Abstract
Background
The measurement of Compensatory Reserve (CRM) has been established to accurately measure the body’s total integrated capacity to compensate for physiological states of reduced central blood volume, and predict hemodynamic decompensation associated with inadequate tissue oxygenation. We previously demonstrated that African American (AA) women have a higher tolerance to reductions in central blood volume. Therefore, we tested the hypothesis that the CRM would identify racial differences during simulated hemorrhage, prior to the onset of traditional signs/symptoms.
Methods
We performed a retrospective analysis during simulated hemorrhage using lower body negative pressure in 23 AA (22 ± 1years; 24 ± 1kg/m2) and 31 white women (WW; 20 ± 1years; 23 ± 1kg/m2). Beat-by-beat blood pressure (BP) and heart rate (HR) were recorded during progressive lower body negative pressure to presyncope. BP waveforms were analyzed using a machine-learning algorithm to derive the CRM at each lower body negative pressure stage.
Results
Resting mean arterial BP (AA, 78 ± 3 vs. WW, 74 ± 2mmHg) and HR (AA, 68 ± 2 vs. WW, 65 ± 2bpm) were similar between groups. The CRM progressively decreased during LBNP in both groups, however the rate of decline in the CRM was less (P<0.05) in AA. The CRM was 4% higher in AA at −15mmHg lower body negative pressure and progressively increased to 21% higher at −50mmHg lower body negative pressure (P<0.05). However changes in BP and HR were not different between groups.
Conclusions
These data support the notion that the greater tolerance to simulated hemorrhage induced by lower body negative pressure in AA women can be explained by their greater capacity to protect the reserve to compensate for progressive central hypovolemia compared to WW independent of standard vital signs.
Keywords: blood pressure, hemorrhage, compensatory reserve
BACKGROUND
The number of women in the military and armed services has steadily increased over the past ~40 years (1). To date, approximately 15–18% of military service personnel are women. Not only has our military seen a growth in gender diversity, but racial diversity among enlisted service personnel has increased such that >30% of our military is racially diverse (1). To this end, there is a greater racial diversity within women compared to men in the military; approximately 50% of women serving in the army and navy are from minority races versus only ~30% of men (1), and one-third of enlisted women are African American (AA) (2). Thus, understanding the dynamics of hemorrhage physiology in women may benefit treatment and triage of injury on the battlefield.
Hemorrhage is a significant problem on the battlefield, and the leading cause of preventable death in our military; 25% of deaths in OIF/OEF due to hemorrhage are potentially survivable (3, 4). Thus, it is increasingly important to identify those individuals who are at greatest risk for development of shock by accurately predicting the early onset of hemodynamic collapse. Because women are more likely to have a lower tolerance to blood loss compared to men (5), it calls into question triage strategies to ensure survival from shock for women. Several mechanisms are activated during hemorrhage to compensate for shifts in blood volume to maintain tissue perfusion. For example, reflex increases in heart rate, vasoconstriction, and respiration occur to maintain blood pressure and perfusion to the tissue. Importantly, there is no single reflex mechanism that is solely responsible, but individual strategies for integrating the sum of all compensatory mechanisms is what ultimately determines the level of tissue perfusion and tolerance to central hypovolemia (6). This is why traditional vital signs such as heart rate or blood pressure do not accurately predict hemodynamic collapse (7).
The capacity to compensate for physiological conditions that compromise tissue oxygenation during low circulating blood volume states is known as the compensatory reserve (8). The Compensatory Reserve Measurement (CRM) is a novel non-invasive technique recently developed to measure the capacity to compensate by utilizing a robotics machine-learning algorithm to analyze real-time changes in features of arterial blood pressure or flow waveforms during simulated hemorrhage (7, 8). Although mathematically derived from the blood pressure wave form, it is a measure derived from integrated physiological systems (8). As such, the CRM can provide assessments of an integrated sum total of all mechanisms activated in response to changes in central blood volume, and identify individual patterns in hemodynamic decompensation (7, 8). Most important, the CRM rapidly and accurately identifies circulatory compromise and early hemodynamic decompensation during simulated hemorrhage, prior to the onset of changes in traditional vital signs. Thus, the CRM is a useful tool to predict individual tolerance to blood loss (7–9).
Our laboratories have conducted a number of experiments demonstrating that women have a lower tolerance to simulated hemorrhage compared to men (5, 10), and that within women there are varying degrees of tolerance to simulated blood loss (11–13). Furthermore, we found that AA women have a greater tolerance to simulated hemorrhage compared to white women (WW) of similar age, BMI and resting blood pressure (11). Although heart rate increased more in AA women at presyncope, the hemodynamic responses to reductions in central blood volume were similar between races, indicating that these traditional vital signs were not able to predict impending cardiovascular collapse. Accordingly, the purpose of this study was to perform a retrospective analysis of arterial waveform features to determine the validity of the CRM in predicting hemodynamic collapse in women, and also to determine whether the CRM was specific enough to distinguish racial differences during simulated hemorrhage. As such, we hypothesized that the CRM algorithm would predict racial differences in response to central hypovolemia, and more specifically, that the CRM would predict hemodynamic decompensation in WW before AA women.
