Abstract
Introduction
The new Kidney Allocation System (KAS) prioritizes patients based on date of dialysis initiation or wait-listing, whichever is earlier. We hypothesized that this change would increase transplant rates for patients with prolonged pre-transplant dialysis times (DT) and aimed to assess the impact of prolonged DT on post-transplant outcomes
Methods
We used United Network for Organ Sharing registry data to assess outcomes for patients added to the renal transplant waitlist from Jan 1 1998 – Dec 31 2010 and patients transplanted from Jan 1 1998 – Dec 3 2012.
Results
Compared with patients transplanted preemptively, patients with <5 years, 5–9 years, and ≥10 years DT had progressively decreased graft and patient survival (p<0.001). The rates of short-term complications including delayed graft function, graft loss within 30 days, and patient death within 30 days were significantly higher in cohorts with ≥10 years DT than in cohorts with less DT (p<0.001).
Conclusions
Patients with pre-transplant DT of ≥10 years had worse outcomes than patients pre-emptively transplanted or transplanted with shorter DT. Durations of dialysis dependence beyond 10 years were associated with further deterioration in short-term but not long-term post-transplant outcomes.
Keywords: Dialysis, end stage renal disease, graft failure, graft survival, kidney transplantation
Introduction
Kidney transplant offers a survival benefit compared to dialysis (1, 2), but there are substantial socioeconomic and racial disparities in access to transplantation (3–6). Multiple studies have demonstrated that African-American, female, lower income, and less well-educated patients are referred to the waiting list later than other patients (3–9). The new Kidney Allocation System (KAS) was introduced on December 4, 2014 in part to mitigate such disparities. The new KAS credits waitlist time from chronic dialysis initiation or date of waitlisting, whichever is earlier (10, 11). As a result, patients with multiple years of dialysis time (DT) whose referral for transplant had been delayed gained additional waitlist priority vs. the prior allocation system. In concert with new priority for highly-sensitized (panel reactive antibody [PRA] ≥ 98%) patients (another major modification of the allocation system), recalculated waitlist time resulted in a “reordering” of the waitlist and patients with delayed referral to transplantation gaining improved access to organs (12).
Pre-emptive transplantation before the initiation of dialysis is associated with graft survival benefit compared to transplantation after the initiation of dialysis (13–15). Several studies have demonstrated a dose-response relationship between duration of dialysis and adverse post-transplant outcomes, but they focused their analysis on periods of dialysis of <6 years (16–22). Notably, two of these studies concluded that a long interval of dialysis exposure prior to waitlisting was, compared to the interval of time after waitlisting, a stronger predictor of death after transplant (21, 22). This difference was attributed to time to waitlisting reflecting not only the risk of dialysis but also access to healthcare and degree of comorbidity. Prior work on the effect of prolonged DT prior to transplantation was derived from single center studies and reached contradictory conclusions (23, 24).
Given the absence of clear data on the relationship between DT ≥10 years and transplant outcomes and the substantial changes in waitlist priority with KAS, we aimed to answer three questions. First, how frequently did patients with prolonged DT (≥10 years) receive deceased donor renal transplants in the era before the implementation of the new KAS and how did this frequency compare to patients with less substantial dialysis exposure? Second, is dialysis exposure longer than 10 years associated with worse post-transplant outcomes? Finally, to what extent is prolonged dialysis exposure for waitlisted patients the result of difficulty finding compatible organs for highly sensitized patients? These questions are crucial to contextualize the assessment of patterns of organ use and post-transplant outcomes following the implementation the new KAS.
Patients and Methods
Data Source
Analyses were conducted using a Standard Transplant Analysis and Research (STAR) file provided by the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network (OPTN).
Study Populations
The study included two cohorts of patients. The first, the waitlist cohort, included patients ≥ 18 years of age who were placed on the renal transplant waitlist from Jan 1 1998 – Dec 31 2010. Patients listed as receiving renal replacement therapy but missing the date of dialysis initiation were excluded. Prior renal transplant recipients being relisted or re-transplanted were excluded to avoid misclassification of their degree of dialysis exposure over multiple listings. Multi-organ transplant candidates were excluded because allocation occurs through separate policy. The second, the transplant cohort, included patients ≥ 18 years of age who a received renal transplant between Jan 1 1998 – Dec 3 2015. Patients listed as receiving renal replacement therapy but missing the date of dialysis initiation were again excluded. Patients with history of prior renal transplant or receiving multiple-organs were excluded for the reasons listed above. Living donor renal transplant recipients were excluded in the transplant cohort because the majority are directed rather than distributed through the allocation system.
Analytic Approach
Outcomes of renal transplant candidates were analyzed within the waitlist cohort. The primary outcomes were 1) transplantation and 2) death or delisting. The primary exposure was DT at waitlist registration, calculated as the difference between the date of dialysis initiation and the date of placement on the waitlist. Patients were followed from date of registration on the waitlist until transplant, death or delisting, or the end of follow-up (Dec 3 2014). The ending date was selected to ensure at least four years of follow-up data prior to the initiation of the new KAS (Dec 4 2014). A multivariable model for the outcome from waitlisting was fit using Fine -Grey competing risk regression (25). Variables included major determinants of access to transplantation including blood group and UNOS-defined geographic region.
