate of cognitive decline (Early et al., 2013; Fletcher et al., 2017; Gross et al., 2015; Mungas et al., 2009). At baseline, brain disease accounts for a smaller percentage of the variation in cognitive status than non-brain disease factors, such as education, early life experiences, and socioeconomic status. In contrast, cognitive decline is less influenced by non-brain factors and more influenced by progressive brain changes. Our findings also provide evidence that ethnoracial group differences exist in the relationships between MRI-based baseline and longitudinal indicators of brain health and changes in cognitive functioning. These differences suggest that the pathogenesis of cognitive decline may vary by race and ethnicity. This implies that race and ethnicity may serve as proxies for fixed (e.g., APOE genotype) and modifiable (e.g., cardiovascular disease risk and metabolic) factors affecting cognitive decline and dementia.
Public Significance Statement.
Dementia in older adults is driven by systematic brain changes. However, the associations between changes in brain structure and cognitive decline are not uniform across ethnic and racial groups. Uniquely salient associations between brain structure and cognitive decline were found for global gray matter volume changes in African Americans, baseline white matter hyperintensities for Hispanics, and baseline white matter hyperintensities, global gray matter volume changes, and regional temporal lobe volume changes in Whites.
Acknowledgments
This work was supported by multiple grants from the National Institute on Aging (NIA) (P30 AG10129, R01 AG021028, and R01 AG047827 C DeCarli, PI; R01 AG10220, D Mungas, PI; R01 AG031563 B Reed/D Mungas, PI; R01 AG031252 S Tomaszewski Farias, PI). Analysis and manuscript development were supported by a NIA Resource Centers for Minority Aging Research grant (P30 AG043097, L Hinton, PI).
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. doi: 10.1007/BF02294359. [DOI] [Google Scholar]
- Aljabar P, Heckemann R, Hammers A, Hajnal J, Rueckert D. Multi-atlas based segmentation of brain images: Atlas selection and its effect on accuracy. NeuroImage. 2009;46(3):726–738. doi: 10.1016/j.neuroimage.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging and Alzheimer’s Association workgroup. Alzheimer’s & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bollen KA, Curran PJ. Latent Curve Models: A Structural Equation Perspective. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2006. [DOI] [Google Scholar]
- Brickman AM. Contemplating Alzheimer’s disease and the contribution of white matter hyperintensities. Current Neurology and Neuroscience Reports. 2013;13(12):415. doi: 10.1007/s11910-013-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui HC, Ramirez-Gomez L. Clinical and imaging features of mixed Alzheimer and vascular pathologies. Alzheimer’s Research & Therapy. 2015;7:21. doi: 10.1186/s13195-015-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane PK, Narasimhalu K, Gibbons LE, Pedraza O, Mehta KM, Tang Y, Mungas DM. Composite scores for executive function items: Demographic heterogeneity and relationships with quantitative magnetic resonance imaging. Journal of the International Neuropsychological Society. 2008;14(5):746–59. doi: 10.1017/S1355617708081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): Exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Reed BR, Jagust W, Martinez O, Ortega M, Mungas D. Brain behavior relationships among African Americans, whites, and Hispanics. Alzheimer Disease and Associated Disorders. 2008;22(4):382–391. doi: 10.1097/WAD.0b013e318185e7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Venegas C, Downer B, Langa KM, Wong R. Racial and ethnic differences in cognitive function among older adults in the USA. International Journal of Geriatric Psychiatry. 2016;31(9):1004–1012. doi: 10.1002/gps.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early DR, Widaman KF, Harvey D, Beckett L, Park LQ, Farias ST, Mungas D. Demographic predictors of cognitive change in ethnically diverse older persons. Psychology and Aging. 2013;28(3):633–645. doi: 10.1037/a0031645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DA, Bennett DA, Wilson RS, Bienias JL, Morris MC, Scherr PA, Schneider J. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Archives of Neurology. 2003;60(2):185–9. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- Fletcher E. Using prior information to enhance sensitivity of longitudinal brain change computation. In: Chen CH, editor. Frontiers of Medical Imaging. Hackensack, NJ: World Scientific; 2014. pp. 63–81. [Google Scholar]
