Abstract
Background:
The high pretreatment plasma fibrinogen has been widely reported to be a possible biomarker for predicting prognosis in hepatocellular carcinoma (HCC) and pancreatic carcinoma (PC), but persuasive conclusion has not been made yet. Thus, we herein conducted a meta-analysis to comprehensively assess the prognostic value of high pretreatment plasma fibrinogen in patients with HCC and PC.
Method:
We systematically searched PubMed, EMBASE, and Web of Science to identify eligible studies from inception to November 10, 2017.
Results:
Finally, a total of 12 publications with 13 studies were included. Of these eligible studies, 5 publications with 6 studies were about pancreatic cancer and 7 were about HCC. The pooled analysis indicated that high plasm fibrinogen level was significantly related to worse overall survival (OS) in HCC [hazard ratio (HR) = 1.87; 95% confidence interval (CI): 1.55–2.24; P < .01]. Similarly, from our result, it was found that high plasm fibrinogen was also significantly associated with worse OS in PC (HR = 1.56; 95% CI: 1.13–2.15; P < .01).
Conclusion:
Taken together, our meta-analysis confirmed that high plasma fibrinogen level could predict worse survival in HCC and PC.
Keywords: hepatocellular carcinoma, meta-analysis, pancreatic cancer, plasma fibrinogen, prognostic
1. Introduction
Hepatocellular carcinoma (HCC) and pancreatic carcinoma (PC) are common malignancies in China. HCC and PC are the major causes of cancer-related death worldwide and despondently patients with PC just have a 5-year survival less than 8%.[1,2] Radical surgery is the most effective therapy for HCC and PC, but postoperative recurrence or metastatic disease are barriers to prolonged survival. Despite the great advancement made in the diagnosis and treatment of cancer in the past decades, Patients with HCC and PC still have unfavorable prognosis. Up to now, many serum biomarkers, such as α-fetoprotein (AFP) and (carbohydrate antigen 19-9) CA19-9, have been used to predict the survival of HCC and PC patents. However, due to limited sensitivity and specificity, these markers are not entirely reliable. Thus, more effective markers for predicting the biological characteristics and guiding individualized comprehensive treatments are urgently needed.
Plasma fibrinogen is an acute-phase soluble glycoprotein, and is a glycoprotein synthesized by liver epithelium via stimulation of interleukin (IL)-6 and IL-1b.[3–5] Fibrinogen is a 350-kDa glycoprotein that comprises 2 sets of 3 different polypeptide chains, a, b, and c, and plays a major role in blood clotting and circulation via its interaction with platelets. Fibrinogen is widely known for its association with the maintenance of hemostatic function, but recent substantial evidence indicates that plasma fibrinogen also plays critical roles in tumor progression.[6–9] Recently, several studies showed that hyperfibrinogenemia is prevalent in patients with HCC and PC and is closely associated with metastasis, resistance to chemo-radiotherapy, disease progression, and poor prognosis.[10–12] Moreover, many studies have also tried to explore the potential mechanisms for the association between high plasm fibrinogen and poor survival in cancers. For instance, it was suggested that fibrinogen could contribute to tumor cell invasion and proliferation, angiogenesis and epithelial-to-mesenchymal transition.[13–15] However, a large part of clinical studies were performed with small sample size, the statistical power of which was not strong enough to draw a persuasive conclusion regarding the prognostic significance of high plasm fibrinogen in HCC and PC patients. Up to now, there is no meta-analysis specifically focusing on investigating the prognostic value of plasma fibrinogen in patients with HCC and PC. Therefore, in order to provide more reliable evidence for the independently prognostic value of plasma fibrinogen in patients with HCC and PC, we herein performed a systematic review and a meta-analysis of relevant studies exploring the relationship between plasma fibrinogen and prognosis of patients with HCC and PC.
2. Materials and methods
2.1. Ethics and dissemination
Ethical approval and informed consent are not required, as the study will be a literature review and will not involve direct contact with patients or alterations to patient care.
2.2. Literature search strategy and selection criteria
We performed the systematic search by retrieving PubMed, EMBASE, and Web of Science for eligible studies assessing prognostic value of pretreatment plasma fibrinogen in HCC and pancreatic cancer from inception to November, 10, 2017. The combination of the following terms: (“pancreatic” OR “hepatocellular” OR liver), (cancer OR tumor OR carcinoma OR malignant or malignancy), (prognosis OR prognostic OR survival OR outcome), and (“fibrinogen OR fibrinogenemia”) generated the search strategy.