Outcomes of kidney transplant recipients were analyzed within the transplant cohort using patients who received renal allografts between Jan 1 1998 – Dec 31 2012. The primary outcome was graft survival defined as time from initial transplant to initiation of maintenance renal replacement therapy, retransplant, or death (i.e. all-cause allograft failure). The mortality outcome is determined by the SRTR from reports from transplant centers as well as verified external sources, such as the Social Security Administration Death Master File (26). The secondary outcome was patient survival. The primary exposure was DT at transplant, calculated as the difference between the date of dialysis initiation and the date of transplantation. To categorize patients based on DT, 3-year graft survival was graphically inspected by years of DT (Supplemental Figure 1). Because the data did not reveal clear cutpoints, patients were stratified by DT in 5 years increments to ensure sufficient numbers for analysis in each category. Patients were followed from date of transplant until death or end of follow-up (Mar 31 2016). The ending date was selected to insure at least three years of follow-up data on transplantation outcomes. Subjects were censored if they had a functioning graft at the end of follow-up or at 12.5 years.
Post-transplant graft or patient survival was examined using Kaplan-Meier curves and compared using a log-rank test for equality of survivor functions. A multivariable model for graft failure was fit using Cox regression. The proportional hazards assumption was confirmed with graphical inspection of log-log plots. We adjusted for recipient, donor and allograft characteristics that were selected based on prior literature and clinical judgment (27–33). Covariates included gender, age, ethnicity, PRA, etiology of end stage renal disease (ESRD), KDPI (calculated from the 2013 reference population), degree of human leukocyte antigen (HLA) mismatch, diabetes status (as reported on transplant candidate registrations), and hepatitis C virus (HCV) status (as reported on transplant recipient registrations).
Finally, the transplant cohort was used to compared the distribution of deceased donor renal allografts to patients with different DT in the year before KAS (Dec 4 2013 – Dec 3 2014) and the year after KAS (Dec 4 2014 – Dec 3 2015) implementation. The primary exposure was DT at transplantation, again calculated from the difference between the date of dialysis initiation and the date of transplant. All analyses have follow-up data until Mar 31 2016.
Statistical analyses were performed with STATA SE for MAC OS X version 14.2 (StataCorp LP, College Station, TX). Continuous variables were compared between groups using the Student’s t test or Analysis of Variance, as appropriate. Categorical variables were compared using χ2 or the Fisher exact test, as appropriate.
Missing data
Two covariates had data missing from >1% of patients: PRA (46% in the waitlist cohort and 28% in the transplant cohort) and HCV status (8% in the transplant cohort). For each of these variables, sensitivity analyses were performed where patients with missing data were first assigned to the lowest category (0% PRA and HCV negative, respectively) and then to the highest category (98–100% PRA and HCV positive, respectively). For final analysis, multiple imputation was used to generate PRA, and HCV status values for individuals with missing values. PRA data was more complete in the year before and the year after KAS implementation with <1% of patients missing data.
Results
Impact of Dialysis Time on Likelihood of Renal Transplantation: Waitlist Cohort
Between Jan 1 1998 – Dec 31 2010, a total of 257,551 patients were added to the renal transplant waitlist and met criteria for inclusion. The majority of patients had either not started dialysis (n=65,972, 26%) or had been on dialysis for <5 years (n=175,893, 71%, Table 1). Five percent (n=12,953) had 5–9 years of DT, 1% (n=2,016) had 10–14 years, 0.2% (n=462) had 15–19 years, and 0.1% (n=262) had ≥20 years of dialysis at the time of listing. High PRA (≥86%) patients were relatively more frequent among patients with DT ≥10 years (p<0.01, Supplemental Table 1), although they composed <10% of registered patients in all cohorts and a substantial number of records were missing PRA data.
Table 1.
Outcome of Waitlisted Patients by Length of Dialysis at Registration*
| Outcome | No Dialysis | <5 yr | 5–9 yr | 10–14 yr | 15–19 yr | ≥20 yr | p |
|---|---|---|---|---|---|---|---|
|
| |||||||
| n=65,968 | n=175,890 | n=12,953 | n=2,016 | n=462 | n=262 | ||
| Deceased Donor Transplant | 25,998 (39%) | 75,488 (43%) | 6,021(47%) | 909 (45%) | 185 (40%) | 101 (39%) | |
| Living Donor Transplant | 19,147 (29%) | 22,839 (13%) | 691 (5%) | 96 (5%) | 23 (5%) | 23 (9%) | <0.001 |
| Death or Delisted | 17,869 (27%) | 71,149 (40%) | 5,851 (45%) | 943 (47%) | 230 (50%) | 134 (51%) | |
| Waitlisted | 2,954 (5%) | 6,414 (4%) | 390 (3%) | 68 (3%) | 23 (5%) | 4 (2%) | |
Patients were followed from date of registration on the waitlist until transplant, death or delisting, or the end of follow-up (Dec 3 2014)
As of the conclusion of the period of analysis (31 Mar 2016), pre-emptively listed patients were significantly more likely to have received a transplant than patients on dialysis at the time of listing (68% vs. 55%, p <0.001, Table 1). The difference in likelihood of receiving a living donor transplant was particularly pronounced at 29% in pre-emptively listed patients and 12% in those on dialysis (p<0.001). Pre-emptively listed patients were also significantly less likely to have died or been delisted without transplant (27% vs. 41%, p<0.001). As DT at listing increased, patients were progressively less likely to have received a transplant and progressively more likely to have died or been delisted by the end of follow-up. In competing risk regression analysis with death or delisting categorized as a competing risk, increasing durations of dialysis dependence were associated with decreased subhazard ratios (SHR) of receiving a transplant (Supplemental Table 2). This association persisted after adjusting for blood group and UNOS-defined geographic region. PRA was omitted from this model because of the degree of missing data, but, in sensitivity analyses, PRA did not substantially impact the SHR associated with duration of pre-registration dialysis dependence.
