Abstract
Rationale:
Testicular Leydig cell tumor (LCT) is a rare neoplasm. It commonly presents as a painless testicular mass with or without endocrine changes. Histological and immunohistochemical examination play important roles in differentiating LCT from testicular germ cell tumors.
Patient concerns:
We highlight the imaging phenotype, as well as the pathological findings of a case of LCT in a 62-year-old male.
Diagnoses:
Preoperative noncontrast CT scan of the abdomen revealed a 7.0 × 6.4 × 5.3 cm oval mass with heterogeneous density, located in the right testis. Pelvic noncontrast MRI showed a heterogeneous mass on T1-weighted and T2-weighted images. The solid part of the tumor exhibited high signal on the diffusion-weighted imaging, and an obvious enhancement on the contrast-enhanced MR imaging. Ultrasonography examination demonstrated a large mixed echogenic space occupying lesion involving the whole right testis with multiple cystic areas and increased vascularity. This patient underwent radical orchiectomy. The pathologic diagnosis was LCT.
Interventions:
This patient underwent operative resection of the tumor. Due to the negative resection margins and absence of distant metastases, the patient did not receive additional radiotherapy or chemotherapy.
Outcomes:
Four months after the surgery, the follow-up CT-scan did not reveal any local recurrence and distant metastases.
Lessons:
This case improves our ability to detect and diagnose LCT by summarizing its imaging characteristics as well as reviewing the literature. Additionally, we described the state-of-the-art management of the management of this rare tumor.
Keywords: Leydig cell tumor, medical imaging, pathology, testis
1. Introduction
Leydig cell tumor (LCT) is a rare testicular tumor, with malignant potential.[1] To the best of our knowledge, most LCTs are presented as case report or as small series in the English literature. About 3% cases of LCT are bilateral,[1] while 10% are malignant with metastatic forms, particular to the inguinal lymph nodes and extranodal organs, including the liver, lungs, and bones.[2] Histologically, the tumor consists of the proliferation of large polygonal tumor cells with granular eosinophilic cytoplasms.[3] LCT has a range of imaging manifestations, some overlapping with other testicular tumors. Because of this, it is difficult to make accurate diagnosis without immunohistochemistry. The treatment is surgical resection for both benign and malignant LCT.[1,4]
Herein, we report a case of LCT located in the right testis occurring in a 62-year-old male. The aim of this report was to better our understanding of testicular LCT by summarizing its characteristics (i.e., imaging phenotype, pathology) as well as reviewing the literature.
2. Case report
In September 2017, a 62-year-old male was admitted to the urology department with a huge painless mass in the right testis of 8-month duration. One month before admission, the lesion started to grow rapidly. On physical examination, the patient had a regular pulse of 76 beats/min, a temperature of 36.8°C, and a respiratory rate of 16 breaths/min. Nor other sigh, including gynecomastia or hypercortisolism, was observed. The penis and pubic hair were normally developed.
His routine laboratory data such as complete blood cell count, renal function tests, liver function tests, and urinalysis were negative. The serum germ cell tumor markers [alpha-fetoprotein (AFP) and β-human chorionic gonadotropin (β-HCG)] showed no significant abnormalities. Other tumor markers [carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), prostate-specific antigen (PSA), carbohydrate antigen 125 (CA 125), CA 199, and CA 724] were within normal range.
Preoperative noncontrast CT scan of the abdomen revealed a 7.0 × 6.4 × 5.3 cm oval mass with heterogeneous density (CT value, 0∼42 HU), located in the right testis (Fig. 1). The mass consisted of multiple small cystic lesions and bleeding focus, but did not contain any fat or calcification. The right testicular mass was completely surrounded by a massive hydrocele. The left testis showed normal size, density, position, and contour. There was no evidence of metastasis to either lymph nodes or other organs.
Figure 1.
