Abstract
Background
Liver transplants account for a high number of procedures with major investments from all stakeholders involved; however, limited studies address liver transplant population heterogeneity pre-transplant predictive of post-transplant survival.
Objective
To identify novel and meaningful patient clusters predictive of mortality that explains the heterogeneity of liver transplant population, taking a holistic approach.
Methods
A retrospective cohort study of 344 adult patients who underwent liver transplantation between 2008 through 2014. Predictors were summarized severity scores for comorbidities and other suboptimal health states grouped into 11 body systems, the primary reason for transplantation, demographics/environmental factors, and Model for End Liver Disease (MELD) score. Logistic regression was used to compute the severity scores, hierarchical clustering with weighted Euclidean distance for clustering, Lasso-penalized regression for characterizing the clusters and Kaplan-Meier analysis to compare survival across the clusters.
Results
Cluster #1 included patients with more severe circulatory problems. Cluster #2 represented older patients with more severe primary disease, while Cluster #3 contained healthiest patients. Clusters #4 and #5 represented patients with musculoskeletal (e.g. pain) and endocrine problems (e.g. malnutrition), respectively. There was a statistically significant difference for mortality between clusters (p value <.001).
Conclusions
This study developed a novel methodology to address heterogeneous and high dimensional liver transplant population characteristics in a single study predictive of survival. A holistic approach for data modeling and additional psychosocial risk factors have the potential to address holistically nursing challenges on liver transplant care and research.
Keywords: Holistic nursing, Liver Transplantation, Heterogeneity, Predictive Modeling, Survival
Transplantation increasingly has been applied as a treatment of choice for severe and end-stage liver diseases. The United States (US) accounted for 7,851 liver transplants in 2016, representing 23% of all transplants in the country, and is the leader worldwide in the number of procedures annually (UNOS, 2014; GODT, 2012). Liver recipient overall survival rates range from 55.6% to 86.5%, depending on recipient status before transplantation and years of follow-up after transplantation (UNOS, 2017). However, the liver transplant population is highly heterogeneous in terms of clinical presentation, non-clinical characteristics, and outcomes. Despite this known heterogeneity, few studies have undertaken a comprehensive investigation of the broad array of potential predictors and the impact of their combinations on patient outcomes (de Kroon, Drent, van den Berg, Haagsma, & Groningen, 2007; Sullivan, Radosevich, & Lake, 2014); and, to our knowledge, no study attempted to bring this heterogeneity to the surface.
A critical review of the literature showed that there is rigorous research addressing physiological risk factors predictive of survival, such as cirrhosis, hepatocellular carcinoma, and hepatitis C (Cho et al., 2001; Dickson et al., 2011; Patel et al., 2012), but further research is needed to include additional risk factors that represent a holistic perspective for this population and strengthen the predictive value of those models. A holistic approach supports the focus on the whole person, the connection of mind, body, and spirit rather than just on illness and specific diseases with the goal of achieving well-being (Walter, 2017). For instance, well-being is a concept that has recently been adopted to entail the whole-person perspective and share common elements across countries and cultures (Gallup, 2014; Kreitzer, 2012; Monsen, Peters, Schlesner, Vanderboom, & Holland, 2015). A framework to map liver transplant characteristics holistically was developed and is reported elsewhere (Pruinelli et al., 2016). Considering this framework, even important non-physiological predictors, such as patient alcohol abuse and psychosocial problems, seldom have been included in statistical models for this population (Cho et al., 2001; Dickson et al., 2011; Fan et al., 2009; Gleisner et al., 2010; Pruinelli, 2016). Further, due to the progression of liver diseases to worsening states while patients are waiting for a liver transplant, several suboptimal health states are developed before transplantation, such as malnutrition, sleep disturbance, and musculoskeletal problems. Thus, there is an urgent need to adopt a holistic approach to explore the association of a broad array of pre-transplant risk factors with survival to better characterize the liver transplant population heterogeneity.
The liver transplant population represents a mix of different risk factors that have different outcomes. Addressing population variability is the aim of Precision Medicine Initiative (PMI), launched in 2015 by the US government (NIH, 2016). The program is an emerging approach for disease treatment and prevention that takes into account individual variability of biological, environmental, and behavioral influences on disease progressions. Similarly, the Symptom Science Model (SSM)(NINR, 2016) was established to guide research in predicting which patients are most at-risk for adverse symptoms and conditions. Both initiatives emphasize developing personalized health strategies for disease and symptom management based not only on clinical, but also on physical and psychosocial parameters. To this end, the inclusion of a variety of factors representing a holistic approach is central to identify how outcomes vary according to patient’s characteristics. Much of this variability can be explained by identifying patient groups with very distinct risk factors. Survival rates vary according to these risk factors, and consequently, vary highly across different groups presenting with different risk factors. On the other hand, since the risk factors are similar within each group, survival rates are also going to be relatively similar. The PMI program seeks to reach all diseases, but to our knowledge, other than common diseases, such as diabetes and heart diseases, no efforts have been addressed for the liver transplant population. To this end, PMI premises applied to the liver transplant population can support research that comprises liver transplant heterogeneity and a holistic approach to risk factors; thus, providing more effective ways of understanding this population.