The selection of all the included studies was finished according to the following criteria: perspective or retrospective cohort study; the studies assessed the link between pretreatment fibrinogen and prognosis in HCC or pancreatic cancer; the articles with full text were published in English; hazard ratio (HR) with its 95% confidence interval (CI) or survival curve were available. Moreover, the following criteria were used to exclude unqualified studies: case reports, reviews, comments, and systematic review; studies about non-digestive system tumors; patients were not divided into 2 groups of low fibrinogen group and high fibrinogen group; no adequate data could be extracted to estimate the HR and 95% CI.
2.3. Data extraction and quality assessment
Data were extracted by 2 independent researchers (RJ and QR) through reviewing full texts of all the eligible studies. Another independent researcher (Yongning Zhou) would join in the discussion to reconcile inconsistencies. The following information was obtained: the first author's name, publication date, region of study, study design, study period, type of cancer, tumor stage, clinical setting, the number of patients, a mean age of patients, treatment methods, cut-off of fibrinogen, and outcome measures. If the HRs for survival outcomes were not provided directly, the Engauge Digitizer version 4.1 (http://digitizer.sourceforge.net/, freely down-loaded software) was used to calculate the survival data from the Kaplan-Meier curves.
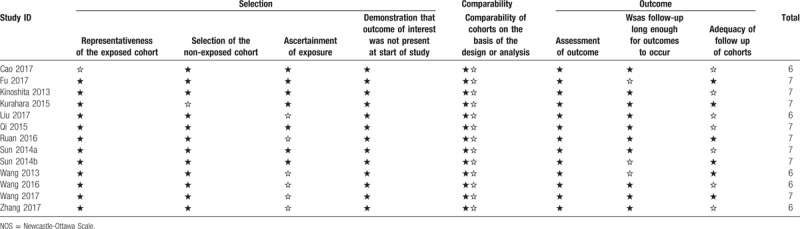
The quality assessment of all the included studies was performed by 2 independent researchers (SB and YW) according to Newcastle-Ottawa Scale (NOS), in which aspects regarding selection, comparability and outcome were assessed.[16] The scores obtained from NOS varied from 0 to 9 and a score of 6 or more is defined as high quality.
2.4. Statistical analysis
The statistical analyses of this review were fulfilled by means of Stata version 12.0 (Stata Corporation, College Station, TX). Pooled HRs with 95% CIs were used to estimate the quantitative aggregation of survival results. The heterogeneity across studies was assessed using the Cochran's Q and Higgins I2 statistics. The occurrence of P < .05 and I2 > 50% indicates a significant heterogeneity, while I2 < 50% means no heterogeneity or moderate heterogeneity. Random effects model was applied when statistical heterogeneity was observed (P < .05, I2 > 50%). HR > 1(low fibrinogen used as reference) stands for higher risk of poor outcomes for high fibrinogen, and it was proposed to be statistically significant if the 95% CI did not contains 1 and P value is less than .05. Sensitivity analysis was performed by sequentially omitting single study step by step. If the pooled results do not significantly alter when single study is omitted, it suggests the pooled results are robust.
3. Results
3.1. Study search and study characteristics
In all, 297 articles were identified based on search strategy, with 76 from PubMed, 130 from EMBASE, and 91 from Web of Science. After duplicate publications were removed and the remaining abstracts and full-texts of the references were meticulously reviewed, 12 publications[11,12,17–26] with 13 studies were finally identified to be eligible for the pooled analysis of the prognostic value of plasma fibrinogen in HCC and PC. The search strategy and selection process were shown in Figure 1.
Figure 1.
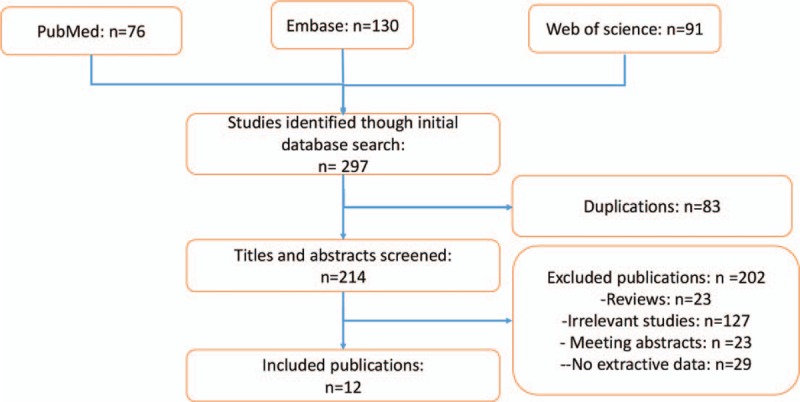
Flow diagram of study selection.