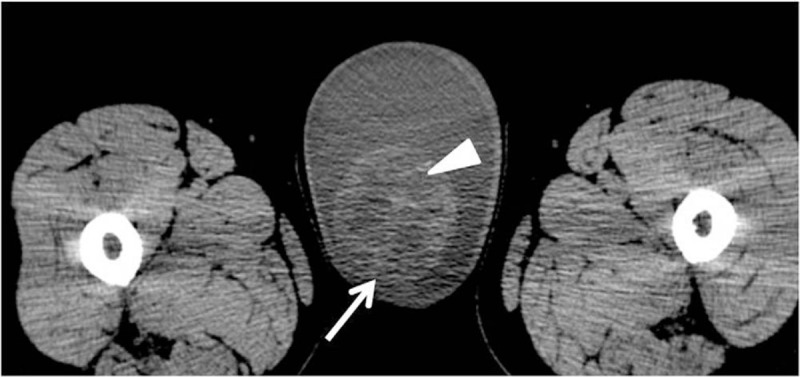
(A) Unenhanced CT scan shows a mass (white arrow) with cystic and bleeding focus (white triangle) in the right testis.
Pelvic noncontrast MRI showed a heterogeneous mass with low to high signal intensity on both T1-weighted images and T2-weighted images when compared with the signal in left testis (Fig. 2A, B). The lesion exhibited low to high signal intensity on the diffusion-weighted images (Fig. 2C). The tumor had an unclear capsule with several nodules on the surface. On contrast-enhanced MR images (Fig. 2D–F), an obvious enhancement was observed in the solid part of the tumor. Cystic areas within the tumor demonstrated no contrast enhancement.
Figure 2.
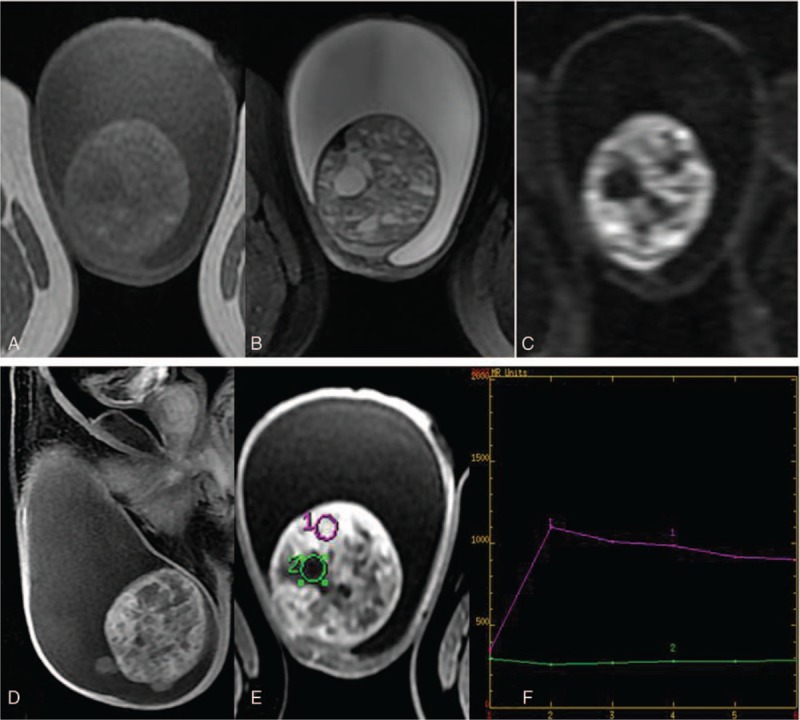
(A–C) The tumor presents as low to high signal on T1WI and T2WI with diffusion restriction. (D–F) On contrast MR imaging, the solid part of the tumor shows a continuous enhancement pattern.
A testicular ultrasound examination (Fig. 3) demonstrated a large mixed echogenic space occupying lesion involving the whole right testis with some cystic areas and increased vascularity.
Figure 3.