Cutting-edge statistical approaches, such as data mining techniques, can be used to analyze a wide variety of patient characteristics and discover unique groups within the liver transplant population that represents this heterogeneity, which might otherwise remain unknown (Tan, Steinbach, & Kumar, 2006). Specifically, clustering methods can be used to discover groups (clusters) of patients such that patients in the same group have similar characteristics to each other, while patients in different clusters are distinctly different. These clusters can represent different subcategories of a disease and/or suboptimal health states, such as novel groupings of liver transplant patient characteristics (Dey et al., 2015; Lanzola, Parimbelli, Micieli, Cavallini, & Quaglini, 2014; Tan et al., 2006), and these may explain why specific groups have different outcomes. This study aimed to apply a hierarchical clustering analysis to identify novel and meaningful patient clusters, incorporating comorbidities and suboptimal health states, to explain the heterogeneity of liver transplant population that are predictive of all-cause mortality.
Methods
Cohort Source and Selection
Data from the Organ Transplant Tracking Registry (OTTR) (OTTR, 2014) of a Midwest institution was used. After approval from the Institutional Review Board, a dataset containing all adult patients 18 years and older who underwent liver transplantation between 01/01/2008 and 12/31/2014, with associated follow-up data through 2/28/2016, was transferred to a secure environment for data analysis.
Inclusion criteria were patients who received their first liver transplant. Patients who received combined transplantation were excluded to prevent bias. Data included: pre-transplant recipient information (date of transplant, reason for transplantation, Model for End Liver Disease (MELD) score), laboratory results (creatinine, bilirubin, international normalized ratio (INR)), problem list, containing comorbidities and suboptimal health states, sociodemographic (age, gender, and race) and environmental information (smoking status and waiting time at list), and post-transplant vital status (dead or alive). For this study, suboptimal health states are a physiological and/or physical state between health and disease, a subclinical problem that could be reversible, not progressing to a disease (Wang & Yan, 2012). Risk factors were pre-transplant recipient conditions, and the outcome was post-transplant all-cause mortality.
Data were assessed for quality based on the current literature and domain knowledge. Duplicate records were removed and missing MELD scores were computed based on data available from other tables; if data was not available for computation, patients were excluded.
Statistical Analysis
Overview
To address the large number of variables (174 variables) and a comparatively modest sample size (344 patients), problems, comorbidities, or suboptimal health states were summarized by body system and then converted into severity scores. By using severity scores, the total number of variables was reduced to 14 and these scores were used to perform clustering rather than the raw data. The analysis approach addressed the varying degree of each variable importance in patient similarity by defining the notion of similarity through a metric that assigns weights to variables, proportional to the variable’s ability to predict mortality. This approach also takes the correlation among variables into account. Below is a detailed explanation of each of these steps.
Constructing severity scores
A severity score was computed for every patient and every body system. The severity score is a number, the log odds of mortality that the problems in the body system confer for the patient. In total, fourteen severity scores were constructed: one for each of the 11 body systems, one for the primary disease (reason for transplant), one for demographics and environmental factors, and the original MELD score was used, which is already a severity score.
The severity scores were computed as follows. All comorbidities and suboptimal health states from the problem table were extracted and categorized into body systems as defined in OTTR. The model for the severity score was a logistic regression model (LRM), which was constructed for each body system separately. The outcome (dependent variable) was all-cause mortality and the independent variables were the various problems in a body system. Variables were selected based on statistical and clinical significance. Clinical significance was determined based on literature review and whether characteristics represented any of the holistic framework dimensions for liver transplant patients (Pruinelli et al., 2016). Five smaller and similar body systems were combined into two models (gastrointestinal and pancreas; and eyes, ears, nose, and throat, with skin and subcutaneous), and one system (gynecologic) was excluded due to the lack of statistical and clinical significance. Backwards elimination was used for statistical significance. The coefficients derived from the LRM models were used for building severity scores for each body system where values were weighted to each patient according to the LRM results. That means, even in cases where the coefficient was high (e.g. peripheral arterial disease with a coefficient of 1.788) and the p-value was not significant, the coefficient was considered and was weighted for each patient. The severity score can thus be interpreted as the log odds of mortality given the problems the patient has associated with the body system in question. A bootstrap simulation with 1,000 replications was used to estimate the predictive validity of each LRM model.
A similar LRM model was built for the primary disease (reason of transplantation) and for demographic/environmental variables. Demographics and environmental variables were combined in one severity score because they represented a small number of variables and were not associated with any body system in OTTR. The resultant data set for analysis had 14 severity scores, including the MELD score, and was the input for the clustering algorithm.
Clustering analysis
The clustering analysis (Tan et al., 2006) had two steps: 1) define a similarity (or dissimilarity) metric that can assign weights to variables that reflect their importance, and 2) perform the clustering using this similarity metric. For step 1, the weighted Euclidean distance was selected as the metric of (dis)similarity. The weights were computed using a LRM with the severity scores as independent variables and all-cause mortality as the dependent variable. There was a weight associated with each severity score (the coefficient of the variable in the model). For step 2, the agglomerative clustering analysis was employed. Agglomerative clustering iteratively merges patient groups that are relatively homogeneous (there is little variability in the clinical presentation within the groups) to form larger groups that are more heterogeneous (having more clinical variability). Initially each patient is considered a small but very homogeneous cluster, then in each iteration, the two most similar clusters are merged until ultimately all patients form a single highly heterogeneous cluster (the entire population). The homogeneity was measured using Wald’s within-cluster sum squared error.
Interpreting the clustering results
To further characterize the clusters, for each cluster, Lasso-penalized logistic regression models were built, “predicting” whether each patient belongs to a particular cluster or a different one. A high positive coefficient for a risk score indicates that the problems in the body system represented by the risk score are prevalent in the cluster; high negative coefficient indicates that the corresponding body system problems have low prevalence; and zero coefficients indicate that the cluster is no different from the population in terms of the corresponding body system. The Lasso penalty induces sparsity (setting coefficients to zero); thus, providing a concise characterization of the clusters.