The basic characteristics of the included studies are summarized in Table 1.[1–12] A total of 5 publications with 6 studies were about PC and 7 studies were about HCC. A total of 11 studies were from China, and 2 studies were from in Japan. The accrual period of 13 studies ranged from 2000 to 2014, and the number of participants ranged from 41 to 539. All of the included studies were retrospectively designed. The cut-off values of high plasm fibrinogen varied from 2.345 to 4.0 g/L. In regard to the outcomes of interest, all of eligible studies have investigated the relationship between high plasm fibrinogen and overall survival (OS), and 5 studies analyzed the association between disease-free survival (DFS) and high plasm fibrinogen in HCC. The scores of the eligible studies from the NOS ranged from 6 to 7, indicating that the included studies was moderate to high quality (Table 2).
Table 1.
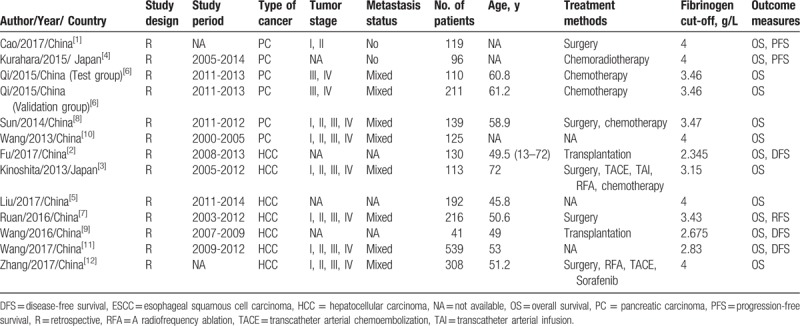
Table 2.
The NOS quality assessment of the included studies.
3.2. Prognostic value of high plasm fibrinogen in HCC
3.2.1. High plasm fibrinogen and OS in HCC
All of the included 7 studies investigated the relationship between high plasm fibrinogen and OS in HCC. The pooled analysis indicated that a high plasm fibrinogen was significantly related to worse OS in HCC (HR = 1.87; 95% CI: 1.55–2.24; P < .01) (Fig. 2).
Figure 2.
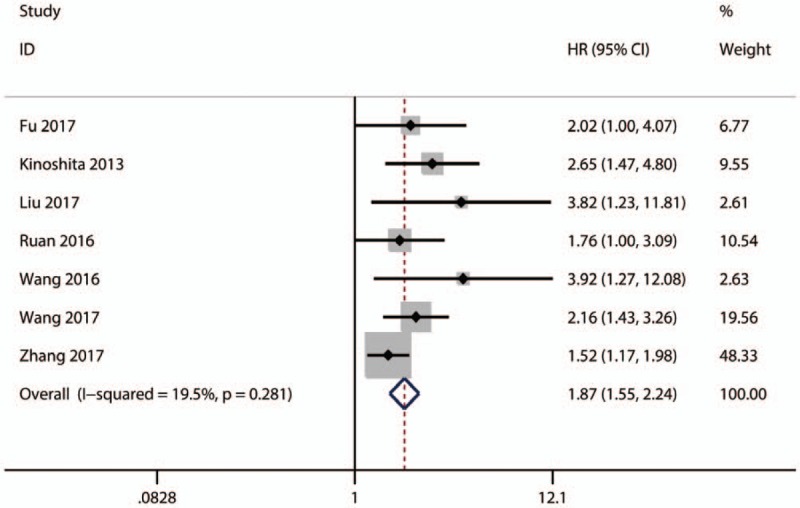
Results of pooled hazard ratios of OS of patients with high plasm fibrinogen in HCC and PC. HCC = hepatocellular carcinoma, PC = pancreatic carcinoma, OS = overall survival.
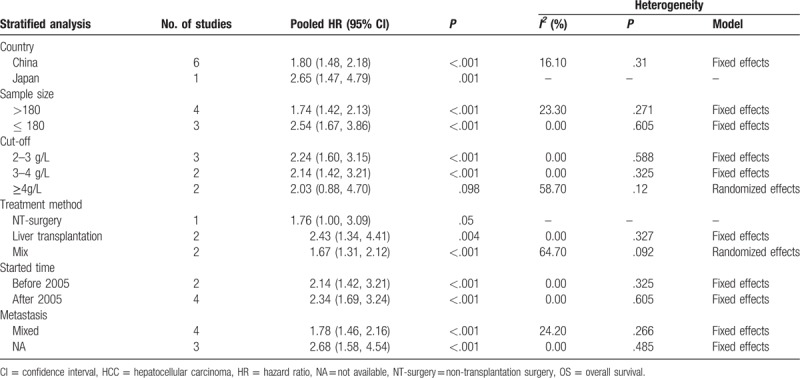
Although no significant heterogeneity was observed (I2 = 19.5%, P = .281) among those studies about OS, the subgroup analyses by country, sample size, cut-off value, treatment method, started time, and metastasis status were performed to test the robustness of our pooled result. From the results of subgroup analysis, we observed that there were significant associations between high fibrinogen level and poor OS in China (HR = 1. 08; 95% CI: 1.48–2.18; P < .001) (Table 3). Additionally, the relationship of high fibrinogen level to OS was also significant in sample size (>180 or ≤180) (HR = 1.74; 95% CI: 1.42–2.13; P < .001 or HR = 2.54; 95% CI: 1.67–3.86; P < .001) (Table 3), cut-off value (2–3 g/L and 3–4 g/L) (HR = 2.24; 95% CI: 1.60–3.15; P < .001 and HR = 2.03; 95% CI: 0.88–4.70; P < .001.) (Table 3), treatment method (liver transplantation or mix) (HR = 2.43; 95% CI: 1. 34–4.41; P = .004 or HR = 1.67; 95% CI: 1.31–2.12; P < .001) (Table 3), started time (before 2005 or after 2005) (HR = 2.14; 95% CI: 1.42–3.21; P < .001 or HR = 2.34; 95% CI: 1.69–3.24; P < .001)(Table 3), and metastasis (yes or mix) (HR = 1.78; 95% CI: 1.46–2.16; P < .001 or HR = 2.68; 95% CI: 1.58–4.54; P < .001) (Table 3). However, no significant relationship was found in the subgroup of cut-off value≥ 4 g/L. Anyway, the results of subgroup analyses indicated that the pooled HR for OS was stable and reliable.
