Abstract
Background
Kidney transplantation holds much promise as a treatment of choice for patients with end-stage kidney disease. The impact of cold ischemia time (CIT) on acute renal transplant rejection (ARTR) remains to be fully studied in a large cohort of renal transplant patients.
Methods
From the Organ Procurement and Transplantation Network database, we analyzed 63 798 deceased donor renal transplants performed between 2000 and 2010. We assessed the association between CIT and ARTR. We also evaluated the association between recipient age and ARTR.
Results
Six thousand eight hundred two (11%) patients were clinically diagnosed with ARTR. Longer CIT was associated with an increased risk of ARTR. After multivariable adjustment, compared with recipients with CIT < 12 hours, the relative risk of ARTR was 1.13 (95% confidence interval, 1.04-1.23) in recipients with CIT ≥ 24 hours. The association of CIT and ARTR was more pronounced in patients undergoing retransplantation: compared with recipients with CIT less than 12 hours, the relative risk of ARTR was 1.66 (95% confidence interval, 1.01-2.73) in recipients with CIT of 24 hours or longer. Additionally, older age was associated with a decreased risk of ARTR. Compared with recipients aged 18 to 29 years, the relative risk of ARTR was 0.50 (95% confidence interval, 0.45-0.57) in recipients 60 years or older. Longer CIT was also associated with increased risk of death-censored graft loss. Compared with recipients with CIT less than 12 hours, the hazard ratio of death-censored graft loss was 1.22 (95% confidence interval, 1.14-1.30) in recipients with CIT of 24 hours or longer.
Conclusions
Prolonged CIT is associated with an increased risk of ARTR and death-censored graft loss. Older age was associated with a lower risk of ARTR.
The number of patients in need of kidney transplantation continues to increase.1 In 2014, the number of prevalent cases of end-stage kidney disease (ESKD) in the United States was 678383, and 70% of those patients were treated with dialysis. In that same year, 17914 kidney transplants were performed in the United States, whereas 88231 candidates were on the waiting list. The number of incident ESKD cases in the United States was 120688 in 2014, and patients aged 65 years and older had the highest ESKD incidence rate among all age groups.2 The scarcity of viable kidneys for transplantation has led clinicians to reconsider previously unacceptable durations of cold ischemia time (CIT). However, ischemia depletes energy production, inactivates ion channels, and eventually leads to cell death.3 After reperfusion of the donor organ, ischemia-reperfusion injury (IRI) induces an inflammatory response in the allograft.4,5 As a result, allografts with long CIT have been linked with increased immunogenicity and acute allograft rejection.6–8
However, the results of prior studies on CIT and renal transplant outcomes have been inconsistent. One study of 14 000 renal transplants from extended criteria donors reported no association between CIT and acute renal transplant rejection (ARTR),9 whereas another study of 611 transplant recipients reported a higher incidence of ARTR with increasing CIT.10 Small studies demonstrating a link between CIT and ARTR also were not adequately powered to identify long-term outcomes in patients of diverse age ranges.11,12
Moreover, aging is associated with declining immunity in renal transplant recipients.13,14 This is explained by a smaller population of lymphocyte progenitors and decreased number of T and B cells in older compared with younger recipients.15 Animal studies have shown a decreased memory T cell response to CD28 costimulation in older mice16 and in humans, vaccinated elderly individuals had reduced responses to influenza virus.17
The association between CIT and graft loss has been investigated in prior studies. Salahudeen et al18 showed a significant association between prolonged CIT and decreased long-term survival of cadaveric renal allografts in 6465 recipients. However, 2 other studies with smaller cohorts found that CIT does not have a significant association with graft loss in deceased donor renal transplant recipients.19,20
We therefore examined the association between CIT and ARTR in a large cohort of renal transplant recipients. In this study, we hypothesize that recipients of kidneys with prolonged CIT are more prone to ARTR. Furthermore, due to declining immunity associated with aging, older renal transplant recipients have a lower risk of acute rejection compared with younger recipients. Also, we hypothesize that due to the deleterious effects of IRI on the graft tissue, recipients of kidneys with prolonged CIT have a higher risk of graft loss.