Outcomes after Transplant by Pre-Operative Dialysis Time: Transplant Cohort
Between Jan 1 1998 and Dec 31 2012, 109,079 patients received a renal transplant and met criteria for analysis. Demographic characteristics by pre-transplant DT are summarized in Table 2. The cohorts of patients with longer DT were younger, had a lower prevalence of diabetes, and had a higher prevalence of female gender, black ethnicity, high PRA, and hepatitis C infection (p<0.001). The greater sensitization in long DT cohorts occurred primarily due to increased frequencies of patients in the 21–85% PRA range; increases in the 86–97% and 98–100% PRA categories also occurred with longer DT but the magnitude of the increase was smaller. These results must be viewed in the context of missing PRA data for 26% of patients. The kidney donor profile index (KDPI) of transplanted organs was higher in patients on dialysis compared to those with no dialysis exposure but the magnitude of the difference was small (p<0.001).
Table 2.
Demographic and Clinical Characteristics of 109,097 Deceased Donor Renal Transplant Recipients, Categorized by Length of Dialysis Dependence
| Characteristic | No Dialysis | <5 yr | 5–9 yr | 10–14 yr | 15–19 yr | ≥20 yr | p |
|---|---|---|---|---|---|---|---|
|
| |||||||
| n=10,360 | n=72,723 | n=22,894 | n=2,473 | n=451 | n=178 | ||
| Age, median (IQ range) | 55 (46–63) | 54 (43–62) | 52 (42–60) | 49 (40–58) | 49 (40–57) | 51 (38–64) | |
| Age, n (%) | |||||||
| 18–25 | 322 (3%) | 2,718 (4%) | 464 (2%) | 18 (0.8%) | 6 (1%) | 2 (1%) | |
| 26–40 | 1,326 (13%) | 11,763 (16%) | 4,534 (20%) | 625 (26%) | 108 (24%) | 22 (13%) | <0.001 |
| 41–60 | 4,739 (46%) | 33,132 (46%) | 11,103 (49%) | 1,241 (52%) | 248 (56%) | 111 (64%) | |
| >60 | 3,819 (37%) | 23,920 (33%) | 6,350 (28%) | 522 (22%) | 80 (18%) | 39 (22%) | |
| Male, n (%) | 5,603 (54%) | 44,881 (62%) | 13,765 (60%) | 1,081 (44%) | 186 (41%) | 81 (45%) | <0.001 |
| Ethnicity, n (%) | |||||||
| White | 7,036 (68%) | 37,137 (51%) | 5,922 (26%) | 476 (19%) | 94 (21%) | 62 (35%) | |
| Black | 1,960 (19%) | 20,872 (29%) | 10,171 (44%) | 1,339 (54%) | 224 (50%) | 82 (46%) | <0.001 |
| Other | 1,364 (13%) | 14,710 (20%) | 6,801 (30%) | 658 (27%) | 133 (30%) | 34 (19%) | |
| Etiology of ESRD, n (%) | |||||||
| Diabetes | 2,027 (20%) | 20,522 (28%) | 5,565 (24%) | 287 (12%) | 14 (3%) | 18 (10%) | |
| Hypertension | 2,012 (19%) | 17,337 (24%) | 7,348 (32%) | 941 (39%) | 155 (35%) | 50 (28%) | |
| Glomerulonephritis | 602 (6%) | 4,512 (6%) | 1,488 (7%) | 205 (8%) | 51 (12%) | 28 (16%) | <0.001 |
| Polycystic Kidney Disease | 1,723 (17%) | 6,734 (9%) | 1,353 (6%) | 153 (6%) | 29 (7%) | 14 (8%) | |
| Other | 3,988 (39%) | 23,559 (32%) | 7,018 (31%) | 860 (35%) | 191 (43%) | 66 (38%) | |
| PRA, % (n) | |||||||
| 0–20 | 6,218 (60%) | 45,418 (663%) | 11,896 (52%) | 1,103 (45%) | 192 (43%) | 75 (42%) | |
| 21–85 | 801 (8%) | 6,142 (9%) | 2,506 (11%) | 325 (13%) | 61 (14%) | 33 (19%) | |
| 86–97 | 203 (2%) | 1,571 (2%) | 644 (3%) | 85 (3%) | 25 (6%) | 6 (3%) | <0.001 |
| 98–100 | 73 (0.7%) | 785 (1%) | 412 (2%) | 77 (3%) | 10 (4%) | 9 (5%) | |
| Missing | 3,065 (30%) | 18,807 (26%) | 7,436 (33%) | 883 (36%) | 157 (35%) | 55 (31%) | |
| Zero HLA Mismatch, n (%) | 1,695 (16%) | 7,667 (11%) | 928 (4%) | 106 (4%) | 28 (6%) | 15 (8%) | <0.001 |
| KDPI, median (IQ range) | 44 (21–61) | 47 (23–71) | 48 (25–71) | 45 (23–68) | 46 (23–65) | 52 (25–75) | <0.001 |
| DM, n(%) | |||||||
| Yes | 2,780 (27%) | 25,851 (36%) | 6,906 (31%) | 386 (16%) | 26 (6%) | 26 (15%) | <0.001 |
| HCV, n (%) | |||||||
| Seropositive | 387 (4%) | 3,831 (6%) | 1,460 (7%) | 231 (10%) | 65 (15%) | 33 (20%) | <0.001 |
| Missing | 374 (4%) | 1,367 (2%) | 1459 (2%) | 52 (2%) | 11 (3%) | 5 (3%) | |
IQ: interquartile; ESRD: end stage renal disease; PRA: panel reactive antibody; KDPI: kidney donor profile index; DM: diabetes mellitus; HCV: hepatitis C virus.