METHODS
Subjects
Women were recruited from the greater New Haven, CT, area, using advertisements posted across the city and Yale campus. Women were excluded if they smoked, were obese (BMI>30kg/m2), had hypertension, or any other chronic disease. A total of 54 young women were included in the analysis, including 23 AA and 31 white women, from previous (11–13) and ongoing studies. All women provided written informed consent, which conformed to the guidelines contained in the Declaration of Helsinki; all experimental procedures were approved by the Human Investigational Committee at Yale School of Medicine (approval number 0512000875).
Experimental Protocol
Lower body negative pressure (LBNP) is an accurate model of simulated hemorrhage (14, 15). All women completed a LBNP test to determine individual tolerance to progressive central hypovolemia as previously described (11–13). Women were instructed to refrain from exercise and alcohol 24 hours prior to the testing, caffeine for 12 hours, and food for at least four hours prior to testing. All LBNP testing occurred in a temperature controlled room (27°C, <30% relative humidity). After being instrumented for measurements of heart rate (single-lead ECG), beat-by-beat blood pressure (BP; Finometer; Finipres, Amsterdam, The Netherlands), and respiration (Pneumotrace, UFI; Morro Bay, CA), women lay supine with their legs inside the LBNP box, which was sealed at the level of the iliac crest. A 21-gauge catheter was placed in the antecubital vein of the left arm. Because changes in vasoactive hormones are associated with BP regulation during hypovolemia and may explain differences in tolerance to central hypovolemia (10, 16), blood samples were drawn at baseline and test termination/pre-syncope (described below). Five-minutes of baseline measurements were recorded after 30 minutes of supine rest. The LBNP test was performed by applying negative pressure in 3 minute intervals at the following stages: −15mmHg, −20mmHg, −30mmHg, −40mmHg, etc. in 10 mmHg increments until presyncope, up to a total of 10 levels and a maximal LBNP level of −100mmHg. The test was terminated upon expression of presyncopal symptoms based on any one of the following criteria: a decrease in systolic BP<80 mmHg; a decrease in systolic BP to <90 associated with symptoms of lightheadedness, nausea, sweating or diaphoresis; or progressive symptoms of presyncope accompanied by a request from the subject to terminate the test. We calculated a cumulative stress index (CSI) for each woman by summing the product of the negative pressure (in mmHg) and the time (in minutes) spent at each stage. Thus, a more negative CSI indicates a greater tolerance to simulated hemorrhage.
Blood Analysis
An aliquot of whole blood was transferred into pre-chilled K+ EDTA tubes for the analysis of plasma renin activity (PRA). A separate aliquot was placed in a pre-chilled K+ EDTA tube containing EGTA and glutathione for the analysis of norepinephrine (NE) and epinephrine (EPI). A final aliquot was placed in a tube with no additives for the measurement of serum aldosterone (ALDO). All samples were centrifuged, the plasma or serum pipetted off, and frozen at −80°C until analysis. Concentrations of PRA and ALDO were measured using competitive binding radioimmunoassays. Intra- and inter- assay coefficients of variation were as follows: 2.1 and 2.6%, respectively, for PRA (Diasorin, Stillwater, MN, USA); 1.7 and 1.8%, respectively, for ALDO (Siemens Healthcare Diagnostics). Epinephrine and NE were analyzed using high-performance liquid chromatography with electrochemical detection (Colorchem Detector, ESA Corp., Acton, MA, USA).
Data Analysis and Statistics
Systolic, diastolic, mean arterial BP, and HR for each level of LBNP were determined by averaging the final 2 minutes of data at each stage. Blood pressure waveforms (Finometer) were separately analyzed using a machine-learning algorithm that has previously been validated to predict hemodynamic decompensation on an individual basis (7, 17–19). Briefly stated, blood pressure waveforms are analyzed on a beat-by-beat basis to generate a CRM value for each heartbeat. Each waveform is assessed by a machine learning algorithm, which assesses 200 distinct waveform features, such as frequency, amplitude and degree of separation of the ejected and reflected waves, and their subtle beat-to-beat changes over time compared with a large reference library of previously learned waveforms representing the transition from normovolemia to hypovolemia (8). In this way the algorithm “learns” the status of the individual based on the degree to which each set of waveform features match the reference set. This unique approach to assessing waveforms provides highly sensitive and specific estimates of each individual’s capacity to compensate for physiological challenges in real-time (17, 19, 20). Baseline waveforms are analyzed within a 30-heartbeat window to generate the initial CRM value. Subsequent CRM values are calculated on a beat-to-beat basis, and the algorithm normalizes these values (100% to 0%) to indicate the proportion of compensatory reserve remaining prior to decompensation (represented by a CRM of 0). Like BP and HR, an average CRM was calculated with the final 2 minutes of data for each 3-minute LBNP stage.
Subject characteristics were compared using independent t-tests. A 2-way repeated measures ANOVA was used to compare traditional hemodynamic responses during the LBNP test up to −50mmHg since this stage was when syncopal symptoms began in some women. Data are presented as mean ± 95% CI. Additionally, Cox proportional hazards regression models for repeated measures (21) were also used to test the hypotheses that (a) CRM would be negatively associated with time-to-presyncope and (b) that race would be independently associated with time-to-presyncope, such that AA women would generally have longer time-to-presyncope than WW. For time-to-presyncope analyses CRM was analyzed as a continuous, time-varying covariate. Based on the LBNP protocol termination criteria for presycope, each individual participant was subsequently classified as ‘compensating’ as long as they continued through each progressive level of LBNP, and ‘decompensating’ at the time of LBNP termination with the onset of presyncope. Hazard ratios were used to compare rates of pre-syncope over the time course of the experiments between AA and WW. A hazard ratio of 1 indicates that there is no difference in the rate of presyncope, while a hazard ratio greater than 1 indicates one group has a greater rate of presyncope and a hazard ratio less than 1 indicates one group has a lower rate of presyncope. Cumulative incidence functions were used to describe the cumulative rate of presyncope over the duration of the LBNP experiment. Significance testing was assessed using two-tailed p values, rather than arbitrary cut-offs. All analyses were performed using SAS v9.2 (Cary, NC).