MATERIALS AND METHODS
Data Sources and Study Population
We used registry data collected by the United Network for Organ Sharing (UNOS), a nonprofit organization that manages the nation’s organ transplant system. UNOS uses Organ Procurement and Transplantation Network (OPTN) data which is collected with the help of professionals from hospitals, histocompatibility laboratories, and organ procurement organizations.21,22
Our analysis includes patients who underwent solitary deceased donor renal transplantation between 2000 and 2010. From 95950 reported transplants, we excluded 32152 transplants because of missing data for CIT and/or ARTR. We excluded 6464 transplants (20%) due to missing data for CIT, 22952 transplants (71%) with missing data for ARTR, and 2736 (9%) transplants with missing data for both CIT and ARTR within 6 months of surgery. We did not observe a pattern of missingness by year. The remaining 63798 renal transplants were included in our analyses. The study group was limited to adult recipients.
The reported clinical and research activities are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.” Informed consent was not required for the registry data.23
CIT, ARTR, and Other Variables
After surgical removal of the organs for transplantation, kidneys are stored in cold solution to preserve their viability.24 CIT is the time between cold storage of the organ and the time it is warmed by having restored blood supply. In the OPTN database, CIT was recorded in hours. For our analysis, we divided CIT into 4 quantiles (<12, 12-17.9, 18-23.9, ≥24 hours). In the OPTN database, ARTR was defined as clinically overt and drug-treated acute graft rejection (Yes/No) in the 6-month posttransplant period.
Age of the transplant recipient and donor was recorded in years in the OPTN database. For our analysis, recipient and donor age were categorized by decade of life. Recipient body mass index (BMI) was categorized into 4 groups (<20, 20-24.9, 25-29.9, ≥30 kg/m2) for the multivariate analysis. Dialysis vintage was also categorized into 4 groups (<6 months, 6 months to <2 years, 2 to <5 years and ≥5 years).25 Percent calculated panel reactive antibody (CPRA) levels were grouped as 0%, 1% to 20%, 21% to 80%, and 81% to 100%.
Statistical Analysis
We studied the association between CIT and ARTR using univariable and multivariable logistic regressions. Odds ratios (with 95% confidence intervals) are used to express differences in the likelihood of acute rejection across different levels of CIT. The group with the shortest CIT (<12 hours CIT) was designated as the reference group. Our multivariable analysis of the association between CIT and ARTR adjusted for age of the recipients and donors, sex of the recipients and donors, ethnicity of the recipients and donors, recipient BMI, HLA mismatching, extended criteria donor, donation after circulatory death, CPRA, cause of death for the donor, dialysis vintage, retransplantation, and year of transplantation. Our regression model evaluating the association between recipient age and ARTR adjusted for the same covariates.
The association between CIT and death-censored graft loss was evaluated with Cox proportional hazards regression models. Graft losses within the first week of transplantation due to vascular surgical complications were excluded from the analyses.26 The CIT less than 12 hours group was designated as the reference group, and our multivariable analysis adjusted for age of the recipients and donors, sex of the recipients and donors, ethnicity of the recipients and donors, diabetes history of the recipients and donors, hypertension history of the recipients and donors, recipient BMI, HLA mismatching, extended criteria donor, donation after circulatory death, CPRA, cause of death for the donor, dialysis vintage, retransplantation, and year of transplantation. The P value for trend was obtained by introducing CIT categories into the regression model and assessing for a linear trend.
Statistical analysis was performed using SAS software, version 9.4 and JMP Pro software, version 13.0.0. (SAS Institute, Inc., Cary, NC).