The rates of delayed graft function (DGF), graft failure within 30 days, and patient death within 30 days were higher among groups with more years of DT (p<0.001, Table 3). In multivariate logistic regression analysis, longer DT remained associated with each of these three early complications (Supplemental Tables 3–5). In Kaplan-Meier analysis of long-term transplant outcomes, pre-emptively transplanted patients had superior graft and overall survival compared to all other groups (p<0.001, Figure 1). Patients with <5 years, 5–9 years and ≥10 years of DT had overlapping graft and overall survival curves. Substratifying further, compared to patients with 10–14 years DT, those with 15–19 years and ≥ 20 years DT had similar graft (p =0.86 and 0.60 respectively, Supplemental Figure 2A) and overall survival (p=0.40 and 0.06, respectively, Supplemental Figure 2B), although sample size was small.
Table 3.
Early Outcomes of 109,079 Deceased Donor Renal Transplant Recipients, Categorized by Length of Dialysis Dependence
| Characteristic | No Dialysis |
<5 yr | 5–9 yr | 10–14 yr | 15–19 yr | ≥20 yr | p |
|---|---|---|---|---|---|---|---|
|
| |||||||
| n=10,360 | n=72,723 | n=22,894 | n=2,473 | n=451 | n=178 | ||
| DGF, n (%) | 847 (8%) | 17,733 (24%) | 7,546 (33%) | 858 (35%) | 180 (40%) | 64 (36%) | <0.001 |
| Graft failure within 30 days, n (%) | 267 (3%) | 2,567 (4%) | 909 (4%) | 107 (4%) | 27 (6%) | 14 (8%) | <0.001 |
| Patient death within 30 days, n (%) | 87 (0.8%) | 958 (1%) | 374 (2%) | 39 (2%) | 8 (2%) | 3 (2%) | <0.001 |
Figure 1.
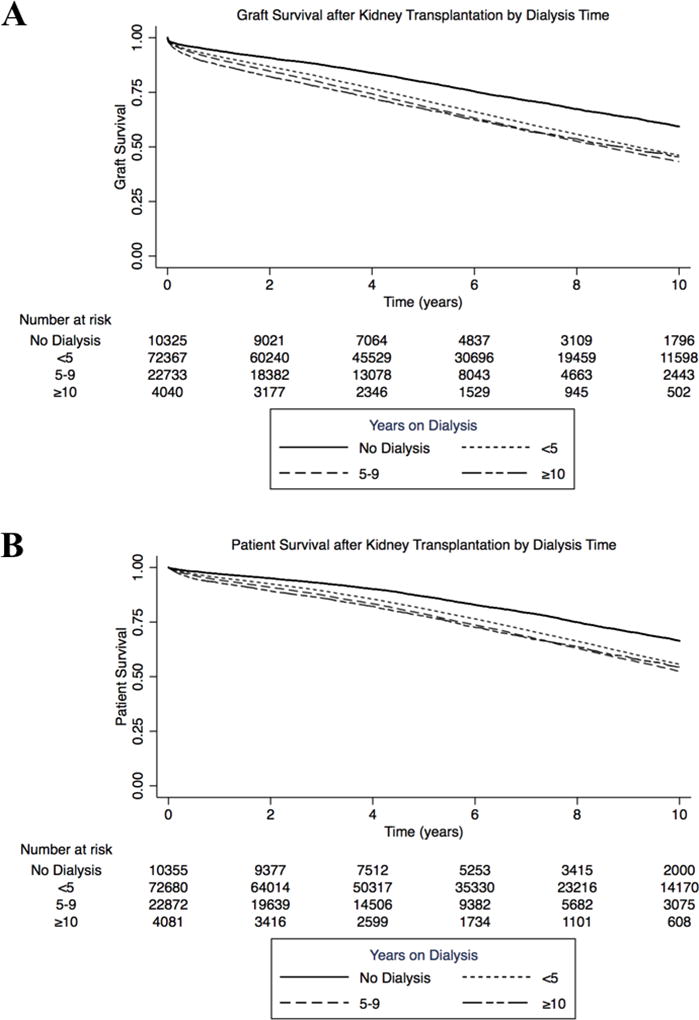
Renal allograft and overall survival by length of pre-transplant dialysis. A. Patients without dialysis exposure had superior graft survival to patients with any DT (p<0.001). B. Patients without dialysis exposure had superior overall survival to patients with any DT (p<0.001).
In multivariable Cox regression analysis, longer DT remained associated with progressively greater hazard of graft failure (Table 4). The magnitude of difference between cohorts was highest between the pre-emptive transplantation group and <5 years dialysis (HR 1.00 [ref] vs. 1.37 [1.34 – 1.41]) and the 5–9 years (HR 1.57 [1.52 −1.61]) groups. Longer duration of DT was associated with less pronounced increases in the HR with minimal differences between 10–14 years, 15–19 years, and ≥20 years groups (HR 1.76 [1.68 – 1.85] vs. 1.62 [1.46 – 1.81] vs. 1.60 [1.37 – 1.8] respectively with overlapping 95% C.I.).