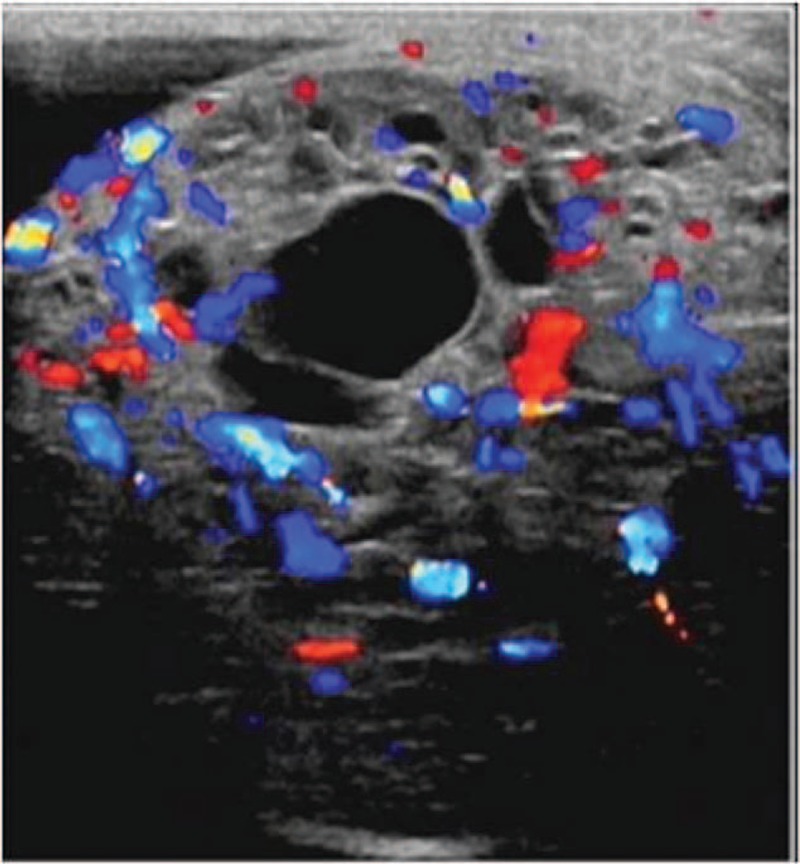
Ultrasonography of the testis shows increased blood flow around the mixed echogenic mass.
This patient underwent radical orchiectomy. The gross tumor specimen after resection measured 8 × 7 × 5.5 cm. Postoperative pathology showed that the tumor had central hemorrhage and necrosis, but no vascular invasion. The spermatic cord, scrotal skin, and surgical margins were free of malignancy. Microscopically, the mass cells arranged in sheets separated by fibrovascular septae cells with abundant eosinophilic cytoplasm and prominent pleomorphic nuclei (Fig. 4A). The immunostaining (Fig. 4B) showed that the tumor cells were positive for inhibin and negative for pan-cytokeratin, cytokeratins 8/18, AFP, HCG, cluster of differentiation 30 (CD 30), CD 99, and S-100. The proliferation index, expressed as a percentage of Ki-67 antigen-positive nuclei, was around 2% (Fig. 4C). On the basis of the pathologic and immunohistochemical findings, the testicular mass was diagnosed as Leydig cell tumor.
Figure 4.
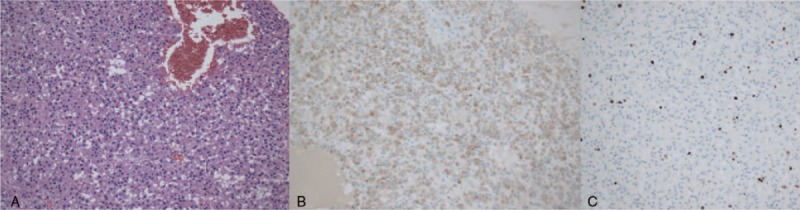
(A) The tumor is predominantly comprised of proliferating tumor cells with granular eosinophilic cytoplasm arranged in sheets pattern (H&E, × 200). (B) Immunohistochemical stain for inhibin. (C) The proliferation index, expressed as a percentage of Ki-67 antigen-positive nuclei, is around 2%.
Four months after the surgery, the follow-up CT-scan did not reveal any local recurrence and distant metastases.
3. Discussion
Primary testicular tumors are rare, accounting for 1% to 1.5% of all tumors in men.[3,5,6] There are 2 members of primary testicular tumors family: germ cell tumors and sex cord/stromal tumors.[3,6] Among the sex cord/stromal tumors, LCT is the most common histologic type, representing 1% to 3% of all testicular tumors in adults[7] and 0.4% to 9% in prepubertal children.[8] The etiology of LCT remains unclear. The genetic risk factors for LCT in adult and child were different.[7] Activating mutation in the guanine nucleotide binding protein α gene is a cause of adult LCT, by driving tumor development and causing hyperactivity of the testosterone biosynthetic pathway.[9] In child, Boot et al[10] have observed activating mutations in the luteinizing hormone receptor, which may result in Leydig cell hyperplasia, lead to familial male-limited gonadotropin-independent precocious puberty due to excess testosterone production.