RESULTS
Demographics
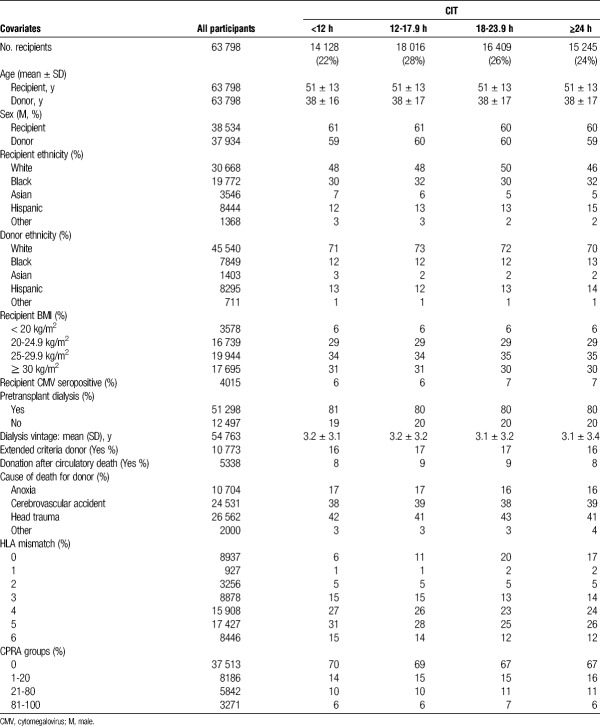
Recipient and donor demographic characteristics are shown in Table 1. All characteristics are stratified by CIT group. The mean recipient age was 51 ± 13 years and mean donor age was 38 ± 17 years for the entire study period. The sex distribution was the same for recipients and donors (61% male and 39% female).
TABLE 1.
Baseline characteristics of recipients and donors in the OPTN database between 2000 and 2010
Association Between CIT and ARTR
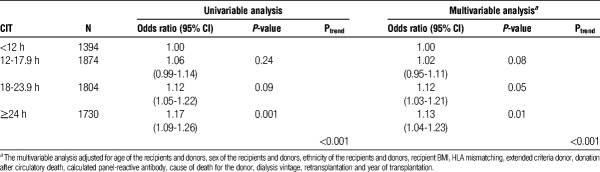
The mean CIT was 18.3 ± 8.4 hours and the highest recorded CIT was 60 hours. Six thousand eight hundred two patients were clinically diagnosed with ARTR, and the overall incidence of ARTR within 6 months of surgery was 11%. During the study period, 15245 renal transplantations were performed with CIT of 24 hours or longer. The relative risk of ARTR was higher for each recipient group with CIT of 12 hours or longer compared with recipients with CIT less than 12 hours. Compared with recipients with CIT less than 12 hours, the relative risk of ARTR was 1.17 (95% confidence interval, 1.09-1.26) in recipients with CIT of 24 hours or longer (Table 2).
TABLE 2.
Association of CIT with ARTR in the OPTN database between 2000 and 2010 (N = 6802)
After adjusting for covariates, the relative risk of ARTR was higher for each group with CIT of 12 hours or longer compared with recipients with CIT less than 12 hours. Compared with recipients with CIT < 12 hours, the relative risk of ARTR was 1.13 (95% confidence interval 1.04, 1.23) in recipients with CIT ≥ 24 hours (Table 2). The relative risk of CIT groups for ARTR remained similar for the CIT groups after adjusting for kidney biopsies. Compared with recipients with CIT less than 12 hours, the relative risk of ARTR was 1.14 (95% confidence interval, 1.05-1.24) in recipients with CIT of 24 hours or longer.
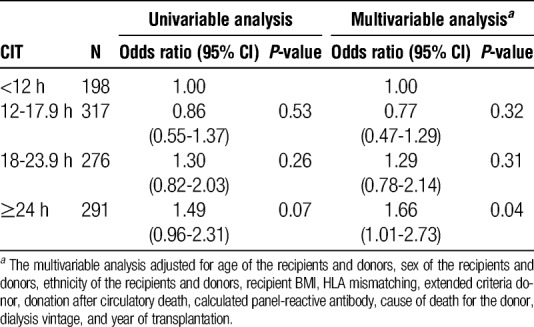
The association between CIT and ARTR was also evaluated among recipients with retransplantation since those recipients carry a higher risk for ARTR. Among recipients with retransplantation, the association between CIT and ARTR was more pronounced in recipients with CIT of 24 hours or longer. Compared with recipients with CIT less than 12 hours, the relative risk of ARTR was 1.66 (95% confidence interval 1.01, 2.73) in recipients with CIT of 24 hours or longer (Table 3).