Table 4.
Multivariable Cox regression model of impact of dialysis time on graft survival after deceased donor kidney transplantation
| HR (95% C.I.) | |||
|---|---|---|---|
| Parameter | Univariate | Demographic Adjustment | Full Adjustment |
| Dialysis Time | |||
| None | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| <5 years | 1.45 (1.42 – 1.39) | 1.47 (1.43 – 1.50) | 1.37 (1.34 – 1.41) |
| 5–9 years | 1.58 (1.54 – 1.62) | 1.65 (1.61 – 1.69) | 1.57 (1.52 – 1.61) |
| 10–14 years | 1.68 (1.61 – 1.76) | 1.79 (1.71 – 1.87) | 1.76 (1.68 – 1.85) |
| 15–19 years | 1.48 (1.35 – 1.63) | 1.64 (1.49 – 1.81) | 1.62 (1.46 – 1.81) |
| ≥20 years | 1.63 (1.41 – 1.88) | 1.72 (1.50 – 1.99) | 1.60 (1.37 – 1.88) |
| Male | 1.13 (1.11 – 1.14) | 1.08 (1.06 – 1.10) | |
| Age | |||
| 18–25 | 1.42 (1.36 – 1.47) | 1.64 (1.58 – 1.71) | |
| 26–40 | 1.00 (ref) | 1.06 (1.04 −1.09) | |
| 41–60 | 1.06 (1.04 – 1.08) | 1.00 (ref) | |
| >60 | 1.63 (1.60. – 1.66) | 1.36 (1.33 – 1.38) | |
| Ethnicity | |||
| White | 1.29 (1.27 – 1.31) | 1.44 (1.42 – 1.47) | |
| Black | 1.54 (1.51 – 1.56) | 1.48 (1.45 – 1.51) | |
| Other | 1.00 (ref) | 1.00 (ref) | |
| PRA | |||
| 0–20 | 1.00 (ref) | ||
| 21–85 | 1.05 (1.03 – 1..07) | ||
| 86–97 | 1.11 (1.06 – 1.15) | ||
| 98–100 | 1.13 (1.07 – 1.19) | ||
| Etiology of ESRD | |||
| Diabetes | 1.60 (1.55 – 1.67) | ||
| Hypertension | 1.59 (1.54 – 1.64) | ||
| Glomerulonephritis | 1.33 (1.28 – 1.38) | ||
| Polycystic Kidney Disease | 1.00 (ref) | ||
| Other | 1.40 (1.36 – 1.45) | ||
| KDPI | |||
| 1–25 | 1.00 (ref) | ||
| 26–50 | 1.17 (1.14 – 1.19) | ||
| 51–75 | 1.39 (1.37 – 1.42) | ||
| 76–99 | 1.89 (1.85 – 1.92) | ||
| Zero HLA Mismatch | 0.90 (0.88 – 0.92) | ||
| DM | 1.27 (1.24 – 1.31) | ||
| HCV | 1.28 (1.25 – 1.32) |
HR: hazard ratio; CI: confidence interval; PRA: panel reactive antibody; KDPI: kidney donor profile index; DM: diabetes mellitus; HCV: hepatitis C virus.
Changes in Renal Transplantation Patterns with new Kidney Allocation System
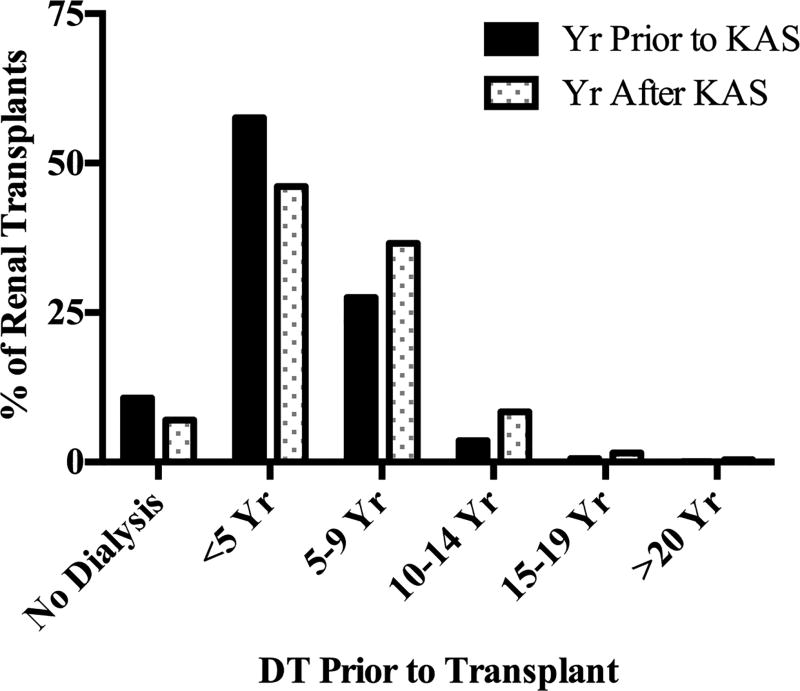
The total number of deceased donor kidney transplants was nearly identical in the year prior to and the year following implementation of KAS (9,036 and 9,031, respectively, Supplemental Table 6). However, patients with DT of 5–9 years, 10–14 years, 15–19 years, and ≥20 years received a substantially higher proportion of the allografts transplanted, and patients with no dialysis exposure or 1–4 years DT received a reduced share of transplants after KAS (p<0.001, Supplemental Table 6, Figure 2). The magnitude of the increase was greatest in patients with 5–9 years DT (28% to 37% of renal transplants, p<0.001). The same trend was observed comparing the year after KAS to any year in the decade before KAS implementation (Supplemental Table 6). Interestingly, among patients placed on the waitlist in the year prior to and the year following KAS implementation, the number with ≥10 years of dialysis time at time of registration was nearly identical (1% vs. 1%, p=0.37)
Figure 2.