Survival Analysis
A Kaplan-Meier analysis was used to estimate survival for each identified clusters of patients, considering its characteristics, and log-rank and Flemington-Herrington tests were used to compare survival estimates between clusters.
Statistical analysis was performed using RStudio, version 3.1.3. Descriptive statistics are presented as mean and IQR for continuous variables. Categorical data are presented as percentages.
Results
From a total of 358 patients that underwent liver transplantation for the first time during the study period, 344 patients were included in this analysis. Fourteen patients were excluded due to a missing MELD score and insufficient laboratory data available for computation.
The majority of patients were male (70%), Caucasian (82.2%), and non-smokers at the time of transplant (86%) (Table 1). The mean age was 55.2 (IQR = 50.9 – 61.7), with a mean waiting list time pre-transplant of 320.5 days (IQR = 27 – 410). The mean MELD score at the time of transplant was 30.18 (IQR = 25 – 36).
Table 1.
Generalized linear models for severity score models by organ system including pre-transplant risk factors.
| Generalized linear models | n (%) | Mean(IQR) | Coefficient | p value |
|---|---|---|---|---|
| Demographics/Environment | ||||
|
| ||||
| Age < 50.90 (1st quartile) | 87 | (28.2) | −0.531 | .12 |
| Age > 61.67 (3rd quartile) | 97 | (28.1) | 0.083 | .77 |
| Gender male | 241 | (70.0) | 0.301 | .30 |
| Race Caucasian | 283 | (82.2) | 0.388 | .28 |
| Smoking status at time of transplant | 48 | (13.9) | 0.138 | .69 |
| Waiting list time in days | 320.5 | (27–410.2) | <−0.0001 | .72 |
|
| ||||
| Primary disease reason for transplantation | ||||
|
| ||||
| Hepatocellular carcinoma and Cirrhosis | 55 | (15.9) | 0.375 | .30 |
| Alcoholic cirrhosis with hepatitis C | 30 | (8.7) | 0.757 | .07 |
| Cirrhosis: type C | 58 | (16.8) | 0.485 | .16 |
| Hepatocellular carcinoma | 8 | (2.3) | 0.939 | .21 |
| Cirrhosis: type B HBSAG+ | 14 | (4.0) | 0.862 | .14 |
|
| ||||
| Blood | ||||
|
| ||||
| Low hemoglobin | 138 | (40.1) | 0.157 | .55 |
| High lipids | 64 | (18.6) | −0.352 | .31 |
| Infection: non-viral | 56 | (16.2) | 0.273 | .41 |
| Low platelet | 70 | (20.3) | −0.453 | .18 |
|
| ||||
| Circulatory | ||||
|
| ||||
| Arrhythmia | 42 | (12.2) | −0.420 | .33 |
| Peripheral vascular disease | 6 | (1.7) | 1.788 | .06 |
| Coronary artery disease (CAD) | 22 | (6.3) | 1.458 | .004** |
| Varicose vein requiring surgery | 5 | (1.4) | 0.802 | .43 |
| Hypotensive crisis | 17 | (4.9) | −1.170 | .16 |
| Angina | 6 | (1.7) | −2.046 | .12 |
|
| ||||
| Endocrine | ||||
|
| ||||
| Failure to thrive or malnutrition requiring treatment | 34 | (9.8) | 0.562 | .15 |
| Adrenal insufficiency | 5 | (1.4) | 2.735 | .02* |
| Electrolyte imbalance requiring treatment | 5 | (1.4) | −1.324 | .37 |
|
| ||||
| Gastrointestinal & pancreas: native | ||||
|
| ||||
| Portal hypertensive gastropathy | 96 | (27.9) | 0.430 | .11 |
| Gastrointestinal benign mass or cyst not infected | 53 | (15.4) | 0.263 | .44 |
| Pancreas benign mass or cyst not infected | 9 | (2.6) | 0.367 | .61 |
| Pancreatitis: acute | 11 | (3.1) | −0.337 | .67 |
|
| ||||
| Kidney & ureter: native | ||||
|
| ||||
| Pre-transplant hemodialysis | 61 | (17.7) | 0.397 | .21 |
| Dysfunction with no dialysis | 81 | (23.5) | −0.210 | .49 |
| Bleed | 7 | (2.0) | 0.889 | .25 |
| Benign mass or cyst not infected | 16 | (4.6) | 0.719 | .18 |
|
| ||||
| Liver & biliary | ||||
|
| ||||
| Ascites | 138 | (40.1) | −0.267 | .31 |
| Biliary stones and/or calculi | 50 | (14.5) | −0.320 | .40 |
| Benign mass or cyst not infected | 14 | (4.0) | 0.647 | .26 |
| Cholangitis | 15 | (4.3) | −0.699 | .36 |
| Bile leak | 4 | (1.1) | 1.184 | .24 |
|
| ||||
| Respiratory | ||||
|
| ||||
| Acute respiratory distress syndrome (ARDS) | 52 | (15.1) | −0.051 | .88 |
| Asthma | 36 | (10.4) | 0.449 | .25 |
| Chronic obstructive pulmonary disease (COPD) | 26 | (7.5) | −0.398 | .45 |
|
| ||||
| Neurologic | ||||
|
| ||||
| Encephalopathy: hepatic | 245 | (71.2) | 0.462 | .12 |
| Sleep apnea | 28 | (8.1) | 0.481 | .26 |
| Seizures and/or epilepsy | 16 | (4.6) | 0.424 | .44 |
|
| ||||
| Mental illness | ||||
|
| ||||
| Alcohol abuse | 163 | (47.3) | 0.123 | .67 |
| Iv drug abuse | 84 | (24.4) | 0.220 | .50 |
| Depression requiring treatment | 105 | (30.5) | 0.205 | .44 |
| Bipolar disorder | 7 | (2.0) | −0.787 | .47 |
|
| ||||
| Musculoskeletal | ||||
|
| ||||
| Pain of unknown etiology | 23 | (6.6) | 0.886 | .05 |
| Carpal tunnel syndrome | 11 | (3.1) | −1.479 | .17 |
| Fibromyalgia | 6 | (1.7) | 1.452 | .09 |
|
| ||||
| Extremities (ears, nose, eyes, skin) | ||||
|
| ||||
| Hearing loss | 10 | (2.9) | −1.191 | .26 |
| Diabetic Retinopathy | 5 | (1.4) | 0.832 | .36 |
| Skin or subcutaneous infection: non-viral | 41 | (11.9) | 0.649 | .07 |
Note: Significant levels:
p value < 0.01,
p value < 0.05.