TABLE 3.
Association of CIT with ARTR in the OPTN database for recipients with retransplantation between 2000 and 2010 (N = 1082)
Age of the Renal Transplant Recipient and Risk of ARTR
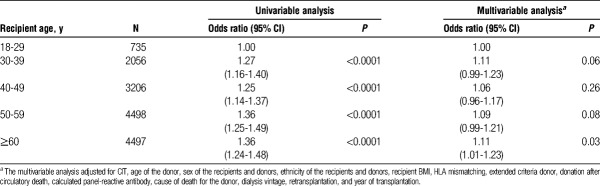
We assessed the association between recipient age and ARTR. After multivariable adjustment, the relative risk of ARTR was lower for recipients 30 years or older compared with recipients 18 to 29 years old. Compared with recipients aged 18 to 29 years, the relative risk of ARTR was 0.87 (95% confidence interval, 0.77-0.98) in recipients aged 30 to 39 years, 0.73 (95% confidence interval, 0.65-0.82) in recipients aged 40 to 49 years, 0.61 (95% confidence interval, 0.54-0.68) in recipients aged 50 to 59 years, and 0.50 (95% confidence interval, 0.45-0.57) in recipients 60 years or older (Figure 1).
FIGURE 1.
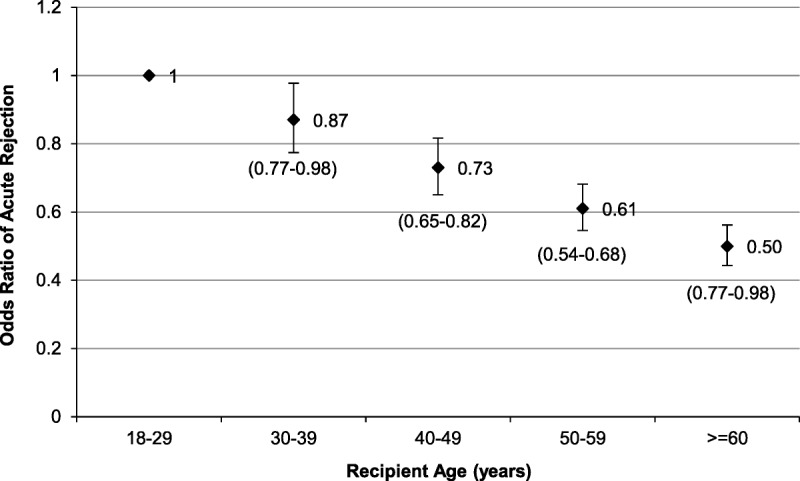
Risk of ARTR declines with older recipient age. Recipients were grouped by decade of life and the association of age with ARTR was evaluated with logistic regression analysis. The odds ratio of ARTR was lower among older recipients compared with younger recipients (P value <0.001). The multivariable analysis adjusted for CIT, age of the donors, sex of the recipients and donors, ethnicity of the recipients and donors, recipient BMI, HLA mismatching, extended criteria donor, donation after circulatory death, CPRA, cause of death for the donor, dialysis vintage, retransplantation, and year of transplantation. Confidence intervals for the odds ratios are shown below the data points.