Changes in Kidney Allocation by DT with KAS. In the first year following implementation of KAS, patients with DT of 5–9 years, 10–14 years, 15–19 years, and ≥20 years received a significantly higher proportion of the allografts transplanted and patients with no dialysis exposure or 1–4 years DT received a significantly reduced share of transplants compared to the year before implementation (p<0.01).
Highly sensitized patients (PRA 98–100%) received a significantly greater proportion of renal allografts in the year following KAS implementation compared to the year prior (7% vs. 2%, p<0.001, Supplemental Table 7). However, the increase in transplants to highly sensitized recipients accounted for only 7% for the total increase in transplants to patients with ≥10 years DT.
Discussion
Prolonged DT patients exemplify the challenge of balancing equity of access to transplantation with utility of organ allocation. The present study examining how patients with prolonged DT fared while before, during and after their transplantation adds several novel findings to this challenge. First, these results demonstrate that patients with ≥10 years of DT experience a much higher incidence of short-term post-transplant complications but do not have continued detrimental dose effects of DT on long-term graft function. Second, high PRA is infrequent in prolonged DT patients, suggesting that the primary causes of the accumulation of DT ≥ 10 are likely separate from the immunological challenge of HLA incompatibility. Finally, the study confirms that prolonged DT patient are gaining increased access to renal transplantation under the new KAS policy (12). These findings reveal that the KAS achieved gains in equity at limited cost to short-term utility.
Prior reports of post-transplant outcomes in patients with extended periods of dialysis dependence focused on patients with <6 years of DT (16–22). Patients with ≥10 years of pre-transplant dialysis dependence have not been discussed in prior literature or SRTR yearly reports, possibly because of the small number of transplants within this group before the KAS policy change. However, nearly 1000 kidney transplants annually have been performed in this cohort after KAS, and it is important to understand expected outcomes and ramifications for the transplant system. Among this group, we observed no decrements in long-term graft survival between those with 10–14, 15–19, and ≥20 years of DT in the pre-KAS era. The absence of dose effect within these groups likely reflects inherent selection of patients able to survive a prolonged dialysis burden and remain transplant candidates (i.e. unmeasured confounding). Transplant recipients with ≥10 years of DT had an almost 50% lower prevalence of diabetes (and by extrapolation, associated comorbidities) compared to recipients with <10 years of DT. Moreover, despite spending years on dialysis, recipients with ≥10 years of DT had a slightly younger age at the time of transplant, indicating younger age at the onset of renal failure and perhaps higher fitness at dialysis initiation.
However, short-term post-transplant complications—including DGF, graft failure within 30 days, and patient death within 30 days—were markedly elevated in patients with ≥10 years DT and increased between the 10–14 years, 15–19 years, and ≥20 years cohorts. This finding suggests that adverse perioperative events and residual unidentified comorbidities (e.g. vascular calcifications or heart disease) may be more common in prolonged DT patients. Careful cardiovascular screening and perioperative management, pre-operative vascular assessment, and assessment of the global health of waitlisted patients may help mitigate these early surgical complications (34–36).
Although our study examined only deceased donor transplants, the dual findings of detrimental effects of prolonged DT on short- and long-term post-transplant outcomes and the decreased renal allograft allocation to patients with no or <5 years of DT in the new KAS has important implications for living donor transplantation. As barriers to preemptive deceased donor transplants increase, living donor transplantation becomes an even more important as a source of preemptive transplants and as a way to secure the superior post-transplant outcomes that result from avoiding dialysis.
This study has several limitations. First, the group of prolonged DT patients who are listed-for or received renal transplantation are a highly selected group of patients. The multivariable analyses described in the paper only partially adjust for this inherent selection bias and the outcomes described may under-estimate the detrimental effects of prolonged DT in less rigorously selected patients. Important aspects of patient selection cannot be elucidated from SRTR data, as many long-term dialysis patients may never have been referred to transplant evaluation or may have been declined for listing and, in either case, would not appear in SRTR data. This absence limits analysis to candidates who at least were listed for transplant and may provide the most optimistic outcomes that could be expected from this cohort if selection practices significantly change. Second, this study does not identify the cause of the long dialysis times seen in some patients. Poor medical compliance, socioeconomic status, late referral, geography, distance to a transplant center, and lack of awareness of transplantation benefits may all reduce access to transplantation (37–40). These factors are not determinable using registry data but should motivate studies using more granular data. Moreover, these factors may impact post-transplant outcomes and may confound the analysis. Third, our analysis included incomplete records. We have addressed the missing data to estimate maximum effect of missing data on associations between study outcomes and the primary exposure of interest – dialysis time. Fourth, the long-term outcomes of patients transplanted after the new KAS implementation may be different from pre-KAS patients because of potential differences in patient selection between these periods. The new priority provides substantial incentive to transplant centers to list long DT patients; the prospect of rapidly securing an allograft for these patients may increase programmatic risk tolerance and willingness to list high-risk patients and may unmask differences in outcome that were not evident with prior patient selection patterns. Insufficient follow-up time is available to directly assess these possible impacts on center practice.