Table 3.
Results of subgroup analysis of pooled HRs for OS of patients with high plasm fibrinogen in HCC.
3.2.2. High plasm fibrinogen and DFS in HCC
Only 3 studies explored the association between high plasm fibrinogen and DFS in HCC. The pooled result showed that there was an inverse correlation between high plasm fibrinogen and DFS (HR = 2.45; 95% CI: 1.71–3.49; P < .01) (Fig. 3).
Figure 3.
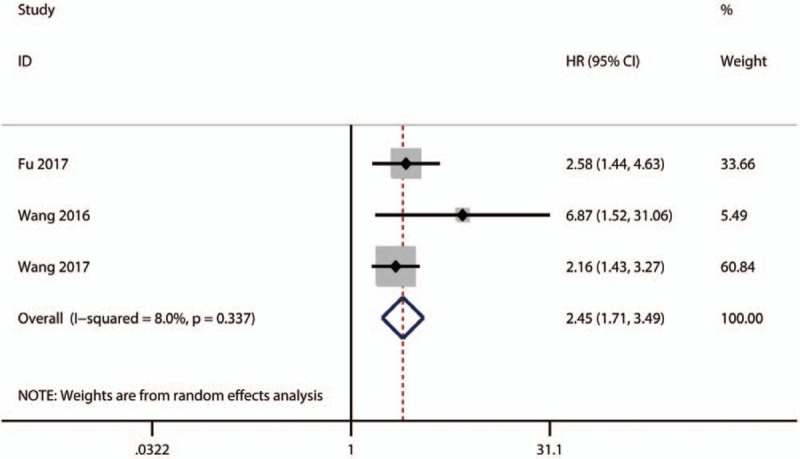
Results of pooled HR of DFS of patients with high plasm fibrinogenin HCC. DFS = disease-free survival, HCC = hepatocellular carcinoma, HR = hazard ratios.
3.3. Prognostic value of high plasm fibrinogen in PC
3.3.1. High plasm fibrinogen and OS in PC
A total of 5 included publications with 6 studies investigated the relationship between high plasm fibrinogen and OS in PC. A random-effect model was conducted to calculate HR and 95% CI due to the severe heterogeneity (I2 = 75.5%, P = .001) among those studies. The pooled result indicated that high plasm fibrinogen was significantly related to worse OS in PC (HR = 1.56; 95% CI: 1.13–2.15; P < .01) (Fig. 4).
Figure 4.
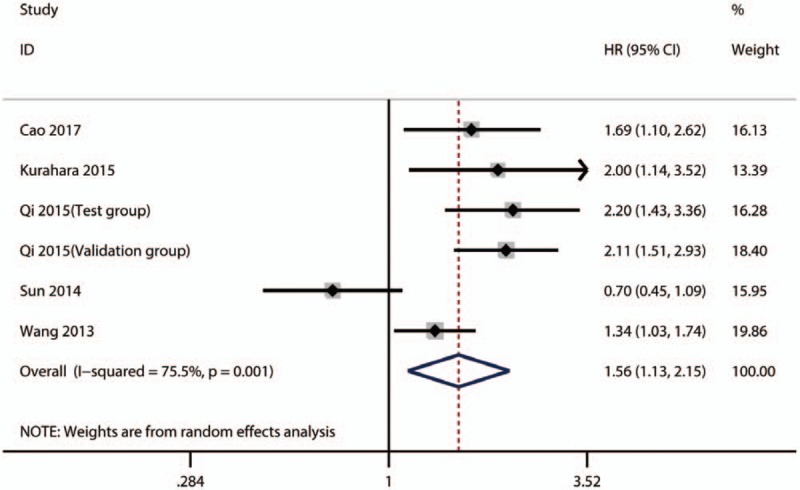
Results of pooled HRs of OS of patients with high plasm fibrinogen in PC. HR = hazard ratios, PC = pancreatic carcinoma, OS = overall survival.