Age of the Renal Transplant Recipient and Delayed Graft Function
In our study group, the incidence of overall delayed graft function (DGF) was 24%. The incidence of DGF was 19% in recipients aged 18 to 29 years, 23% in recipients aged 30 to 39 years, 23% in recipients 40 to 49 years, 24% in recipients 50 to 59 years, and 24% in recipients 60 years or older. We evaluated the association between recipient age and DGF. In univariable analysis, older age was associated with an increased risk of DGF (Table 4). However, after multivariable adjustment, only recipients 60 years or older had an increased risk of DGF compared with younger recipients (18-29 years old). The relative risk of DGF in recipients 60 years or older was 1.11 (95% confidence interval, 1.01-1.23) compared with recipients aged 18 to 29 years (Table 4). Furthermore, after adjustment for DGF and other variables, the relative risk for the association between recipient age and ARTR remained essentially unchanged compared with the analysis without adjustment for DGF. Compared with recipients aged 18 to 29 years, the relative risk for ARTR was 0.84 (95% confidence interval 0.75, 0.96) in recipients aged 30 to 39 years, 0.72 (95% confidence interval, 0.64-0.80) in recipients aged 40 to 49 years, 0.58 (95% confidence interval 0.52, 0.65) in recipients aged 50 to 59 years, and 0.48 (95% confidence interval 0.43, 0.54) in recipients ≥60 years old.
TABLE 4.
Association of recipient age with DGF in the OPTN database between 2000 and 2010 (N = 14 992)
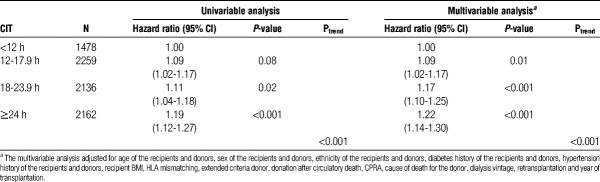
CIT and Death-Censored Graft Loss
We evaluated the association between CIT and death-censored graft loss. 8035 recipients experienced graft loss during the study period. The relative risk of death-censored graft loss was higher for each recipient group with CIT of 12 hours or longer compared with recipients with CIT less than 12 hours. Compared with recipients with CIT less than 12 hours, the hazard ratio of death-censored graft loss was 1.19 (95% confidence interval, 1.12-1.27) in recipients with CIT of 24 hours or longer (Table 5). After multivariable adjustment, compared with recipients with CIT < 12 hours, the hazard ratio of death-censored graft loss was 1.22 (95% confidence interval, 1.14-1.30) in recipients with CIT of 24 hours or longer (Table 5).
TABLE 5.
Association of CIT with death-censored graft loss in the OPTN database between 2000 and 2010 (N = 8035)
DISCUSSION
In our study, we found that the occurrence of ARTR increases with CIT among 63 798 recipients undergoing deceased donor renal transplantation. Additionally, the risk of ARTR is decreased in older recipients.
IRI has received increasing attention as an instigator of alloimmune responses and the main cause of intragraft inflammatory responses.27 An increase in proinflammatory cytokines results in a proinflammatory milieu that tips the balance in favor of effector immune responses as opposed to regulatory immune responses.28,29
The association of CIT with renal transplant outcomes has been inconsistent in the literature. One study reported no significant difference in the incidence of ARTR despite varied CIT exposure time among 45 patients.30 Another study of 40 paired renal transplantations did not report any difference in graft rejection rates despite significant differences in CIT exposure time between paired transplantations.31 On the other hand, a European study of 859 deceased donor renal transplants reported an independent positive association between CIT and ARTR.32 Moreover, this inconsistency applies to studies on graft survival as well.9,33
Interestingly, when we examined the association between CIT and ARTR among the subset of recipients with retransplantation, the association between CIT and ARTR was more pronounced in recipients of highly ischemic kidneys (CIT ≥ 24 hours). This finding might suggest that IRI can further potentiate the antigraft alloimmune responses in sensitized hosts. These data may imply using kidneys with shorter ischemia time for patients undergoing retransplantation.