Severity Score Models
Table 1 presents the severity score models for demographics/environmental factors, primary disease, and each of the 11 body systems after backward elimination was applied. Each segment in the table describes the model for the corresponding body system, and represent the coefficients used to compute the severity scores during the next step of the analysis. Coefficients denote the log odds of mortality for each model. For example, considering the endocrine body system model, failure to thrive or malnutrition requiring treatment and adrenal insufficiency increases the log odds of mortality by 0.56 and 2.73, respectively, while electrolyte imbalance requiring treatment decreases by 1.32 [log(odds) = 0.56 + 2.73 – 1.32 = 1.97]. Overall, patients with endocrine body system comorbidities and suboptimal health states, such as malnutrition and electrolyte imbalance, had 7.17 [exp(1.97)] times greater odds of dying than patients who did not have endocrine body system comorbidities.
Clustering Results
As the first step of clustering, weights were computed for the weighted Euclidean distance using a second LRM (Table 2). For example, taking the two extreme coefficients from Table 2, mental and circulatory body systems results showed that for each unit increase in the severity of mental problems, the log odds of mortality increased by 0.58, while a unit increase in the severity of circulatory problems increased the risk of mortality by 3.717. Since the severity scores are on the same scale, the coefficients are directly comparable: circulatory problems are 6 (3.717/.58=6.379) times more predictive of all-cause mortality than mental illness. During clustering, these weighted coefficients, reflect this difference in importance: a unit of dissimilarity in circulatory system-related problems between two patients will make them approximately 6 times as dissimilar as a unit difference in mental illness. The agglomerative coefficient, which measures the quality of the cluster structure in the data, was 0.95 (1=very clear structure, 0=no structure was found).
Table 2.
Parameter estimates for severity score models (n=344).
| Severity score models | Coefficient | Std. Error | p value |
|---|---|---|---|
| Demographics/Environmental | 4.532 | 2.623 | .08 |
| Primary disease reason for transplantation | 4.580 | 2.411 | .05 |
| MELD | −0.003 | 0.015 | .82 |
| Comorbidities conditions | |||
| Blood | 4.950 | 3.212 | .12 |
| Circulatory | 3.717 | 1.329 | .005** |
| Endocrine | 4.206 | 1.907 | .02* |
| Gastrointestinal & pancreas: native | 3.534 | 2.807 | .20 |
| Kidney & ureter: native | 3.930 | 2.615 | .13 |
| Liver & Biliary | 4.215 | 2.640 | .11 |
| Respiratory | 4.854 | 4.685 | .30 |
| Neurologic | 1.213 | 3.059 | .69 |
| Mental illness | 0.580 | 4.216 | .89 |
| Musculoskeletal | 4.117 | 1.993 | .03* |
| Extremities (ears, nose, eyes, skin) | 2.964 | 2.577 | .25 |
Note: Significance level:
p value < 0.01,
p value < 0.05.
Interpretation of the Clustering
To identify patient characteristics that distinguish the clusters, Figure 1 displays results of the discriminative analysis through the Lasso-penalized regression with the 14 variables used as independent variables. The figure depicts 14 groups of 5 color bars, each group corresponding to severity scores and each bar representing a cluster. The vertical axes represent the Lasso coefficient results and the horizontal axes the severity scores assigned to each of the clusters.
Figure 1.
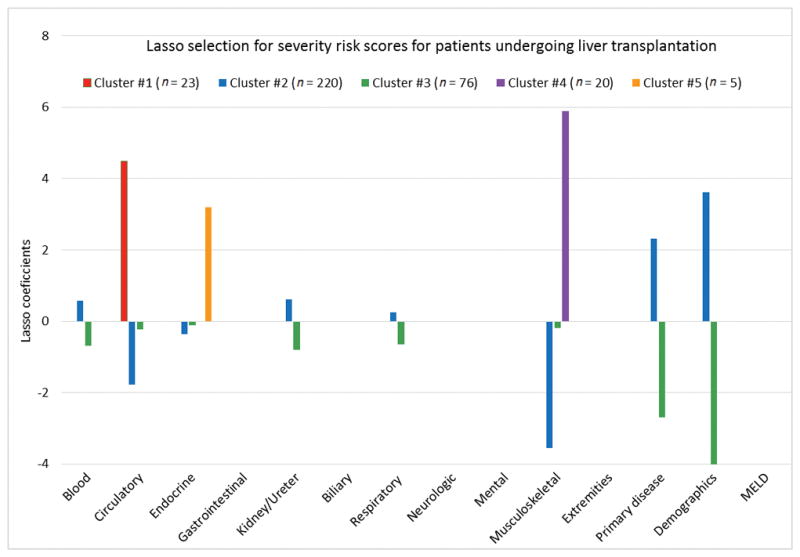
Lasso penalized regression coefficients for severity score of predictors of death for patients undergoing liver transplantation (n=344).