RESULTS
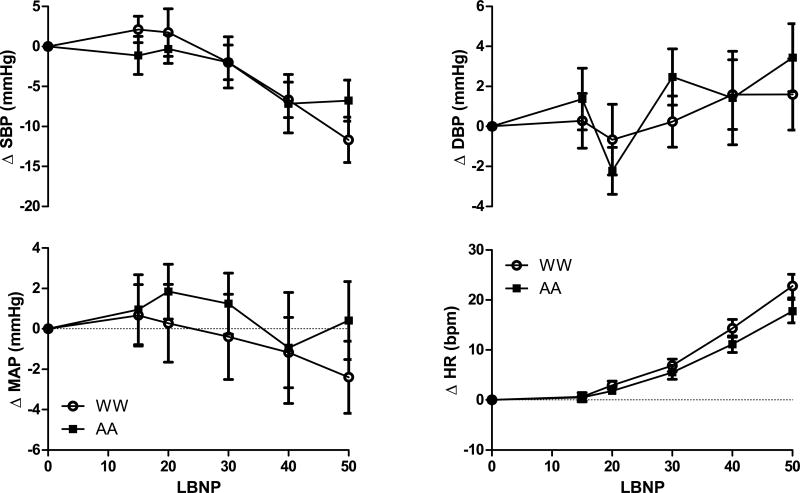
Participant characteristics are listed in Table 1. Women were well matched with respect to age, BMI, and resting hemodynamics. As expected (11), AA women had a higher CSI, indicating greater tolerance to progressive LBNP. Mean arterial BP and diastolic BP were unchanged through −50mmHg of LBNP, and there were no differences between AA and WW (Table 2, Figure 1). Systolic BP decreased and HR increased through −50mmHg of the LBNP, but responses were similar between AA and WW (Table 2, Figure 1). In the subset of women on whom we had blood samples, (19 AA, 26 WW), the change in PRA (AA Δ 1.4, 95% CI 0.8–2.0 vs. WW Δ 1.4, 95%CI 0.8–2.0 ng Ang I/mL/h; P=0.97), ALDO (AA Δ 3.34, 95%CI −7.91–14.59 vs. WW −Δ 2.08, 95%CI −12.57–8.41 pg/mL; P=0.49), NE (AA Δ 142, 95%CI 93–191 vs. WW Δ 109, 95%CI 76–142 pg/mL; P=0.29), and EPI (AA Δ 43, 95%CI 18–68 vs. WW Δ 20, 95%CI 12–28 pg/mL; P=0.08) from baseline to presyncope were not statistically distinguishable between groups.
Table 1.
Participant Characteristics
| AA (n=23) | WW (n=31) | P-value | |
|---|---|---|---|
| Age (years) | 20 ± 1 | 22 ± 1 | 0.11 |
| Height (cm) | 164 ± 2 | 168 ± 1 | 0.06 |
| Mass (kg) | 65 ± 8 | 64 ± 6 | 0.81 |
| BMI (kg/m2) | 24 ± 1 | 23 ± 1 | 0.16 |
| Systolic BP (mmHg) | 119 ± 6 | 117 ± 5 | 0.66 |
| Diastolic BP (mmHg) | 59 ± 4 | 55 ± 4 | 0.19 |
| Mean Arterial BP (mmHg) | 77 ± 4 | 74 ± 4 | 0.30 |
| Heart Rate (bpm) | 68 ± 3 | 65 ± 3 | 0.18 |
| CSI (mmHg•min) | −820 ± 172 | −631 ± 88 | 0.02 |
BMI, body mass index; BP, blood pressure; CSI, cumulative stress index
Mean ± 95% Confidence Interval
Table 2.