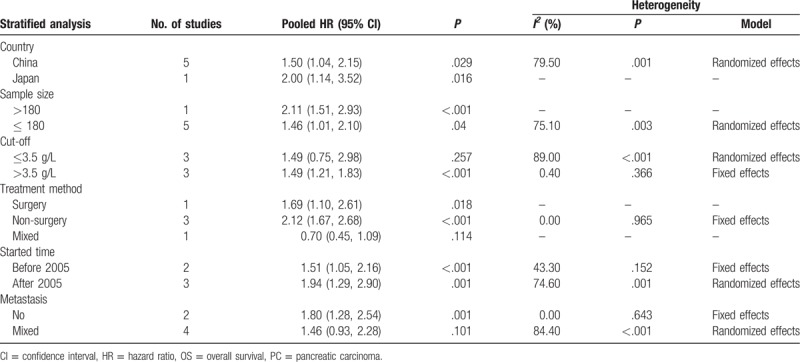
Due to substantial heterogeneity, we performed subgroup analyses by country, sample size, cut-off value, treatment method, started time, and metastasis status to explore the potential sources of heterogeneity. From the results of subgroup analysis, we observed a significant association between high plasm fibrinogen and worse OS in country (China) (HR = 1. 50; 95% CI: 1.04–2.15; P = .029) (Table 4). Additionally, the relationship between high plasm fibrinogen and unfavorable OS was significant in the subgroups of sample size (>180) (HR = 2.14; 95% CI: 1.65–2.78; P < .001) (Table 4), cut-off value (>3.5 g/L) (HR = 1.49; 95% CI: 1.21–1.83; P < .001) (Table 4), treatment method (non-surgery) (HR = 2.12; 95% CI: 1. 67–2.68; P < .001)(Table 4), started time (before 2005 or after 2005) (HR = 1.51; 95% CI: 1.05–2.16; P < .001 or HR = 1.94; 95% CI: 1.29–2.90; P = .001)(Table 4), and no metastasis (HR = 1.80; 95% CI: 1.28–2.54; P = .001) (Table 4). Nevertheless, there were no substantial associations between high plasm fibrinogen and worse OS in the subgroups of cut-off value <3.5 g/L, mixed treatment and mixed metastasis status. With respect to heterogeneity, only in the subgroup of treatment no significant heterogeneity remained, which indicated that treatment might partly account for the heterogeneity of pooled HR for OS in patients with PC. In general, our subgroup analyses indicated that those factors might not be responsible for the sources of the heterogeneity, except for treatment method, and meanwhile verified the robustness of the pooled HR for OS in patients with PC.
Table 4.
Results of subgroup analysis of pooled HRs for OS of patients with high plasm fibrinogen in PC.
3.4. Sensitivity analysis
In order to further verify the robustness of the pooled HR for OS in HCC and PC, sensitivity analyses were conducted to assess the influence of each individual study on the pooled HRs for OS by omitting single study in each step. The results showed that the pooled HRs for of the association between high plasm fibrinogen and OS of patients with HCC (Fig. 5) and PC (Fig. 6) did not alter substantially, when any individual study was omitted, implying that the pooled results of our meta-analysis are robust.
Figure 5.
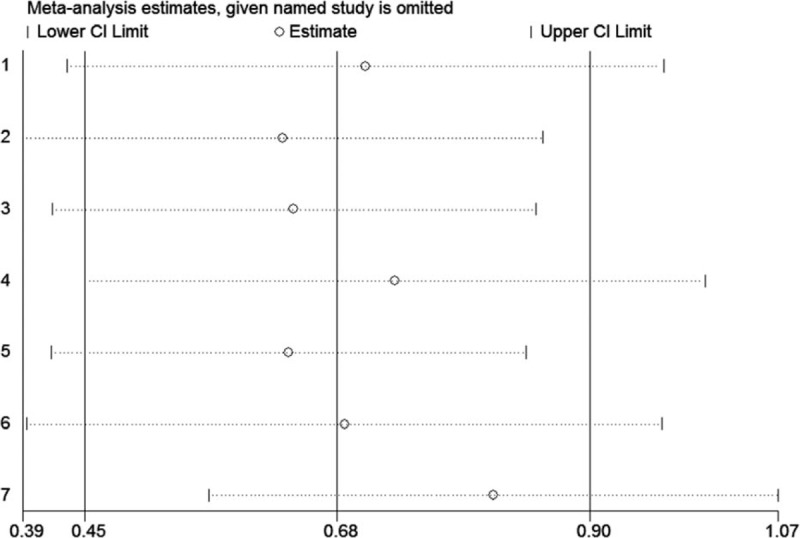
Sensitivity analysis of the pooled effects of OS in HCC. HCC = hepatocellular carcinoma, OS = overall survival.