Lasso results showed that there was a single driver for patient assignment to three of the five clusters (#1, #4, #5). Cluster #1 represents patients with a higher circulatory system severity score, Cluster #4 with higher musculoskeletal system severity score, and Cluster #5 with higher endocrine system severity score. In contrast, Lasso results for Cluster #3 showed negative values for multiple severity scores, suggesting that Cluster #3 consisted of healthier patients (those with lower severity score for the indicated body systems). Finally, results showed that Cluster #2 represents patients whose risk of mortality is driven by demographics/environmental factors, primary disease reason for transplantation, and have low severity for circulatory and musculoskeletal body systems. Severity scores for gastrointestinal, biliary, neurologic, mental, extremities systems, and meld score were not predictive of membership for any cluster in this population.
Survival Analysis
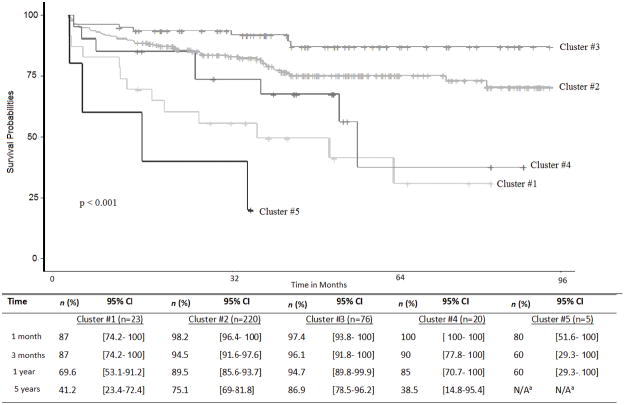
The mean follow-up time for patients alive at the end of the follow-up was 1523 days (n = 262, IQR = 1093) and the mean for those who had died was 609 days (n = 82, IQR = 996). Figure 2 shows the survival curves for the five clusters of patients, and the estimated survival rate for these same clusters. Cluster #3 and Cluster #5, respectively, had the best and worst survival probabilities considering a long-term follow-up of 5 years, and all-cause mortality, after liver transplantation (Figure 2). Patients in Cluster #4 had the worst survival at long term follow-up, 37.5% at 5 years. The log-rank and Flemington-Herrington tests showed statistical significance between clusters (p value < 0.001).
Figure 2.
Survival probabilities and estimates survival rates after liver transplantation for different cluster of patients (n=344).
Discussion
Important heterogeneity exists in patients undergoing liver transplant in terms of their clinical presentation, their non-clinical characteristics, and their outcomes in predicting survival. Recipients have a variety of risk factors, and according to these factors, survival rates vary greatly. In this data-driven study, a hierarchical clustering analysis was applied to identify novel and meaningful patient clusters to better understand and characterize this heterogeneity, and to discover knowledge that is unknown in transplant research. A holistic perspective was employed to achieve this goal which took into account a broad range of clinical and non-clinical factors, psychosocial risk factors, including environmental factors. However, consideration of a broad range of risk factors with a relatively modest sample size made off-the-shelf cluster analysis challenging and required the development of a novel, robust analytic strategy to overcome these challenges. Following the proposed strategy, hierarchical clustering analysis was able to identify clusters of patients who share similar characteristics that are predictive of all-cause mortality and explain liver transplant population heterogeneity. Understanding this heterogeneity and its impact on disease progressions and health deterioration has the potential to improve the allocation process and tailored nursing and multiprofessional interventions, increasing personalized and holistic care to this population.
Cluster #1 was determined by circulatory system severity and highly predictive of survival after liver transplantation, and patients had poor survival probability at 5 years after transplantation. Cardiac evaluations pre-transplantation already follow strict guidelines due to the higher risk of mortality and morbidity for patients undergoing liver transplantation with such problems (UNOS, 2017); however, additional investigation and validation of these findings are needed to determine the appropriate interventions to be performed before undergoing transplantation that may improve outcomes (Skaro et al., 2014). Patients with circulatory problems, such as peripheral artery disease, have impaired quality of life and several atypical symptoms that impact the overall well-being (Regensteiner et al., 2008; Schorr, Peden-McAlpine, Treat-Jacobson, & Lindquist, 2015; WHO, 2013). These patients may benefit from multidisciplinary interventions such as exercise training that has shown positive results in another populations.
The Musculoskeletal system incorporated pain and comorbidities that are often associated with pain, such as fibromyalgia and carpal tunnel diseases, that to our knowledge are not considered as predictors of survival for this population. Pain is reported by more than a half (65%) of the adults in US with increased cost (Gaskin & Richard, 2012; Nahin, 2015), but no studies have investigated underlying pain characteristics and related factors, specifically in the liver transplant population. For instance, a study reported that frailty, a measure that consider not just severity of the disease, is a better indicator than MELD in predicting health outcomes for this population, an issue that is increasingly becoming recognized as a major determinant of patient survival (Derck et al., 2015). This study showed similar results, that patients with musculoskeletal problems behaved differently and tended to cluster in the same group (Cluster #4), and had very poor survival at 5-years follow-up (37.5%). It may be that tailored nursing interventions to pain management may increase patients’ comfort and help patients to cope with the strict treatment plan before and after transplantation.