Hemodynamics during LBNP
| Baseline | −15 | −20 | −30 | −40 | −50 | |
|---|---|---|---|---|---|---|
| MAP (mmHg) | ||||||
| AA | 77 ± 4 | 78 ± 6 | 79 ± 4 | 78 ± 4 | 76 ± 6 | 77 ± 4 |
| White | 74 ± 4 | 74 ± 4 | 74 ± 4 | 73 ± 4 | 72 ± 4 | 72 ± 4 |
| SBP (mmHg) | ||||||
| AA | 119 ± 6 | 118 ± 6 | 119 ± 6 | 117 ± 4 | 112 ± 8 | 111 ± 6 |
| White | 117 ± 6 | 119 ± 6 | 119 ± 6 | 115 ± 8 | 110 ± 6† | 106 ± 6† |
| DBP (mmHg) | ||||||
| AA | 59 ± 4 | 60 ± 4 | 61 ± 4 | 61 ± 4 | 60 ± 6 | 62 ± 4 |
| White | 55 ± 4 | 55 ± 4 | 56 ± 4 | 55 ± 4* | 57 ± 4 | 57 ± 4 |
| HR (bpm) | ||||||
| AA | 68 ± 4 | 68 ± 4 | 69 ± 4 | 73 ± 4† | 79 ± 4† | 86 ± 4† |
| White | 65 ± 4 | 65 ± 4 | 67 ± 4 | 71 ± 4† | 79 ± 6† | 86 ± 6† |
| CRM | ||||||
| AA | 0.90 ± 0.02 | 0.85 ± 0.02† | 0.81 ± 0.02† | 0.65 ± 0.02† | 0.48 ± 0.04† | 0.34 ± 0.04† |
| White | 0.91 ± 0.02 | 0.82 ± 0.02*† | 0.77 ± 0.02*† | 0.60 ± 0.02*† | 0.44 ± 0.04*† | 0.28 ± 0.04*† |
LBNP, lower body negative pressure; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; CRM, compensatory reserve measure. Mean ± 95% Confidence Interval.
P≤0.05 vs. AA.
P≤0.05 vs. Baseline.
Figure 1.
Changes in mean, systolic, and diastolic BP along with changes in HR in African-American (AA, closed squares) and white women (WW, open circles) through −50mmHg LBNP. Data expressed as mean and 95% confidence intervals.
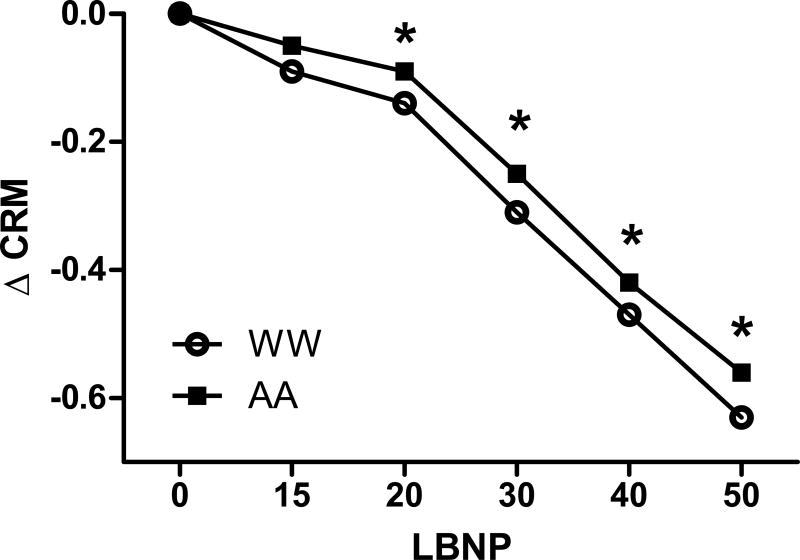
Changes in compensatory reserve are displayed in Figure 2. These results show a pattern of consistent reductions in compensatory reserve as measured by CRM across progressive stages of LBNP for both AA and WW. While AA and WW did not differ in their CRM at baseline, mean values of CRM were between 4% and 21% higher for AA women from −15 mmHg (AA mean 0.85 vs. WW mean 0.82) through −50 mmHg (AA mean 0.34 vs. WW mean 0.28), although only the differences from −20 mmHg through −50 mmHg were statistically significant (P<0.005).
Figure 2.
Least-squared mean values and 95% confidence intervals for CRM among African-American (AA) and white women (WW). * P<0.05 vs WW.
Similarly, average time-to-presyncope was observed to be 1.6 minutes longer (P<0.05) for AA women than for WW. The cumulative incidence function plots (Figure 3) show a greater rate of presyncope for WW beginning after approximately 12 minutes of total LBNP time and extending throughout the remainder of the LBNP experiment. For example, it took approximately 17.5 minutes for 50% of WW to reach presyncope (i.e., decompensation), while it took approximately 20 minutes for 50% of AA women to reach presyncope (Figure 3). The difference in the cumulative presyncope rate was maximized generally between 12 and 15 minutes and then between 16 and 20 minutes, which is consistent with the LBNP stages between roughly −20 mmHg and −50 mmHg, when the differences in CRM values between AA and WW were observed to be the greatest.
Figure 3.
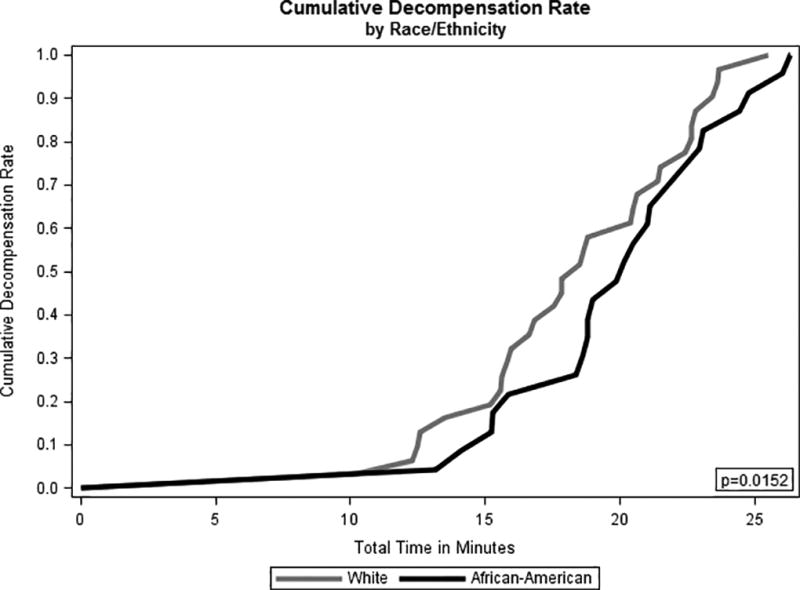
Cumulative incidence function of time-to-presyncope for African-American (AA) and white women (WW).