In conclusion, prolonged pre-operative dialysis exposure is associated with increased risk of graft loss, with dose effects up to 10 years of dialysis time. Prolonged pre-transplant dialysis exposure is also associated with markedly increased early post-transplant complications including DGF, graft failure within 30 days, and patient death within 30 days. The new KAS has increased access to renal transplantation for patients with prolonged pre-operative dialysis exposure with highly HLA sensitized patients comprising only a minority of the increase in prolonged DT transplants. Examinations of the effects of the new KAS on long-term graft outcomes must account for higher-risk recipients prioritized in the new allocation scheme.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01-DK106243 (PI: MHL) and by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- CI
confidence interval
- DGF
delayed graft function
- DM
diabetes mellitus
- DT
dialysis time
- ESRD
end stage renal disease
- HCV
hepatitis C virus
- HLA
human leukocyte antigen
- HR
hazard ratio
- IQ
interquartile
- KAS
kidney allocation system
- KDPI
kidney donor profile index
- OPTN
organ procurement and transplantation network
- PRA
panel reactive antibody
- SHR
subhazard ratio
- STAR
standard transplant analysis and research
- UNOS
united network for organ sharing
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to disclose. The results presented in this paper have not been published previously in whole or part, except in abstract form.
Authors’ Contributions
DDA, AWP, PLA, PPR, and MHL participated in research design.. DDA, DRM, SJC, PLA, DS, RDB, PPR, and MHL participated in writing the manuscript.. DDA, AWP, DRM, SJC, and MHL participated in performance of research. DDA, AWP, PLA, DS, RDB, PPR, and MHL participated in data analysis.
References
- 1.Vollmer WM, Wahl PW, Blagg CR. Survival with dialysis and transplantation in patients with end-stage renal disease. N Engl J Med. 1983;308(26):1553. doi: 10.1056/NEJM198306303082602. [DOI] [PubMed] [Google Scholar]
- 2.Schnuelle P, Lorenz D, Trede M, Van Der Woude FJ. Impact of renal cadaveric transplantation on survival in end-stage renal failure: evidence for reduced mortality risk compared with hemodialysis during long-term follow-up. J Am Soc Nephrol. 1998;9(11):2135. doi: 10.1681/ASN.V9112135. [DOI] [PubMed] [Google Scholar]
- 3.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA. 1998;280(13):1148. doi: 10.1001/jama.280.13.1148. [DOI] [PubMed] [Google Scholar]
- 4.Kasiske BL, London W, Ellison MD. Race and socioeconomic factors influencing early placement on the kidney transplant waiting list. J Am Soc Nephrol. 1998;9(11):2142. doi: 10.1681/ASN.V9112142. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT. Preemptive kidney transplantation: the advantage and the advantaged. J Am Soc Nephrol. 2002;13(5):1358. doi: 10.1097/01.asn.0000013295.11876.c9. [DOI] [PubMed] [Google Scholar]
- 6.Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche HU. Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol. 2011;6(7):1760. doi: 10.2215/CJN.08620910. [DOI] [PubMed] [Google Scholar]
- 7.Patzer RE, Plantinga LC, Paul S, Gander J, Krisher J, Sauls L, et al. Variation in Dialysis Facility Referral for Kidney Transplantation Among Patients With End-Stage Renal Disease in Georgia. JAMA. 2015;314(6):582. doi: 10.1001/jama.2015.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patzer RE, McClellan WM. Influence of race, ethnicity and socioeconomic status on kidney disease. Nat Rev Nephrol. 2012;8(9):533. doi: 10.1038/nrneph.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM. Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol. 2009;20(6):1333. doi: 10.1681/ASN.2008030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Israni AK, Salkowski N, Gustafson S, Snyder JJ, Friedewald JJ, Formica RN, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol. 2014;25(8):1842. doi: 10.1681/ASN.2013070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, et al. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant. 2015;(15 Suppl 2):1. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
- 12.Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, Aeder MI. Changes in Deceased Donor Kidney Transplantation One Year After KAS Implementation. Am J Transplant. 2016;16(6):1834. doi: 10.1111/ajt.13770. [DOI] [PubMed] [Google Scholar]
- 13.Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med. 2001;344(10):726. doi: 10.1056/NEJM200103083441004. [DOI] [PubMed] [Google Scholar]
- 14.Asderakis A, Augustine T, Dyer P, Short C, Campbell B, Parrott NR, et al. Pre-emptive kidney transplantation: the attractive alternative. Nephrol Dial Transplant. 1998;13(7):1799. doi: 10.1093/ndt/13.7.1799. [DOI] [PubMed] [Google Scholar]
- 15.Amaral S, Sayed BA, Kutner N, Patzer RE. Preemptive kidney transplantation is associated with survival benefits among pediatric patients with end-stage renal disease. Kidney Int. 2016;90(5):1100. doi: 10.1016/j.kint.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller MC, Kainz A, Baer H, Oberbauer R. Dialysis Vintage and Outcomes after Kidney Transplantation: A Retrospective Cohort Study. Clin J Am Soc Nephrol. 2016 doi: 10.2215/CJN.04120416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cosio FG, Alamir A, Yim S, Pesavento TE, Falkenhain ME, Henry ML, et al. Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int. 1998;53(3):767. doi: 10.1046/j.1523-1755.1998.00787.x. [DOI] [PubMed] [Google Scholar]