For graft survival, CIT was reported to be an independent predictor of graft survival among 518 deceased donor renal transplant recipients.34,35 Another study reported an association between prolonged CIT and graft failure among 6322 renal transplant recipients.36 On the other hand, no association was found between CIT and graft survival among 38000 living donor renal transplant recipients.37 However, the CIT range in this study was limited to 0 to 8 hours. We expand on these reports by analyzing a much larger study population with a broader range of CIT exposure times (0-60 hours) among deceased donor renal transplant recipients. We found that prolonged CIT is associated with an increased risk of graft loss.
In the literature, DGF is defined with different criteria and studies reported inconsistent associations with long-term renal transplant outcomes. A study with 710 deceased donor renal transplants did not demonstrate a relationship between DGF and ARTR.38 Another study with 734 cadaveric transplants found DGF was associated with acute rejection.39 Additionally, a study from Europe with 1784 deceased donor renal transplants showed that increasing recipient age is a risk factor for ARTR during the DGF period.40 In our data, adjustment for DGF in our multivariable model did not substantially affect the association between recipient age and ARTR.
With an aging chronic kidney disease population, more kidney transplants are being offered to older hosts.14 For this reason, aging immunity has been the subject of intense research.13,41 Aging causes a decline in the immune response.41 In elderly patients, compromised proliferation of lymphocytes42,43 and defective responsiveness of memory T cells to CD28 costimulation16,17 may explain this decline.42,43
Our study is limited by the UNOS registry format in which ARTR is defined clinically. We were unable to examine the association of CIT with ARTR subtypes. We had missing data for CIT and ARTR. A previous study found underreporting of ARTR in the OPTN database, but among the reported cases, the diagnosis was valid.44 The missingness of rejection data varies by the transplant centers44,45 and is not likely to be differential with respect to our exposure variable, so the findings presented are internally valid. We evaluated the association of CIT with death-censored graft loss which does not include death as an outcome. The results for the association between CIT and death-censored graft loss should be interpreted cautiously due to the possibility of differential censoring of patients with a functioning graft at the time of death.
In summary, we report the largest study on the association between CIT and ARTR among renal transplant recipients in the United States and found that longer CIT is associated with increased ARTR and death-censored graft loss. Older recipient age was associated with a decreased risk of ARTR.
Future studies are needed to examine the association between ischemia time and other outcomes, including hospitalizations, financial cost and/or death-censored allograft survival in older transplant recipients.
Footnotes
This work was supported by NIH grant K24 AI116925 (RA).
M.P. and A.D.K. contributed equally to the study.
The authors declare no conflicts of interest.
M.P. participated in the study conception, data analysis and interpretation, article preparation, and critical editing. A.D.K. participated in the study conception, data analysis and interpretation, article preparation, and critical editing. B.C.B. participated in the data analysis and interpretation and article preparation. A.S. participated in the study conception and data analysis and interpretation. S.G.T. participated in the study conception. E.L.M. participated in the study conception and data analysis and interpretation. J.M.P. participated in the data analysis and interpretation, article preparation, and critical editing. R.A. participated in the study conception, data analysis and interpretation, and critical editing.
Correspondence: Reza Abdi, MD, Brigham & Women’s Hospital Transplantation Research Center, EBRC 221 Longwood Ave, 3. Floor Boston, MA 02115. (Rabdi@partners.org).
Prolonged cold ischemia time is associated with an increased risk of acute renal transplant rejection (ARTR) and death-censored graft loss, however older age is associated with a lower risk of ARTR.