Results of Cox proportional hazards analysis of time-to-presyncope (Table 3) show that both the CRM measure and AA race/ethnicity were negatively associated with the rate of presyncope for the women in this experiment. Higher values of CRM were associated with lower rates of presyncope (Hazard Ratio=0.002; 95% CI 0.00–0.01; P<0.0001). Likewise, AA women had a 50% lower rate of presyncope compared with WW (Hazard Ratio=0.50; 95% CI 0.28–0.88; P=0.015).
Table 3.
Results of Cox proportional hazards regression model with a time-varying covariate for CRM (N=54)
| Variables | Hazard Ratio (95% CI) | p value |
|---|---|---|
|
| ||
| CRM | 0.002 (0.00–0.01) | <0.0001 |
| African-American | 0.50 (0.28–0.88) | 0.015 |
| White (ref) | -- | -- |
DISCUSSION
The main novel finding of the current study is that measurement of the compensatory reserve using the CRM algorithm accurately predicts racial differences in response to simulated hemorrhage in women well before the onset of traditional signs and symptoms of presyncope. Importantly, differences in CRM between AA and WW were evident as early as the −20 mmHg LBNP stage; this difference persisted through the −50 mmHg LBNP stage until group comparisons were not valid as a result of diminishing sample size in the WW group due to earlier presyncope compared to the AA group. African-American women exhibited CRM values that were 4% higher at −20 mmHg, increasing to 21% higher values at −50 mmHg. In addition to generally higher CRM values between −20 mmHg and −50 mmHg of LBNP, AA women also had a more than 1½ minute greater average time-to-presyncope than WW. These findings support the hypothesis that analysis of arterial waveform features accurately measures reductions in the capacity of individual women to compensate in response to progressive reductions in central blood volume. Furthermore, these findings also support the hypothesis that AA women exhibit a measurable advantage because of their greater capacity to protect the total reserve to compensate for progressive central hypovolemia against exhaustion compared with WW. Consistent with previously reported responses to LBNP, our subjects demonstrated a gradual elevation in heart rate as a compensatory response to a progressive reduction in central blood volume that contributed to unchanged blood pressures during graded increases in LBNP from −15 to −50 mmHg. Similar to previously reported comparisons between groups with high and low LBNP tolerance (10, 18, 22), the BP and HR responses were not different between AA and WW subject groups.
The CRM is a novel, non-invasive method to identify early hemodynamic decompensation in humans (8, 18–20). Traditional vital signs such as BP and HR are not able to provide an early indication of circulatory compromise because of compensatory mechanisms. In as much as LBNP mimics progressive blood loss (8, 14, 15, 23, 24), persistent use of standard monitoring technologies may place a bleeding patient at risk of irreversible shock in the face of hemorrhage if clinical assessment tools are incapable of recognizing the complex integrated response associated with compensation (8, 18). Our data support these previous findings, as mean arterial BP was consistent throughout the initial stages of the LBNP test (through −50mmHg), and there were also no differences between AA and WW in BP or HR. Activation of vasoconstricting hormones such as NE or PRA that help compensate for the loss in blood volume were also similar between AA and WW during LBNP. Although prior studies have suggested changes in vasoactive hormones partially contribute to differences in tolerance to central hypovolemia (10, 11, 16), our data suggest that differences in compensatory responses and tolerance to hypovolemia between AA and WW in the present study could not be explained by influences of the measured vasoactive hormones. However, mean values of CRM decreased in an early and consistent manner across progressive levels of LBNP, indicating increasingly diminished capacity to compensate with increased exposure to central hypovolemia. Although both groups started at the same CRM values at baseline, reductions in CRM values for AA women remained less than WW in response to progressive LBNP exposure. Therefore, the CRM was able to accurately delineate the utilization rate of the compensatory reserve between AA and WW during LBNP whereas traditional vital signs were not. Consistent with previous findings (7, 8, 17, 18), the results of this study demonstrate that overall, the measurement of the compensatory reserve is a strong predictor of tolerance to simulated hemorrhage because of superior sensitivity (i.e., greater alterations) and specificity (i.e., distinguishing AA from WW), and extends these findings to predict racial differences in tolerance to reductions in central blood volume in women.