- 18.Meier-Kriesche HU, Port FK, Ojo AO, Rudich SM, Hanson JA, Cibrik DM, et al. Effect of waiting time on renal transplant outcome. Kidney Int. 2000;58(3):1311. doi: 10.1046/j.1523-1755.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 19.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002;74(10):1377. doi: 10.1097/00007890-200211270-00005. [DOI] [PubMed] [Google Scholar]
- 20.Helantera I, Salmela K, Kyllonen L, Koskinen P, Gronhagen-Riska C, Finne P. Pretransplant dialysis duration and risk of death after kidney transplantation in the current era. Transplantation. 2014;98(4):458. doi: 10.1097/TP.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 21.Harhay MN, Hill AS, Wang W, Even-Shoshan O, Mussell AS, Bloom RD, et al. Measures of Global Health Status on Dialysis Signal Early Rehospitalization Risk after Kidney Transplantation. PLoS One. 2016;11(6):e0156532. doi: 10.1371/journal.pone.0156532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Walraven C, Austin PC, Knoll G. Predicting potential survival benefit of renal transplantation in patients with chronic kidney disease. CMAJ. 2010;182(7):666. doi: 10.1503/cmaj.091661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishikawa H, Ichikawa Y, Arichi N, Tokugawa S, Yoshioka I, Nishimura K, et al. Kidney transplantation in patients receiving dialysis treatment for more than 10 years. Transplant Proc. 2006;38(10):3445. doi: 10.1016/j.transproceed.2006.10.138. [DOI] [PubMed] [Google Scholar]
- 24.Ushigome H, Sakai K, Suzuki T, Nobori S, Yoshizawa A, Akioka K, et al. Kidney transplantation for patients on long-term hemodialysis. Transplant Proc. 2008;40(7):2297. doi: 10.1016/j.transproceed.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Fine JPG, Robert J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496. [Google Scholar]
- 26.Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK, et al. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando) 2013;27(2):50. doi: 10.1016/j.trre.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Aufhauser DD, Jr, Wang Z, Murken DR, Bhatti TR, Wang Y, Ge G, et al. Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J Clin Invest. 2016;126(5):1968. doi: 10.1172/JCI84712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88(2):231. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 29.Isaacs RB, Nock SL, Spencer CE, Connors AF, Jr, Wang XQ, Sawyer R, et al. Racial disparities in renal transplant outcomes. Am J Kidney Dis. 1999;34(4):706. doi: 10.1016/S0272-6386(99)70397-5. [DOI] [PubMed] [Google Scholar]
- 30.Basu A, Falcone J, Dvorchik I, Tan H, Schonder K, Marsh JW, et al. Outcomes of renal transplantation in recipients with peak panel reactive antibody >30% under tacrolimus-based immunosuppression. Ann Transplant. 2011;16(3):5. doi: 10.12659/aot.881988. [DOI] [PubMed] [Google Scholar]
- 31.Clayton PA, McDonald SP, Snyder JJ, Salkowski N, Chadban SJ. External validation of the estimated posttransplant survival score for allocation of deceased donor kidneys in the United States. Am J Transplant. 2014;14(8):1922. doi: 10.1111/ajt.12761. [DOI] [PubMed] [Google Scholar]
- 32.Goodkin DA, Bieber B, Gillespie B, Robinson BM, Jadoul M. Hepatitis C infection is very rarely treated among hemodialysis patients. Am J Nephrol. 2013;38(5):405. doi: 10.1159/000355615. [DOI] [PubMed] [Google Scholar]
- 33.Goodkin DA, Bieber B, Jadoul M, Martin P, Kanda E, Pisoni RL. Mortality, Hospitalization, and Quality of Life among Patients with Hepatitis C Infection on Hemodialysis. Clin J Am Soc Nephrol. 2017;12(2):287. doi: 10.2215/CJN.07940716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reese PP, Shults J, Bloom RD, Mussell A, Harhay MN, Abt P, et al. Functional status, time to transplantation, and survival benefit of kidney transplantation among wait-listed candidates. Am J Kidney Dis. 2015;66(5):837. doi: 10.1053/j.ajkd.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAdams-DeMarco MA, King EA, Luo X, Haugen C, DiBrito S, Shaffer A, et al. Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Ann Surg. 2016 doi: 10.1097/SLA.0000000000002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147(2):190. doi: 10.1001/archsurg.2011.1229. [DOI] [PubMed] [Google Scholar]
- 37.Mucsi I, Bansal A, Jeannette M, Famure O, Li Y, Novak M, et al. Mental Health and Behavioural Barriers in Access to Kidney Transplantation: A Canadian Cohort Study. Transplantation. 2016 doi: 10.1097/TP.0000000000001362. [DOI] [PubMed] [Google Scholar]
- 38.Tong A, Hanson CS, Chapman JR, Halleck F, Budde K, Papachristou C, et al. The preferences and perspectives of nephrologists on patients’ access to kidney transplantation: a systematic review. Transplantation. 2014;98(7):682. doi: 10.1097/TP.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 39.Gander J, Browne T, Plantinga L, Pastan SO, Sauls L, Krisher J, et al. Dialysis Facility Transplant Philosophy and Access to Kidney Transplantation in the Southeast. Am J Nephrol. 2015;41(6):504. doi: 10.1159/000438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hao H, Lovasik BP, Pastan SO, Chang HH, Chowdhury R, Patzer RE. Geographic variation and neighborhood factors are associated with low rates of pre-end-stage renal disease nephrology care. Kidney Int. 2015;88(3):614. doi: 10.1038/ki.2015.118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.