Clinically, LCT presents as a potentially malignant testicular mass with or without endocrine abnormalities such as loss of libido and gynecomastia.[1] There are 2 age peaks in the incidence of LCT: 30 to 60 years in adult and 3 to 9 years in child.[1] According to the review by Efthimiou et al,[11] 480 cases of LCT have been reported in the English literature and 29.2% of them are presented as testicular mass, 12.5% with gynecomastia. The mechanism of hormonal disorders is likely to be excess production of testosterone and estrogens by the tumor.[8] In the current report, the tumor was 8 × 7 × 5.5 cm in size involving the whole right testis. The patient did not present with any endocrine changes. About 10% cases of LCT are malignancy. Some authors[7,8,12] suggested that 5 clinical features allow the identification of malignant LCT, including presence of endocrine changes, older patients (more than 40 years), tumor size greater than 5 cm, infiltrative margins, and areas of hemorrhage and necrosis extending beyond testicular parenchyma. In our case report, 3 features (patient's age, tumor size, range of hemorrhage, and necrosis) were consistent with the above criteria.
LCT arises from the interstitial cells of Leydig adjacent to the seminiferous tubules. Histological and immunohistochemical findings are crucial for the diagnosis of LCT. Histologically, LCT is characterized by the proliferation of large polygonal tumor cells with granular eosinophilic cytoplasms and prominent nucleoli arranged in sheets pattern.[3] Immunologically, LCT is associated with an almost uniformly positive expression for inhibin and Melan-A.[13] The expression of calretinin and vimentin varies.[5] In contrast, LCT is distinguished from germ cell tumors by the negative immunostaining with lactate dehydrogenase (LDH), AFP, and HCG.[1] In the present case, the tumor cells were positive for inhibin, but there was no immunoreactivity for cytokeratin, AFP, HCG, CD, and S-100.
The typical imaging phenotype of LCT can be deciphered from a comprehensive literature review of published reports. Ultrasonography is considered the initial investigative method for the diagnosis of LCT and shows hypoechoic mass with a heterogeneous enhancement pattern or peripheral hypervascularity.[11,14,15] With high tissue resolution, MRI seems to be superior to ultrasonography in demonstrating the testicular lesions.[16] By comparing MRI findings in 104 patients with different testicular pathologies, Fernandez et al[17] concluded that “marked and maintained enhancement” was the characteristic sign of LCT in differentiation from other testicular tumors. Similar result was also mentioned in the case report by Obembe and Patel.[14] In our current report, radiological imaging findings described a heterogeneous soft tissue mass (with necrosis and hemorrhage) involving the whole right testis without fat or calcification. The substantial part of the tumor showed high signal on diffusion-weighted imaging (due to lesion hypercellularity), as well as obvious enhancement on contrast-enhanced imaging (due to vascularity of the tumor). By CT-scan, we did not find any metastases to the nearby lymph nodes or distant parts of the body such as the liver, lungs, or bones.
Surgical resection remains the gold standard treatment for both benign and malignant LCT.[1,5] Metastatic LCT responds poorly to additional systemic chemotherapy or radiation.[18,19] Regular long-term follow-up is recommended to exclude recurrence or metastasis. In our current case report, due to the negative resection margins and absence of distant metastases, no further management was suggested.
In conclusion, our case report allows for a comprehensive analysis of the imaging phenotype of LCT on CT-scan, MRI, and ultrasonography. In addition, we described the state-of-the-art management of the management of this rare tumor.
Author contributions
Conceptualization: Jianguo Zhu.
Data curation: Yun Luan, Haige Li.
Formal analysis: Haige Li.
Supervision: Jianguo Zhu.
Visualization: Yun Luan, Haige Li.
Writing – original draft: Jianguo Zhu.
Writing – review & editing: Jianguo Zhu.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, CA = carbohydrate antigen, CD = cluster of differentiation, CEA = carcinoembryonic antigen, CK = cytokeratin, CT = computed tomography, HCG = human chorionic gonadotropin, HU = Hounsfield unit, LCT = Leydig cell tumor, MRI = magnetic resonance imaging, NSE = neuron-specific enolase, PSA = prostate-specific antigen.
This study was approved by the institutional review board at the Second Affiliated Hospital of Nanjing Medical University in China. Patient gave informed consent.
The authors report no conflicts of interest.