Figure 6.
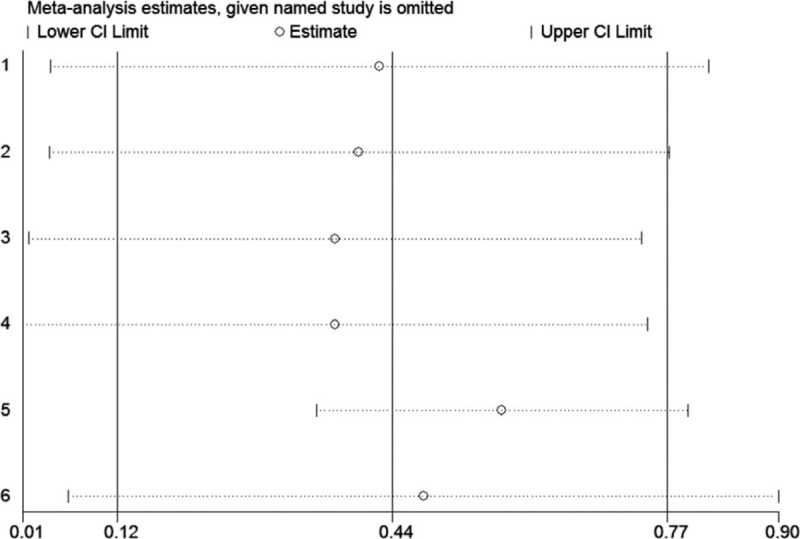
Sensitivity analysis of the pooled effects of OS in PC. PC = pancreatic carcinoma, OS = overall survival.
4. Discussion
Numerous studies have suggested that high plasma fibrinogen was closely correlated with poor prognosis of patients with various cancers.[6,27,28] In addition, the prognostic role of plasma fibrinogen in patients with solid tumors has been previously confirmed by meta-analyses.[29] However, till now, no specific meta-analysis had been conducted to assess the potential of plasma fibrinogen as a prognostic biomarker in HCC and PC. Therefore, we performed a systematic review and meta-analysis to validate the notion that elevated plasma fibrinogen predicts poor oncological outcomes.
In our meta-analysis, a total of 12 publications with 13 studies investigating the association between plasma fibrinogen and the prognosis of patients with HCC or PC were included. The data on OS and DFS of individual studies were pooled into statistical analysis. Overall, our meta-analysis validated that high plasma fibrinogen is a predictor of worse OS and DFS in patients with HCC and PC. Additionally, in general, our subgroup analyses and sensitivity analyses demonstrated the robustness of the pooled results of OS and DFS in patients with HCC and PC. However, in the subgroup analysis by cut-off values, a significant association between high plasm fibrinogen and worse OS in HCC was only observed in the subgroups of cut-off value < 4.0 g/L, but not in subgroup of cut-off value ≥ 4.0 g/L. There is a possible explanation for this inconsistency. Fibrinogen is a kind of protein produced by liver and its production will be reduced when liver dysfunction occurs. Usually, most of patients with HCC have chronic liver diseases and hepatic function abnormality, and hence their plasm fibrinogen may be at a relatively low level compared with those patients with normal liver function. Thus, using a low cut-off value of high plasm fibrinogen in HCC patients may be more sensitive to stratify patients with relatively high plasm fibrinogen and low plasm fibrinogen, under which condition the potentially prognostic values of high plasm fibrinogen in HCC could be truly reflected. On contrast, there was an obvious relationship between high plasm fibrinogen and worse OS in PC in the subgroup of high cut-off value but not in low cut-off value. Therefore, we speculated that it may be more reasonable to use the high cut-off value in PC and low cut-off value in HCC, when assessing the prognostic values of high plasm fibrinogen.