Similarly, in Cluster #5, although a small group, the membership was determined by the endocrine system severity. This specific cluster contains endocrine related problems not usually considered in transplant research, such as failure to thrive or malnutrition requiring medication, adrenal insufficiency, and electrolyte imbalance requiring medication. These problems were statistically significant predictors for survival in this study, and this cluster had the worst survival curve, with no patient surviving at 5 years. This finding points to the need to rethink nutritional and electrolyte imbalance management strategies adopted when treating patients with these problems. These findings are consistent with other studies (Johnson, Overgard, Cohen, & DiBaise, 2013; Kalafateli et al., 2016), and support the need to optimize nutritional status for liver recipients while on the waiting list, either while in the hospital and/or at home. Further efforts should be made on investigating these issues, and care coordination may play an important role when caring for these patients in the continuum of care. Further, adrenal insufficiency was found statistically significant in the respective severity score; and additional research is needed to validate the relationship of this problem pre-transplant with patient survival.
Unlikely Cluster #1, #4 and #5, Cluster #2 membership was determined by a variety of system severities, but these were not statistically predictive during LRM modeling in Table 1. Patients in Cluster #2 were mainly determined by the primary disease reason for transplantation and demographics/environmental factors, and less likely determined by circulatory, endocrine, and musculoskeletal body systems severity scores. Cluster #2 included the majority of the sample of this study and may represent the average liver transplant population that undergoes liver transplantation, as well as survival estimates reflecting national average reported rates. Nurses play an important role on patient education and in the transplantation process. The inclusion of smoking cessation, access to community resources for specific groups based on age, gender and race, as well as advice on alcohol control and disease spread prevention (e.g. Hepatitis C and B) may be some social-behavioral factors that nurses may address when educating these patients while they are waiting for an organ.
From the results, Cluster #3 could be considered the “healthier cluster”. Findings suggest that Cluster #3 represents patients with less severe problems across the various body systems and may represent patients that undergo transplantation in better health compared to the general population of liver transplant patients, and showed a better survival rate over time. It may be that there are other health conditions not considered in this analysis that are predictive of that membership, and even play a protective role in that group. This finding reinforce the need to achieve the best health state possible before transplantation that, consequently, will be reflected in better outcomes. That means, attention should be directed to the combination of factors that together play a critical role on patients’ health instead of treating patients for specific isolated problems. Our approach showed this: a holistic approach was able to identify the heterogeneity that exist in liver transplant population and inform personalized approach to health. Although this study incorporated a great number of risk factors, donor and logistics variables were not considered in this analysis that could provide additional insights, as well as patient’ general health status.
Cluster #3 together with cluster #2 accounted for 86% of our sample and showed a better survival rate, which is comparable with national reports. This emphasizes the need to address specific subgroups that perform poorly after liver transplantation; and consequently, providing better survival rates through tailored interventions pre-transplantation. Future research should also consider additional health outcomes that are important for this population and costly for the health care system, such as length of stay, readmission, and rejection.
Severity scores of the gastrointestinal, biliary, mental, neurologic, and extremities systems and MELD score did not play an important role in determining membership during clustering. The MELD score is well-known for predicting mortality in a 90-day period if patients stay on a waiting list rather than predicting post-transplantation outcomes (Volk, Hernandez, Lok, & Marrero, 2007; Kanwal, Dulai, Spiegel, Yee, & Gralnek, 2005). These findings indicate that clustering analysis, using a holistic approach, is able to predict patient survival after transplantation better than the MELD score. Gastrointestinal and neurologic problems, such as encephalopathy hepatic and portal hypertensive gastropathy, represent comorbidities that highly influence how patient health deteriorates while waiting for transplantation (Negreanu et al., 2005; Taniguchi, 2012). These conditions not just impact patient care, but demand skillful and intensive nursing care. Although alcohol and intravenous drug abuse are commonly reported to impact survival after transplantation (Wiesner, Lombardero, Lake, Everhart, & Detre, 1997; Cowling et al., 2004), our findings did not show this impact; however, this study did not consider abstinence time before transplantation.
The main limitation of this study is the lack of cross-validation to establish that the clusters are meaningful across health systems and not just tailored to the variation in one health system. Additionally, the study sample was of moderate size, from one single center, and was predominantly Caucasian and male. However, this study presented a cutting-edge methodology that successfully used the pre-transplant severity of liver disease and a wide range of comorbidities, suboptimal health states, and environmental risk factors to elucidate the heterogeneity in a patient population that undergo transplantation, and is predictive of all-cause mortality. Further study is needed to investigate certain problems that appeared to be predictive of survival in this study, such as problems from musculoskeletal and endocrine systems; as well as to validate these findings using a larger national cohort.
This study showed that nursing science combined with data science have the potential to uncover complexities in liver transplant heterogeneity, not just holistically addressing risk factors that are critical for the nursing discipline, but also providing new insights in tailoring patient-centered interventions. Nurses and multidisciplinary teams play an important role in managing liver transplant patients in the continuum of care, both before and after transplantation. Understanding that multiple factors influence patient outcomes supports the need for a more holistic view when treating this population. Liver transplant patients have a broad variety of conditions that affect their health and well-being as described in this study. Although the majority of the factors are still physiologic factors, the impact of these factors on how patients interact with their environment and themselves are still insufficiently understood. The burden of the disease may have further implications for their mental and spiritual health, as well as how they overcome difficulties during the treatment process. These factors are not yet considered when treating this population, and additional studies may find innovative ways to incorporate and account for these factors; thus, providing a refined holistic approach when caring for these patients.