REFERENCES
- 1.Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999-2008 Am J Transplant 2010. 10961–972 [DOI] [PubMed] [Google Scholar]
- 2.USRDS 2016. Annual Data Report. Volume 2: End-Stage Renal Disease in United States. 2016. https://www.usrds.org/2016/view/v2_07.aspx. [Google Scholar]
- 3.Kalogeris T, Baines CP, Krenz M. Cell biology of ischemia/reperfusion injury Int Rev Cell Mol Biol 2012. 298229–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Land WG. Injury to allografts: innate immune pathways to acute and chronic rejection Saudi J Kidney Dis Transpl 2005. 16520–539 [PubMed] [Google Scholar]
- 5.Alegre ML, Florquin S, Goldman M. Cellular mechanisms underlying acute graft rejection: time for reassessment Curr Opin Immunol 2007. 19563–568 [DOI] [PubMed] [Google Scholar]
- 6.Takada M, Nadeau KC, Shaw GD. Prevention of late renal changes after initial ischemia/reperfusion injury by blocking early selectin binding Transplantation 1997. 641520–1525 [DOI] [PubMed] [Google Scholar]
- 7.Tilney NL, Guttmann RD. Effects of initial ischemia/reperfusion injury on the transplanted kidney Transplantation 1997. 64945–947 [DOI] [PubMed] [Google Scholar]
- 8.Kupiec-Weglinski JW. Tolerance induction Curr Opin Organ Transplant 2008. 13331–332 [DOI] [PubMed] [Google Scholar]
- 9.Kayler LK, Magliocca J, Zendejas I. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis Am J Transplant 2011. 112647–2656 [DOI] [PubMed] [Google Scholar]
- 10.Mikhalski D, Wissing KM, Ghisdal L. Cold ischemia is a major determinant of acute rejection and renal graft survival in the modern era of immunosuppression Transplantation 2008. 85S3–S9 [DOI] [PubMed] [Google Scholar]
- 11.Halloran P, Aprile M. Factors influencing early renal function in cadaver kidney transplants. A case-control study Transplantation 1988. 45122–127 [DOI] [PubMed] [Google Scholar]
- 12.Bryan CF, Luger AM, Martinez J. Cold ischemia time: an independent predictor of increased HLA class I antibody production after rejection of a primary cadaveric renal allograft Transplantation 2001. 71875–879 [DOI] [PubMed] [Google Scholar]
- 13.Martins PN, Tullius SG, Markmann JF. Immunosenescence and immune response in organ transplantation Int Rev Immunol 2014. 33162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tullius SG, Tran H, Guleria I. The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome Ann Surg 2010. 252662–674 [DOI] [PubMed] [Google Scholar]
- 15.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function Nat Immunol 2004. 5133–139 [DOI] [PubMed] [Google Scholar]
- 16.Engwerda CR, Handwerger BS, Fox BS. Aged T cells are hyporesponsive to costimulation mediated by CD28 J Immunol 1994. 1523740–3747 [PubMed] [Google Scholar]
- 17.Thompson WW, Shay DK, Weintraub E. Influenza-associated hospitalizations in the United States JAMA 2004. 2921333–1340 [DOI] [PubMed] [Google Scholar]
- 18.Salahudeen AK, Haider N, May W. Cold ischemia and the reduced long-term survival of cadaveric renal allografts Kidney Int 2004. 65713–718 [DOI] [PubMed] [Google Scholar]
- 19.Emmanouilidis N, Boeckler J, Ringe BP. Risk balancing of cold ischemic time against night shift surgery possibly reduces rates of reoperation and perioperative graft loss. J Transplant. 2017;2017:5362704. doi: 10.1155/2017/5362704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo CH, Ju JI, Kim MH. Risk factors and long-term outcomes of delayed graft function in deceased donor renal transplantation Ann Surg Treat Res 2015. 89208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UNOS. https://www.unos.org/data/. https://www.unos.org/data/. [Google Scholar]
- 22.OPTN. https://optn.transplant.hrsa.gov/data/about-data/data-collection/. https://optn.transplant.hrsa.gov/data/about-data/data-collection/. [Google Scholar]
- 23. http://www.declarationofistanbul.org/. [Google Scholar]
- 24.Ponticelli CE. The impact of cold ischemia time on renal transplant outcome Kidney Int 2015. 87272–275 [DOI] [PubMed] [Google Scholar]