Although women generally have a lower tolerance to central blood volume loss compared to men (10), there are also varying degrees of tolerance within women (11–13). The mechanisms contributing to these differences within women are multifactorial, and therefore have been the focus of numerous investigations (10, 12, 13, 25–29). The complexity of the total integrative compensatory response reported in women is reflected by fluctuations in sex hormones that may alter peripheral vasoconstriction (12, 13), baroreflex sensitivity (30, 31), or sympathetic outflow (25, 26, 32), thereby contributing to varying levels of tolerance to simulated hemorrhage in women. Consistent with our previous findings (11), the results generated from the retrospective analysis of this larger data set utilized in the present study support the notion that race contributes to differences in tolerance to blood loss among women. We extended our previous findings by demonstrating that measurement of the compensatory reserve provides the most sensitive and specific metric for distinguishing between racial groups of women. Data reported from previous investigations suggest the potential contribution of numerous compensatory responses that could explain increased tolerance in AA women such as greater increase in heart rate (present study), PRA and NE (11), and greater sympathetic transduction for peripheral vasoconstriction (33). However, utilizing measurements of changing features in arterial waveforms underscores the clinical and physiological advantage of obtaining a CRM that represents the integration of the sum total of numerous complex compensatory mechanisms that respond individually to conditions of reduced central blood volume (8). Such an integrative approach is essential in obtaining a valid assessment of clinical status because the physiology of each individual has its own strategy for integrating compensatory mechanisms (6).
Compared to males, females appear to be at greatest risk for acute hemorrhagic shock (10, 34–38). In contrast, there is compelling evidence that females may have a greater survival advantage compared to males in the course of septic shock (39–41), although data obtained from clinical studies have been conflicting (41). One reason for this apparent discrepancy may be because clinical studies have mainly focused on sepsis and complications following trauma or hemorrhage (40). The physiology underlying acute hemorrhage / central hypovolemia is different than sepsis, the latter which is often due to infection (39). Data suggest that the inflammatory and immune responses to sepsis are not appropriately regulated in men, and that estradiol in women plays a primary role in maintaining these appropriate responses (39–41). Thus, while sex differences in survival from sepsis may be influenced by female sex hormones (39–41), the greater risk of hemorrhagic shock in women may reflect differences in the underlying compensatory mechanism(s) that exist between shock caused by absolute acute hypovolemia (hemorrhage) compared to shock associated with relative prolonged hypovolemia (sepsis). In any case, the advantage of the CRM is that irrespective of baseline differences in sex or causes of hypovolemia, CRM can accurately identify impending cardiovascular collapse on an individual basis, and before changes in traditional vital signs. Our data further validate the use of the CRM as a strong tool to be used during triage to distinguish who is in danger of impending cardiovascular collapse.
Limitations
Like any study, our investigation is not without limitation. First, we cannot dismiss the possibility of bias in our sample selection of subjects. We recruited our participants from the greater New Haven, CT, area, using advertisements posted across the city and Yale campus, and adhered to the a priori inclusion/exclusion criteria previously stated. Only women who completed the LBNP protocol and reached presyncope as defined in the methods section were included in the analysis. Thus, to the best of our knowledge, we have no reason to suspect our results are bias, but cannot completely dismiss the possibility. Second, we recognize that the magnitude of difference in the capacity to protect the compensatory reserve between AA and WW appears small, but the results are clinically significant in that they demonstrate the close physiological relationship between the rate of reduction in the capacity to compensate and the patient’s ability to delay the onset of shock. It is important to note that the CRM was able to distinguish early on in the LBNP protocol (−20 mmHg) differences between groups long before signs/symptoms of presyncope were present. This is clinically critical in triage decision support, for earlier time of diagnosis and intervention should be translated to improved patient outcomes (3, 4, 42). CRM data have been collected in the field by Israeli Defense Force medics and assessed retrospectively in an effort to substantiate the value of CRM in early patient status assessment. It was noted that a major advantage of the CRM is that it offers the front line providers with simple, user friendly and intuitive adjuncts for triage (COL Elon Glassberg, personal communication). Thus, findings from the current manuscript lend further support to the accuracy of the CRM to identify impending cardiovascular collapse, and show that this measure is sensitive in women as well as men.
In conclusion, we show that measurement of the compensatory reserve can accurately predict racial differences during reductions in central blood volume in women. Furthermore, the CRM identified these differences early in the process of progressive central hypovolemia (as early as −20mmHg, second LBNP stage) and prior to changes in traditional vital signs. These data lend further support to show that the CRM is a strong predictor of tolerance to simulated hemorrhage and that this superiority in prediction applies to women as well as men. An important clinical translation is that the CRM provides a more sensitive and specific clinical metric for early assessment of cardiovascular collapse in comparison to traditional vital signs and symptoms or changes in hemodynamics. Given the threat of exposure to life-threatening hemorrhage on the battlefield, these findings are important for servicewomen and healthcare providers as the diversity in our military personnel increases with respect to sex and race. In the context of assessing the clinical status in bleeding trauma patients or combat casualties where severe central hypovolemia can be life threatening, such a difference could potentially represent greater opportunity for recognition and initiation of life-saving interventions, and could ultimately correspond to a survival advantage.
Acknowledgments
GRANTS
This study was supported by a National Institutes of Health Research Grant R01 HL071159 (awarded to NSS) and a US Army Medical Research and Materiel Command Grant D-009-2014-USAISR through the Combat Casualty Care Research Program (awarded to VAC).
The authors wish to thank Andrew Grabarek and Cheryl Leone for technical support, and the participants for their time.
Footnotes
AUTHOR CONTRIBUTIONS
M.M.W., K.H., N.S.S., and V.A.C. participated in the conception/design of this study, M.M.W., K.H., J.T.H., and C.D.N. participated in data collection/analysis, M.M.W., K.H., J.T.H., C.D.N., N.S.S., and V.A.C. participated interpretation, and the writing the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship; all those who qualify for authorship are listed.