Conclusion
A novel hierarchical clustering analysis with severity scores was able to group patients with similar characteristics that are predictive of all-cause mortality, and explain liver transplant population heterogeneity, using a holistic perspective. This approach has the potential to identify groups of individuals that may benefit from intensive patient-centered interventions prior to liver transplantation. To this end, the study accounted for individual variability addressing PMI program efforts to enable individualized, holistic patient care. This study highlights the need to assess additional risk factors that are seldom addressed as impacting liver transplant survival, such as pain, adrenal insufficiency, and nutritional and electrolyte imbalance that may be consequence of the liver disease progression. Further, this study showed a new approach to analyze heterogeneous and high dimensional transplant data, and that is possible to model a broad array of risk factors in a single model, using a holistic approach. Finally, this research informs clinical practice toward individualized care for critical populations, such as liver transplantation, and may provide insights into new multidisciplinary interventions to improve outcomes and enable patient-centered care.
Acknowledgments
Lisiane Pruinelli was supported by the 2015/2016 University of Minnesota Doctoral Dissertation Fellowship, and the Midwest Nursing Research Society 2016 Joseph and Jean Buckwalter Grant.
Footnotes
The authors declare no conflict of interest.
Ethical Conduct of Research: This study was approved by the University of Minnesota Institutional Review Board (IRB) number #1502E62121.
Contributor Information
Lisiane Pruinelli, University of Minnesota School of Nursing, Minneapolis, MN.
György J. Simon, University of Minnesota Institute for Health Informatics and School of Medicine, Minneapolis, MN.
Karen A. Monsen, University of Minnesota School of Nursing, Minneapolis, MN.
Timothy Pruett, University of Minnesota Department of Surgery, Minneapolis, MN.
Cynthia R. Gross, University of Minnesota Department of Experimental and Clinical Pharmacology and School of Nursing, Minneapolis, MN.
David M. Radosevich, University of Minnesota School of Public Health, Minneapolis, MN.
Bonnie L. Westra, University of Minnesota School of Nursing and Institute for Health Informatics, Minneapolis, MN.
References
- Cho CS, Knechtle SJ, Heisey DM, Hermina M, Armbrust M, D’Alessandro AM, … Kalayoglu M. Analysis of tumor characteristics and survival in liver transplant recipients with incidentally diagnosed hepatocellular carcinoma. Journal of Gastrointestinal Surgery. 2001;5(6):594–601. doi: 10.1016/s1091-255x(01)80101-3. [DOI] [PubMed] [Google Scholar]
- Cowling T, Jennings LW, Goldstein RM, Sanchez EQ, Chinnakotla S, Klintmalm GB, Levy MF. Societal reintegration after liver transplantation: findings in alcohol-related and non-alcohol-related transplant recipients. Annals of Surgery. 2004;239(1):93–98. doi: 10.1097/01.sla.0000103064.34233.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kroon L, Drent G, van den Berg AP, Haagsma EB, Groningen LTG. Current health status of patients who have survived for more than 15 years after liver transplantation. The Netherlands Journal of Medicine. 2007;65(7):252–258. [PubMed] [Google Scholar]
- Derck JE, Thelen AE, Cron DC, Friedman JF, Gerebics AD, Englesbe MJ, Sonnenday CJ. Quality of life in liver transplant candidates: frailty is a better indicator than severity of liver disease. Transplantation. 2015;99(2):340–4. doi: 10.1097/TP.0000000000000593. [DOI] [PubMed] [Google Scholar]
- Dey S, Cooner J, Delaney CW, Fakhoury J, Kumar V, Simon G, … Westra BL. Mining Patterns Associated With Mobility Outcomes in Home Healthcare. Nursing Research. 2015;64(4):235–245. doi: 10.1097/NNR.0000000000000106. [DOI] [PubMed] [Google Scholar]
- Dickson RC, Pungpapong S, Keaveny AP, Taner CB, Ghabril M, Aranda-Michel J, … Nguyen JH. Improving graft survival for patients undergoing liver transplantation. Clinical Transplantation. 2011;25(3):E345–55. doi: 10.1111/j.1399-0012.2011.01428.x. [DOI] [PubMed] [Google Scholar]
- Fan J, Yang GS, Fu ZR, Peng ZH, Xia Q, Peng CH, … Zhang JJ. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi-center experience in Shanghai, China. Journal of Cancer Research and Clinical Oncology. 2009;135(10):1403–1412. doi: 10.1007/s00432-009-0584-6. [DOI] [PubMed] [Google Scholar]
- Gallup. Gallup healthways wellbeing index. 2014 Retrieved from http://www.well-beingindex.com/
- Gaskin DJ, Richard P. The Economic Costs of Pain in the United States. The Journal of Pain. 2012;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Gleisner AL, Munoz A, Brandao A, Marroni C, Zanotelli ML, Cantisani GG, … Pawlik TM. Survival benefit of liver transplantation and the effect of underlying liver disease. Surgery. 2010;147(3):392–404. doi: 10.1016/j.surg.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Johnson TM, Overgard EB, Cohen AE, DiBaise JK. Nutrition assessment and management in advanced liver disease. Nutrition in Clinical Practice: Official Publication of the American Society for Parenteral and Enteral Nutrition. 2013;28(1):15–29. doi: 10.1177/0884533612469027. [DOI] [PubMed] [Google Scholar]
- Kalafateli M, Mantzoukis K, Choi Yau Y, Mohammad AO, Arora S, Rodrigues S, … Tsochatzis EA. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. Journal of Cachexia, Sarcopenia and Muscle. 2016 doi: 10.1002/jcsm.12095. [DOI] [PMC free article] [PubMed]
- Kanwal F, Dulai GS, Spiegel BM, Yee HF, Gralnek IM. A comparison of liver transplantation outcomes in the pre- vs. post-MELD eras. Alimentary Pharmacology & Therapeutics. 2005;21(2):169–177. doi: 10.1111/j.1365-2036.2005.02321.x. [DOI] [PubMed] [Google Scholar]
- Kreitzer MJ. Spirituality and well-being: focusing on what matters. Western Journal of Nursing Research. 2012;34(6):707–711. doi: 10.1177/0193945912448315. [DOI] [PubMed] [Google Scholar]
- Lanzola G, Parimbelli E, Micieli G, Cavallini A, Quaglini S. Data quality and completeness in a web stroke registry as the basis for data and process mining. Journal of Healthcare Engineering. 2014;5(2):163–184. doi: 10.1260/2040-2295.5.2.163. [DOI] [PubMed] [Google Scholar]
- Maíllo B, Miranda B, Domínguez-Gil B, Carmona M, Valentín M, Alvarez M, … Matesanz R. Global Observatory on donation and transplantation. 2007 Retrieved from http://www.transplant-observatory.org/Pages/home.aspx.