- Fletcher E, Gavett BE, Harvey D, Farias S, Olichney J, Beckett L, Mungas D. Brain volume change and cognitive trajectories. 2017 doi: 10.1037/neu0000447. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Knaack A, Singh B, Lloyd E, Wu E, Carmichael O, Alzheimer’s Disease Neuroimaging Initiative Combining boundary-based methods with tensor-based morphometry in the measurement of longitudinal brain change. IEEE Transactions on Medical Imaging. 2013;32(2):223–236. doi: 10.1109/TMI.2012.2220153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Singh B, Harvey D, Carmichael O, DeCarli C. 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Vol. 2012. IEEE; 2012. Adaptive image segmentation for robust measurement of longitudinal brain tissue change; pp. 5319–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Villeneuve S, Maillard P, Harvey D, Reed B, Jagust W, DeCarli C. β-amyloid, hippocampal atrophy and their relation to longitudinal brain change in cognitively normal individuals. Neurobiology of Aging. 2016;40:173–180. doi: 10.1016/j.neurobiolaging.2016.01.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Bogardus ST, Inouye SK. Dementia and race: Are there differences between African Americans and Caucasians? Journal of the American Geriatrics Society. 2001;49(4):477–84. doi: 10.1046/j.1532-5415.2001.49096.x. [DOI] [PubMed] [Google Scholar]
- Goldstein FC, Zhao L, Steenland K, Levey AI. Inflammation and cognitive functioning in African Americans and Caucasians. International Journal of Geriatric Psychiatry. 2015;30(9):934–941. doi: 10.1002/gps.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootjes L, Teipel SJ, Zebuhr Y, Schwarz R, Leinsinger G, Scheltens P, Hampel H. Regional distribution of white matter hyperintensities in vascular dementia, Alzheimer’s disease and healthy aging. Dementia and Geriatric Cognitive Disorders. 2004;18(2):180–188. doi: 10.1159/000079199. [DOI] [PubMed] [Google Scholar]
- Gross AL, Mungas DM, Crane PK, Gibbons LE, MacKay-Brandt A, Manly JJ, Jones RN. Effects of education and race on cognitive decline: An integrative study of generalizability versus study-specific results. Psychology and Aging. 2015;30(4):863–880. doi: 10.1037/pag0000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: The influence of type 2 diabetes mellitus, stroke and genetic factors. Journal of the American Geriatrics Society. 2003;51(2):169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- Hinton L, Carter K, Reed BR, Beckett L, Lara E, DeCarli C, Mungas D. Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Disease & Associated Disorders. 2010;24(3):1. doi: 10.1097/WAD.0b013e3181c1ee01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ, Greenberg SM. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39(4):1127–1133. doi: 10.1161/STROKEAHA.107.497438.Spatial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard VJ. Reasons underlying racial differences in stroke incidence and mortality. Stroke. 2013;44(6 Suppl 1):S126–8. doi: 10.1161/STROKEAHA.111.000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Gamboa A, Vintimilla R, Cheatwood AJ, Grant A, Trivedi A, O’Bryant SE. Comorbid depression and diabetes as a risk for mild cognitive impairment and Alzheimer’s disease in elderly Mexican Americans. Journal of Alzheimer’s Disease. 2015;47(1):129–136. doi: 10.3233/JAD-142907. [DOI] [PubMed] [Google Scholar]
- Jordanova V, Stewart R, Davies E, Sherwood R, Prince M. Markers of inflammation and cognitive decline in an African-Caribbean population. International Journal of Geriatric Psychiatry. 2007;22(10):966–973. doi: 10.1002/gps.1772. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Lancaster JL, Thompson P, Woods R, Mazziotta J, Hardies J, Fox P. Regional spatial normalization: toward an optimal target. Journal of Computer Assisted Tomography. 2001;25(5):805–16. doi: 10.1097/00004728-200109000-00023. [DOI] [PubMed] [Google Scholar]
- Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: A systematic review. Ethnicity and Disease. 2007;17(1):143–152. [PubMed] [Google Scholar]
- Lee DY, Fletcher E, Martinez O, Zozulya N, Kim J, Tran J, DeCarli C. Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and Alzheimer disease. Stroke. 2010;41(8):1791–1797. doi: 10.1161/STROKEAHA.110.582163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SY, Glymour MM, Zahodne LB, Weiss C, Manly JJ. Role of place in explaining racial heterogeneity in cognitive outcomes among older adults. Journal of the International Neuropsychological Society. 2015;21(9):677–687. doi: 10.1017/S1355617715000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda ER, Haan MN, Kanaya AM, Yaffe K, Neuhaus J. Type 2 diabetes and 10-year risk of dementia and cognitive impairment among older Mexican Americans. Diabetes Care. 2013;36(9):2600–2606. doi: 10.2337/dc12-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose RJ, Brewster P, Marquine MJ, MacKay-Brandt A, Reed B, Farias ST, Mungas D. Early life development in a multiethnic sample and the relation to late life cognition. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2015;70(4):519–531. doi: 10.1093/geronb/gbt126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, Thompson PM. Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer’s disease, mild cognitive impairment, and elderly controls. NeuroImage. 2009;45(1 Suppl):S3–15. doi: 10.1016/j.neuroimage.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas DM, Reed BR, Marshall SC, González HM. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology. 2000;14(2):209–23. doi: 10.1037/0894-4105.14.2.209. [DOI] [PubMed] [Google Scholar]