Conflicts of Interest: None
Meetings the paper was presented: Annual Meeting of Experimental Biology, April 22–26, 2017 in Chicago, IL.
References
- 1.Defense Do. Population Representation in the Military Services: Fiscal Year 2014 Summary Report. Defense Do, editor. [Accessed on September 8, 2017];:1–59. http://www.people.mil/2014.
- 2.Center PR. Women in the U.S. Military: Growing Share, Distinctive Profile. [Accessed on September 8, 2017];2011 [Available from: http://www.pewsocialtrends.org/2011/12/22/women-in-the-u-s-military-growing-share-distinctive-profile/
- 3.Eastridge BJ, Hardin M, Cantrell J, Oetjen-Gerdes L, Zubko T, Mallak C, Wade CE, Simmons J, Mace J, Mabry R, et al. Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma. 2011;71(1 Suppl):S4–8. doi: 10.1097/TA.0b013e318221147b. [DOI] [PubMed] [Google Scholar]
- 4.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–7. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 5.Hinojosa-Laborde C, Aden JK, Goei KA, Convertino VA. Evidence for a higher risk of hypovolemia-induced hemodynamic instability in females: implications for decision support during prehospital triage. Mil Med. 2015;180(3 Suppl):19–23. doi: 10.7205/MILMED-D-14-00394. [DOI] [PubMed] [Google Scholar]
- 6.Carter R, Hinojosa-Laborde C, Convertino VA. Variability in integration of mechanisms associated with high tolerance to progressive reductions in central blood volume: the compensatory reserve. Physiol Rep. 2016;4(4) doi: 10.14814/phy2.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Convertino VA, Moulton SL, Grudic GZ, Rickards CA, Hinojosa-Laborde C, Gerhardt RT, Blackbourne LH, Ryan KL. Use of advanced machine-learning techniques for noninvasive monitoring of hemorrhage. J Trauma. 2011;71(1 Suppl):S25–32. doi: 10.1097/TA.0b013e3182211601. [DOI] [PubMed] [Google Scholar]
- 8.Convertino VA, Wirt MD, Glenn JF, Lein BC. The Compensatory Reserve for Early and Accurate Prediction of Hemodynamic Compromise: A Review of the Underlying Physiology. Shock. 2016;45(6):580–90. doi: 10.1097/SHK.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 9.Muniz GW, Wampler DA, Manifold CA, Grudic GZ, Mulligan J, Moulton S, Gerhardt RT, Convertino VA. Promoting early diagnosis of hemodynamic instability during simulated hemorrhage with the use of a real-time decision-assist algorithm. J Trauma Acute Care Surg. 2013;75(2 Suppl 2):S184–9. doi: 10.1097/TA.0b013e31829b01db. [DOI] [PubMed] [Google Scholar]
- 10.Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol. 1998;275(6 Pt 2):R1909–20. doi: 10.1152/ajpregu.1998.275.6.R1909. [DOI] [PubMed] [Google Scholar]
- 11.Hinds K, Stachenfeld NS. Greater orthostatic tolerance in young black compared with white women. Hypertension. 2010;56(1):75–81. doi: 10.1161/HYPERTENSIONAHA.110.150011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenner MM, Haddadin AS, Taylor HS, Stachenfeld NS. Mechanisms contributing to low orthostatic tolerance in women: the influence of oestradiol. J Physiol. 2013;591(Pt 9):2345–55. doi: 10.1113/jphysiol.2012.247882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenner MM, Taylor HS, Stachenfeld NS. Progesterone enhances adrenergic control of skin blood flow in women with high but not low orthostatic tolerance. J Physiol. 2011;589(4):975–86. doi: 10.1113/jphysiol.2010.194563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinojosa-Laborde C, Howard JT, Mulligan J, Grudic GZ, Convertino VA. Comparison of compensatory reserve during lower-body negative pressure and hemorrhage in nonhuman primates. Am J Physiol Regul Integr Comp Physiol. 2016;310(11):R1154–9. doi: 10.1152/ajpregu.00304.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinojosa-Laborde C, Shade RE, Muniz GW, Bauer C, Goei KA, Pidcoke HF, Chung KK, Cap AP, Convertino VA. Validation of lower body negative pressure as an experimental model of hemorrhage. J Appl Physiol (1985) 2014;116(4):406–15. doi: 10.1152/japplphysiol.00640.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Convertino VA, Sather TM. Vasoactive neuroendocrine responses associated with tolerance to lower body negative pressure in humans. Clin Physiol. 2000;20(3):177–84. doi: 10.1046/j.1365-2281.2000.00244.x. [DOI] [PubMed] [Google Scholar]
- 17.Convertino VA, Howard JT, Hinojosa-Laborde C, Cardin S, Batchelder P, Mulligan J, Grudic GZ, Moulton SL, MacLeod DB. Individual-Specific, Beat-to-beat Trending of Significant Human Blood Loss: The Compensatory Reserve. Shock. 2015;44(Suppl 1):27–32. doi: 10.1097/SHK.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 18.Convertino VA, Grudic G, Mulligan J, Moulton S. Estimation of individual-specific progression to impending cardiovascular instability using arterial waveforms. J Appl Physiol (1985) 2013;115(8):1196–202. doi: 10.1152/japplphysiol.00668.2013. [DOI] [PubMed] [Google Scholar]