References
- [1].Muheilan MM, Shomaf M, Tarawneh E, et al. Leydig cell tumor in grey zone: a case report. Int J Surg Case Rep 2017;35:12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Farkas LM, Szekely JG, Pusztai C, et al. High frequency of metastatic Leydig cell testicular tumours. Oncology 2000;59:118–21. [DOI] [PubMed] [Google Scholar]
- [3].Vukina J, Chism DD, Sharpless JL, et al. Metachronous bilateral testicular Leydig-like tumors leading to the diagnosis of congenital adrenal hyperplasia (adrenogenital syndrome). Case Rep Pathol 2015;2015:459318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Plastini T, Staddon A. Sertoli-Leydig cell tumor with concurrent rhabdomyosarcoma: three case reports and a review of the literature. Case Rep Med 2017;2017:4587296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tahaineh S, Mughli RA, Fallatah M. Giant mixed Sertoli-Leydig-Granulosa sex cord tumor of the testis; clinical, histopathological, and radiological features: a case report. Pan Afr Med J 2017;27:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nitta S, Sakka S, Endo T, et al. [Metachronous bilateral testicular tumors with frequently recurrent hydrocele: a case report]. Hinyokika Kiyo 2017;63:115–8. [DOI] [PubMed] [Google Scholar]
- [7].Gheorghisan-Galateanu AA. Leydig cell tumors of the testis: a case report. BMC Res Notes 2014;7:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mameli C, Selvaggio G, Cerini C, et al. Atypical Leydig cell tumor in children: report of 2 cases. Pediatrics 2016;138:pii: e20160151. [DOI] [PubMed] [Google Scholar]
- [9].Libe R, Fratticci A, Lahlou N, et al. A rare cause of hypertestosteronemia in a 68-year-old patient: a Leydig cell tumor due to a somatic GNAS (guanine nucleotide-binding protein, alpha-stimulating activity polypeptide 1)-activating mutation. J Androl 2012;33:578–84. [DOI] [PubMed] [Google Scholar]
- [10].Boot AM, Lumbroso S, Verhoef-Post M, et al. Mutation analysis of the LH receptor gene in Leydig cell adenoma and hyperplasia and functional and biochemical studies of activating mutations of the LH receptor gene. J Clin Endocrinol Metab 2011;96:E1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Efthimiou I, Mamoulakis C, Papageorgiou G, et al. Unilateral malignant leydig cell tumor of testis in a patient with contralateral cryptorchidism. Urol J 2009;6:60–2. [PubMed] [Google Scholar]
- [12].Al-Agha OM, Axiotis CA. An in-depth look at Leydig cell tumor of the testis. Arch Pathol Lab Med 2007;131:311–7. [DOI] [PubMed] [Google Scholar]
- [13].Mukhopadhyay M, Das C, Sarkar S, et al. Leydig cell tumor of testis in a child: an uncommon presentation. J Indian Assoc Pediatr Surg 2017;22:181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Obembe OO, Patel MD. Value of dynamic, contrast-enhanced MRI & intraoperative ultrasound for management of a nonpalpable, incidental, testicular Leydig-cell tumor. Radiol Case Rep 2010;5:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Prasivoravong J, Barbotin AL, Derveaux A, et al. Leydig cell tumor of the testis with azoospermia and elevated delta4 androstenedione: case report. Basic Clin Androl 2016;26:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Watanabe Y, Dohke M, Ohkubo K, et al. Scrotal disorders: evaluation of testicular enhancement patterns at dynamic contrast-enhanced subtraction MR imaging. Radiology 2000;217:219–27. [DOI] [PubMed] [Google Scholar]
- [17].Fernandez GC, Tardaguila F, Rivas C, et al. Case report: MRI in the diagnosis of testicular Leydig cell tumour. Br J Radiol 2004;77:521–4. [DOI] [PubMed] [Google Scholar]
- [18].Thambi R, Pothen L, Emmanuel KM, et al. Leydig cell tumor of testis with indeterminate features. Indian J Cancer 2015;52:529–30. [DOI] [PubMed] [Google Scholar]
- [19].Markou A, Vale J, Vadgama B, et al. Testicular leydig cell tumor presenting as primary infertility. Hormones (Athens) 2002;1:251–4. [DOI] [PubMed] [Google Scholar]