Several potential mechanisms accounting for the link between high plasma fibrinogen and tumor progression have been explored. Firstly, high plasma fibrinogen may be associated with increased fibrinogen deposits in tumor tissue and serves as an extracellular matrix for tumor cell adhesion or migration, which contribute to tumor metastasis[30] and invasion.[31] Secondly, chronic inflammatory responses plays critical roles in tumor development and progression. In particular, there is a close relationship between fibrinogen and systemic inflammation. A direct evidence for this association is that plasma fibrinogen level is positively related to many inflammatory indices, such as neutrophil-lymphocyte ratio, platelets-lymphocyte ratio, and lymphocyte-monocyte ratio.[19] Besides, it was reported that systemic inflammation could promote fibrinogen release,[32] and fibrinogen could induce the synthesis of interleukin-6 (IL-6).[33] A body of evidence supports that IL-6 is closely associated with tumor progression.[34] Therefore, high plasma fibrinogen induced by chronic inflammation may facilitate tumor progression by inducing IL-6 synthesis. Consistently, fibrinogen-dependent inflammatory response has been reported to be involved in tumor initiation and progression, indicating that high plasm fibrinogen may reflect an active inflammatory tumor microenvironment in favor of the progression of inflammation-related cancers.[15] At last but not least, it has been demonstrated that fibrinogen was capable of binding to several growth factors and vascular endothelial cell growth factors and functions as a reservoir for these factors. More importantly, these growth factors play important roles in promoting cell proliferation, metastasis and angiogenesis, and inhibiting apoptosis of cancer cells.[35–37]
Certainly, our study had several significant limitations. Therefore, the results of this meta-analysis should be interpreted with caution. Firstly, the main limitation in this meta-analysis is that the cut-off values of high plasm fibrinogen were not consistent. In the perspective of statistical significance, this might introduce heterogeneity and bias to our pooled analysis. In addition, it made our meta-analysis fail to provide a precision guidance to clinical practice. Thus, in future more well-designed studies with large sample size are needed to solve this problem. Secondly, only English publications were included in this meta-analysis, which may introduce publication bias to some degree. Thirdly, the HRs and 95% CIs in a few of the included studies were calculated from the survival curves, which were the statistical results of the univariate analysis. The univariate analysis does not take several potential confounding factors into account, which may cause heterogeneity and bias. Last but not least, the treatment methods varied among different studies, which might influence the survival outcomes and thus introduce heterogeneity into our meta-analysis.
In conclusion, our study indicated that the high plasma fibrinogen level predicted worse prognosis of patients with HCC and PC. Considering the aforementioned limitations, more high-quality clinical studies are needed to be performed to confirm the prognostic value of plasma fibrinogen level in patients with HCC and PC.
Author contributions
Data curation: Suyang Bai, Yuping Wang.
Funding acquisition: Qian Ren, Yongning Zhou.
Methodology: Yongning Zhou.
Software: Suyang Bai, Yuping Wang.
Supervision: Yongning Zhou.
Writing – original draft: Rui Ji, Qian Ren.
Writing – review & editing: Yongning Zhou.
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, HCC = hepatocellular carcinoma, HR = hazard ratio, NOS = Newcastle-Ottawa Scale, OR = odds ratio, OS = overall survival, PC = pancreatic carcinoma.
RJ and QR contributed equally to this work.
This study was supported by National Science and Technology Support Program (2014BAI09B02) and the First Hospital of Lanzhou University Foundation (ldyyynqn201201).
The authors declare no conflicts of interest.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tennent GA, Brennan SO, Stangou AJ, et al. Human plasma fibrinogen is synthesized in the liver. Blood 2007;109:1971–4. [DOI] [PubMed] [Google Scholar]
- [4].Weisel JW. Fibrinogen and fibrin. Adv Protein Chem 2005;70:247–99. [DOI] [PubMed] [Google Scholar]
- [5].Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost 2005;3:1894–904. [DOI] [PubMed] [Google Scholar]
- [6].Ghezzi F, Cromi A, Siesto G, et al. Prognostic significance of preoperative plasma fibrinogen in endometrial cancer. Gynecol Oncol 2010;119:309–13. [DOI] [PubMed] [Google Scholar]
- [7].Arigami T, Okumura H, Matsumoto M, et al. Analysis of the fibrinogen and neutrophil-lymphocyte ratio in esophageal squamous cell carcinoma: a promising blood marker of tumor progression and prognosis. Medicine (Baltimore) 2015;94:e1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Son HJ, Park JW, Chang HJ, et al. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Ann Surg Oncol 2013;20:2908–13. [DOI] [PubMed] [Google Scholar]
- [9].Palumbo JS, Degen JL. Mechanisms coupling the hemostatic system to colitis-associated cancer. Thromb Res 2010;125(Suppl 2):S39–43. [DOI] [PubMed] [Google Scholar]
- [10].Gouin-Thibault I, Achkar A, Samama MM. The thrombophilic state in cancer patients. Acta Haematol 2001;106:33–42. [DOI] [PubMed] [Google Scholar]