- 19.Howard JT, Janak JC, Hinojosa-Laborde C, Convertino VA. Specificity of Compensatory Reserve and Tissue Oxygenation as Early Predictors of Tolerance to Progressive Reductions in Central Blood Volume. Shock. 2016;46(3 Suppl 1):68–73. doi: 10.1097/SHK.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 20.Janak JC, Howard JT, Goei KA, Weber R, Muniz GW, Hinojosa-Laborde C, Convertino VA. Predictors of the Onset of Hemodynamic Decompensation during Progressive Central Hypovolemia: Comparison of the Peripheral Perfusion Index, Pulse Pressure Variability, and Compensatory Reserve Index. Shock. 2015;44(6):548–53. doi: 10.1097/SHK.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 21.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–57. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 22.Rickards CA, Ryan KL, Cooke WH, Convertino VA. Tolerance to central hypovolemia: the influence of oscillations in arterial pressure and cerebral blood velocity. J Appl Physiol (1985) 2011;111(4):1048–58. doi: 10.1152/japplphysiol.00231.2011. [DOI] [PubMed] [Google Scholar]
- 23.Cooke WH, Ryan KL, Convertino VA. Lower body negative pressure as a model to study progression to acute hemorrhagic shock in humans. J Appl Physiol (1985) 2004;96(4):1249–61. doi: 10.1152/japplphysiol.01155.2003. [DOI] [PubMed] [Google Scholar]
- 24.Johnson BD, van Helmond N, Curry TB, van Buskirk CM, Convertino VA, Joyner MJ. Reductions in central venous pressure by lower body negative pressure or blood loss elicit similar hemodynamic responses. J Appl Physiol (1985) 2014;117(2):131–41. doi: 10.1152/japplphysiol.00070.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter JR, Klein JC, Schwartz CE. Effects of oral contraceptives on sympathetic nerve activity during orthostatic stress in young, healthy women. Am J Physiol Regul Integr Comp Physiol. 2010;298(1):R9–R14. doi: 10.1152/ajpregu.00554.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab. 2009;297(1):E85–91. doi: 10.1152/ajpendo.00019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol. 2004;286(1):H449–57. doi: 10.1152/ajpheart.00735.2002. [DOI] [PubMed] [Google Scholar]
- 28.Fu Q, Witkowski S, Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation. 2004;110(18):2931–7. doi: 10.1161/01.CIR.0000146384.91715.B5. [DOI] [PubMed] [Google Scholar]
- 29.Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol. 2005;289(1):R109–16. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- 30.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101(8):862–8. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 31.Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation. 2000;102(13):1473–6. doi: 10.1161/01.cir.102.13.1473. [DOI] [PubMed] [Google Scholar]
- 32.Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol. 2009;587(Pt 9):2019–31. doi: 10.1113/jphysiol.2008.168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ray CA, Monahan KD. Sympathetic vascular transduction is augmented in young normotensive blacks. J Appl Physiol (1985) 2002;92(2):651–6. doi: 10.1152/japplphysiol.00788.2001. [DOI] [PubMed] [Google Scholar]
- 34.Convertino VA. Lower body negative pressure as a tool for research in aerospace physiology and military medicine. J Gravit Physiol. 2001;8(2):1–14. [PubMed] [Google Scholar]
- 35.Franke WD, Lee K, Graff SR, Flatau AB. Effects of gender on the autonomic modulation of the cardiovascular responses to lower body negative pressure. Aviat Space Environ Med. 2000;71(6):626–31. [PubMed] [Google Scholar]
- 36.Gotshall RW. Gender differences in tolerance to lower body negative pressure. Aviat Space Environ Med. 2000;71(11):1104–10. [PubMed] [Google Scholar]
- 37.Montgomery LD, Kirk PJ, Payne PA, Gerber RL, Newton SD, Williams BA. Cardiovascular responses of men and women to lower body negative pressure. Aviat Space Environ Med. 1977;48(2):138–45. [PubMed] [Google Scholar]
- 38.White DD, Gotshall RW, Tucker A. Women have lower tolerance to lower body negative pressure than men. J Appl Physiol. 1996;80(4):1138–43. doi: 10.1152/jappl.1996.80.4.1138. [DOI] [PubMed] [Google Scholar]
- 39.Hubbard WJ, Bland KI, Chaudry IH. The ERRor of Our Ways: Estrogen-Related Receptors are About Energy, Not Hormones, and are Potential New Targets for Trauma and Shock. Shock. 2015;44(1):3–15. doi: 10.1097/SHK.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 40.Weniger M, Angele MK, Chaudry IH. The Role and Use of Estrogens Following Trauma. Shock. 2016;46(3 Suppl 1):4–11. doi: 10.1097/SHK.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 41.Weniger M, D'Haese JG, Angele MK, Chaudry IH. Potential therapeutic targets for sepsis in women. Expert Opin Ther Targets. 2015;19(11):1531–43. doi: 10.1517/14728222.2015.1057570. [DOI] [PubMed] [Google Scholar]
- 42.Mason PE, Eadie JS, Holder AD. Prospective observational study of United States (US) Air Force Critical Care Air Transport team operations in Iraq. J Emerg Med. 2011;41(1):8–13. doi: 10.1016/j.jemermed.2008.06.032. [DOI] [PubMed] [Google Scholar]