- Monsen KA, Peters J, Schlesner S, Vanderboom CE, Holland DE. The Gap in Big Data: Getting to Wellbeing, Strengths, and a Whole-person Perspective. Global Advances in Health and Medicine: Improving Healthcare Outcomes Worldwide. 2015;4(3):31–39. doi: 10.7453/gahmj.2015.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahin RL. Estimates of Pain Prevalence and Severity in Adults: United States, 2012. The Journal of Pain. 2015;16(8):769–780. doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negreanu L, Busegeanu C, Trandafir D, Dragomir P, Udeanu M, Fierbinteanu-Braticevici C, Andronescu D. Portal hypertensive gastropathy. Romanian Journal of Internal Medicine = Revue Roumaine de Medecine Interne. 2005;43(1–2):3–8. [PubMed] [Google Scholar]
- NIH. Precision medicine initiative cohort program. 2016 Retrieved from https://www.nih.gov/precision-medicine-initiative-cohort-program.
- NINR. The NINR Strategic Plan: Advancing science, improving lives. 2016 doi: 10.1111/jnu.12286. Retrieved from https://www.mendeley.com/research-papers/ninr-strategic-plan-advancing-science-improving-lives/authors/ [DOI] [PubMed]
- OTTR. Organ Transplant Tracking Registry. 2014 Retrieved from http://www.ottr.com/
- Patel SS, Arrington AK, McKenzie S, Mailey B, Ding M, Lee W, … Kim J. Milan Criteria and UCSF Criteria: A Preliminary Comparative Study of Liver Transplantation Outcomes in the United States. International Journal of Hepatology. 2012;2012:253517. doi: 10.1155/2012/253517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruinelli L, Monsen KA, Gross CR, Radosevich DM, Simon GJ, Westra BL. Predictors of Liver Transplant Patient Survival: A Critical Review Using a Holistic Framework. Progress in Transplantation (Aliso Viejo, Calif) 2016 doi: 10.1177/1526924816680099. [DOI] [PubMed]
- Regensteiner JG, Hiatt WR, Coll JR, Criqui MH, Treat-Jacobson D, McDermott MM, Hirsch AT. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vascular Medicine. 2008;13(1):15–24. doi: 10.1177/1358863X07084911. [DOI] [PubMed] [Google Scholar]
- Schorr EN, Peden-McAlpine C, Treat-Jacobson D, Lindquist R. Characterization of the peripheral artery disease symptom experience. Geriatric Nursing. 2015;36(4):293–300. doi: 10.1016/j.gerinurse.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaro AI, Gallon LG, Lyuksemburg V, Jay CL, Zhao L, Ladner DP, … Gheorghiade M. The impact of coronary artery disease on outcomes after liver transplantation. Journal of Cardiovascular Medicine (Hagerstown, Md) 2014 doi: 10.2459/JCM.0000000000000207. [DOI] [PubMed]
- Sullivan KM, Radosevich DM, Lake JR. Health-related quality of life: two decades after liver transplantation. Liver Transplantation: Official Publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2014;20(6):649–654. doi: 10.1002/lt.23855. [DOI] [PubMed] [Google Scholar]
- Tan P-N, Steinbach M, Kumar V. Introduction to data mining. Pearson Education Inc; 2006. [Google Scholar]
- Taniguchi M. Liver transplantation in the MELD era--analysis of the OPTN/UNOS registry. Clinical Transplants. 2012:41–65. Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=medl&AN=23721009. [PubMed]
- UNOS. United nation for organ sharing. 2017 Retrieved from http://www.unos.org/donation/index.php?topic=data.
- Volk ML, Hernandez JC, Lok AS, Marrero JA. Modified Charlson comorbidity index for predicting survival after liver transplantation. Liver Transplantation: Official Publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2007;13(11):1515–1520. doi: 10.1002/lt.21172. [DOI] [PubMed] [Google Scholar]
- Walter S. Holistic Health. American Holistic Health Association; 2017. Retrieved January 29, 2018, from https://ahha.org/selfhelp-articles/holistic-health/ [Google Scholar]
- Wang W, Yan Y. Suboptimal health: a new health dimension for translational medicine. Clinical and Translational Medicine. 2012;1(1):28. doi: 10.1186/2001-1326-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. World Health Organization; 2013. Retrieved from http://www.who.int/about/definition/en/print.html. [Google Scholar]
- Wiesner RH, Lombardero M, Lake JR, Everhart J, Detre KM. Liver transplantation for end-stage alcoholic liver disease: an assessment of outcomes. Liver Transplantation & Surgery. 1997;3(3):231–239. doi: 10.1002/lt.500030307. Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med4&AN=9346745. [DOI] [PubMed] [Google Scholar]