- [11].Kinoshita A, Onoda H, Imai N, et al. Elevated plasma fibrinogen levels are associated with a poor prognosis in patients with hepatocellular carcinoma. Oncology 2013;85:269–77. [DOI] [PubMed] [Google Scholar]
- [12].Wang H, Gao J, Bai M, et al. The pretreatment platelet and plasma fibrinogen level correlate with tumor progression and metastasis in patients with pancreatic cancer. Platelets 2014;25:382–7. [DOI] [PubMed] [Google Scholar]
- [13].Zhang F, Wang Y, Sun P, et al. Fibrinogen promotes malignant biological tumor behavior involving epithelial-mesenchymal transition via the p-AKT/p-mTOR pathway in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 2017;143:2413–24. [DOI] [PubMed] [Google Scholar]
- [14].Shu YJ, Weng H, Bao RF, et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer 2014;14:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Steinbrecher KA, Horowitz NA, Blevins EA, et al. Colitis-associated cancer is dependent on the interplay between the hemostatic and inflammatory systems and supported by integrin alpha(M)beta(2) engagement of fibrinogen. Cancer Res 2010;70:2634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [17].Cao J, Fu Z, Gao L, et al. Evaluation of serum D-dimer, fibrinogen, and CA19-9 for postoperative monitoring and survival prediction in resectable pancreatic carcinoma. World J Surg Oncol 2017;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kurahara H, Maemura K, Mataki Y, et al. Prognostication by inflammation-based score in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Pancreatology 2015;15:688–93. [DOI] [PubMed] [Google Scholar]
- [19].Qi Q, Geng Y, Sun M, et al. Hyperfibrinogen is associated with the systemic inflammatory response and predicts poor prognosis in advanced pancreatic cancer. Pancreas 2015;44:977–82. [DOI] [PubMed] [Google Scholar]
- [20].Sun W, Ren H, Gao CT, et al. Clinical and prognostic significance of coagulation assays in pancreatic cancer patients with absence of venous thromboembolism. Am J Clin Oncol 2015;38:550–6. [DOI] [PubMed] [Google Scholar]
- [21].Fu SJ, Ji F, Han M, et al. Prognostic value of combined preoperative fibrinogen and neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma after liver transplantation. Oncotarget 2017;8:4301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu Z, Guo H, Gao F, et al. Fibrinogen and D-dimer levels elevate in advanced hepatocellular carcinoma: high pretreatment fibrinogen levels predict poor outcomes. Hepatol Res 2017;47:1108–17. [DOI] [PubMed] [Google Scholar]
- [23].Ruan DY, Lin ZX, Wang TT, et al. Nomogram for preoperative estimation of long-term survival of patients who underwent curative resection with hepatocellular carcinoma beyond Barcelona clinic liver cancer stage A1. Oncotarget 2016;7:61378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang GY, Jiang N, Yi HM, et al. Pretransplant elevated plasma fibrinogen level is a novel prognostic predictor for hepatocellular carcinoma recurrence and patient survival following liver transplantation. Ann Transplant 2016;21:125–30. [DOI] [PubMed] [Google Scholar]
- [25].Wang XP, Mao MJ, He ZL, et al. A retrospective discussion of the prognostic value of combining prothrombin time(PT) and fibrinogen(Fbg) in patients with Hepatocellular carcinoma. J Cancer 2017;8:2079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang X, Long Q. Elevated serum plasma fibrinogen is associated with advanced tumor stage and poor survival in hepatocellular carcinoma patients. Medicine (Baltimore) 2017;96:e6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kijima T, Arigami T, Uchikado Y, et al. Combined fibrinogen and neutrophil-lymphocyte ratio as a prognostic marker of advanced esophageal squamous cell carcinoma. Cancer Sci 2017;108:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Qiu J, Yu Y, Fu Y, et al. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res 2012;38:651–7. [DOI] [PubMed] [Google Scholar]
- [29].Perisanidis C, Psyrri A, Cohen EE, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev 2015;41:960–70. [DOI] [PubMed] [Google Scholar]
- [30].Garcia MG, Bayo J, Bolontrade MF, et al. Hepatocellular carcinoma cells and their fibrotic microenvironment modulate bone marrow-derived mesenchymal stromal cell migration in vitro and in vivo. Mol Pharm 2011;8:1538–48. [DOI] [PubMed] [Google Scholar]
- [31].Zheng S, Shen J, Jiao Y, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci 2009;100:859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Trinchieri G. Cancer immunity: lessons from infectious diseases. J Infect Dis 2015;212(Suppl 1):S67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ridker PM, Howard CP, Walter V, et al. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 2012;126:2739–48. [DOI] [PubMed] [Google Scholar]
- [34].Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol 2014;26:54–74. [DOI] [PubMed] [Google Scholar]
- [35].Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood 2000;96:3772–8. [PubMed] [Google Scholar]
- [36].Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology (Bethesda) 2010;25:85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sahni A, Khorana AA, Baggs RB, et al. FGF-2 binding to fibrin(ogen) is required for augmented angiogenesis. Blood 2006;107:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