- 25.Molnar MZ, Mehrotra R, Duong U. Dialysis modality and outcomes in kidney transplant recipients Clin J Am Soc Nephrol 2012. 7332–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ammi M, Daligault M, Sayegh J. Evaluation of the vascular surgical complications of renal transplantation Ann Vasc Surg 2016. 3323–30 [DOI] [PubMed] [Google Scholar]
- 27.Solhjou Z, Athar H, Xu Q. Emerging therapies targeting intra-organ inflammation in transplantation Am J Transplant 2015. 15305–311 [DOI] [PubMed] [Google Scholar]
- 28.Alegre ML, Leemans J, Le Moine A. The multiple facets of toll-like receptors in transplantation biology Transplantation 2008. 861–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy SP, Porrett PM, Turka LA. Innate immunity in transplant tolerance and rejection Immunol Rev 2011. 24139–48 [DOI] [PubMed] [Google Scholar]
- 30.Bhargava A, Arora S, Marcus RJ. Outcomes of paired-exchange live-donor kidney transplantation: a single-center experience Transplant Proc 2014. 463420–3422 [DOI] [PubMed] [Google Scholar]
- 31.Giessing M, Fuller TF, Friedersdorff F. Comparison of first and second kidney transplants from the same deceased donor Nephrol Dial Transplant 2010. 254055–4061 [DOI] [PubMed] [Google Scholar]
- 32.Kieszek R, Kwiatkowski A, Jedrzejko K. Impact of pretransplant body mass index on early kidney graft function Transplant Proc 2014. 462689–2691 [DOI] [PubMed] [Google Scholar]
- 33.Frei U, Noeldeke J, Machold-Fabrizii V. Prospective age-matching in elderly kidney transplant recipients—a 5-year analysis of the Eurotransplant Senior Program Am J Transplant 2008. 850–57 [DOI] [PubMed] [Google Scholar]
- 34.Quiroga I, McShane P, Koo DD. Major effects of delayed graft function and cold ischaemia time on renal allograft survival Nephrol Dial Transplant 2006. 211689–1696 [DOI] [PubMed] [Google Scholar]
- 35.Debout A, Foucher Y, Trebern-Launay K. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation Kidney Int 2015. 87343–349 [DOI] [PubMed] [Google Scholar]
- 36.Warle MC, Cheung CL, Teerenstra S. Cold ischaemia time and outcome of renal transplantation. Ned Tijdschr Geneeskd. 2010;154:B539. [PubMed] [Google Scholar]
- 37.Simpkins CE, Montgomery RA, Hawxby AM. Cold ischemia time and allograft outcomes in live donor renal transplantation: is live donor organ transport feasible? Am J Transplant 2007. 799–107 [DOI] [PubMed] [Google Scholar]
- 38.McLaren AJ, Jassem W, Gray DW. Delayed graft function: risk factors and the relative effects of early function and acute rejection on long-term survival in cadaveric renal transplantation Clin Transplant 1999. 13266–272 [DOI] [PubMed] [Google Scholar]
- 39.Boom H, Mallat MJ, de Fijter JW. Delayed graft function influences renal function, but not survival Kidney Int 2000. 58859–866 [DOI] [PubMed] [Google Scholar]
- 40.Chaumont M, Racape J, Broeders N. Delayed graft function in kidney transplants: time evolution, role of acute rejection, risk factors, and impact on patient and graft outcome. J Transplant. 2015;2015:163757. doi: 10.1155/2015/163757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seyda M, Quante M, Uehara H. Immunosenescence in renal transplantation: a changing balance of innate and adaptive immunity Curr Opin Organ Transplant 2015. 20417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koch S, Larbi A, Derhovanessian E. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Douziech N, Seres I, Larbi A. Modulation of human lymphocyte proliferative response with aging Exp Gerontol 2002. 37369–387 [DOI] [PubMed] [Google Scholar]
- 44.Potluri VS, Parikh CR, Hall IE. Validating early post-transplant outcomes reported for recipients of deceased donor kidney transplants Clin J Am Soc Nephrol 2016. 11324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillespie BW, Merion RM, Ortiz-Rios E. Database comparison of the adult-to-adult living donor liver transplantation cohort study (A2ALL) and the SRTR U.S. Transplant Registry Am J Transplant 2010. 101621–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]