- Mungas D, Beckett L, Harvey D, Tomaszewski Farias S, Reed B, Carmichael O, DeCarli C. Heterogeneity of cognitive trajectories in diverse older persons. Psychology and Aging. 2010;25(3):606–619. doi: 10.1037/a0019502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Crane PK, Haan MN, González H. Spanish and English Neuropsychological Assessment Scales (SENAS): Further development and psychometric characteristics. Psychological Assessment. 2004;16(4):347–359. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Farias ST, DeCarli C. Age and education effects on relationships of cognitive test scores with brain structure in demographically diverse older persons. Psychology and Aging. 2009;24(1):116–128. doi: 10.1037/a0013421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Haan MN, González H. Spanish and English Neuropsychological Assessment Scales: Relationship to demographics, language, cognition, and independent function. Neuropsychology. 2005a;19(4):466–475. doi: 10.1037/0894-4105.19.4.466. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Tomaszewski Farias S, DeCarli C. Criterion-referenced validity of a neuropsychological test battery: Equivalent performance in elderly Hispanics and non-Hispanic Whites. Journal of the International Neuropsychological Society. 2005b;11(5):620–30. doi: 10.1017/S1355617705050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Widaman KF, Reed BR, Tomaszewski Farias S. Measurement invariance of neuropsychological tests in diverse older persons. Neuropsychology. 2011;25(2):260–9. doi: 10.1037/a0021090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L, Muthén B. Mplus User’s Guide. Los Angeles: Muthén & Muthén; 1998–2015. [Google Scholar]
- Newman DA. Longitudinal modeling with randomly and systematically missing data: A simulation of ad hoc, maximum likelihood, and multiple imputation techniques. Organizational Research Methods. 2003;6(3):328–362. doi: 10.1177/1094428103254673. [DOI] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Hall JR, Cukrowicz KC, Edwards M, Johnson LA, Lefforge D, Dentino A. The differential impact of depressive symptom clusters on cognition in a rural multi-ethnic cohort: A Project FRONTIER study. International Journal of Geriatric Psychiatry. 2011;26(2):199–205. doi: 10.1002/gps.2514. [DOI] [PubMed] [Google Scholar]
- O’Bryant SE, Johnson LA, Balldin V, Edwards M, Barber R, Williams B, Hall J. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer’s disease. Journal of Alzheimer’s Disease. 2013a;33(2):373–379. doi: 10.3233/JAD-2012-121420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Johnson LA, Reisch J, Edwards M, Hall J, Barber R, Singh M. Risk factors for mild cognitive impairment among Mexican Americans. Alzheimer’s & Dementia. 2013b;9(6):622–631. doi: 10.1016/j.jalz.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Xiao G, Barber R, Reisch J, Doody R, Fairchild T, Diaz-Arrastia R. A serum protein-based algorithm for the detection of Alzheimer disease. Archives of Neurology. 2010;67(9):1077–1081. doi: 10.1001/archneurol.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Xiao G, Zhang F, Edwards M, German DC, Yin X, Grammas P. Validation of a serum screen for Alzheimer’s disease across assay platforms, species, and tissues. Journal of Alzheimer’s Disease. 2014;42(4):1325–1335. doi: 10.3233/JAD-141041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert D, Aljabar P, Heckemann RA, Hajnal JV, Hammers A. Diffeomorphic registration using B-splines. Springer; Berlin, Heidelberg: 2006. pp. 702–709. [DOI] [PubMed] [Google Scholar]
- Satorra A, Bentler PM. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66(4):507–514. doi: 10.1007/BF02296192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman N, Llabre M, Cowie CC, Barnhart J, Carnethon M, Gallo LC, Avilés-Santa ML. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Diabetes Care. 2014;37(8):2233–2239. doi: 10.2337/dc13-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Matsumoto S, Kmiecik J, Studholme C, Du A, Ezekiel F, Weiner MW. Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimer’s & Dementia. 2009;5(6):454–62. doi: 10.1016/j.jalz.2009.04.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. The Annals of Statistics. 1978;6(2):461–464. doi: 10.1214/aos/1176344136. [DOI] [Google Scholar]
- Sclove SL. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika. 1987;52(3):333–343. doi: 10.1007/BF02294360. [DOI] [Google Scholar]
- Sisco S, Gross AL, Shih RA, Sachs BC, Glymour MM, Bangen KJ, Manly JJ. The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2015;70(4):557–567. doi: 10.1093/geronb/gbt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/WNL.56.1.49. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Nordberg A, Petersen RC. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Windham BG, Simpson BN, Lirette S, Bridges J, Bielak L, Peyser PA, Mosley TH. Associations between inflammation and cognitive function in African Americans and European Americans. Journal of the American Geriatrics Society. 2014;62(12):2303–2310. doi: 10.1111/jgs.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67(12):2192–8. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Narkhede A, Griffith EY, DeCarli C, Schupf NS, Brickman AM. Structural MRI predictors of late-life cognition differ across African Americans, Hispanics, and Whites. Current Alzheimer research. 2015;12(7):632–9. doi: 10.2174/1567205012666150530203214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Stern Y, Manly JJ. Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology. 2015;29(4):649–657. doi: 10.1037/neu0000141. [DOI] [PMC free article] [PubMed] [Google Scholar]


