Abstract
A limit of detection of 200 CFU/mL of Salmonella typhi spiked in various sample matrices were achieved in 30 min. The sample matrices were raw/unprocessed milk, commercially available milk, juice from packed bottles, fresh juice from carts, potable water, turbid water and calf serum. The complete protocol comprised of three steps: (a) cell lysis (b) nucleic acid amplification and (c) an in situ optical detection. The cell lysis was carried out using a simple heating based protocol, while the loop-mediated isothermal amplification of DNA was carried out by an in-house designed and fabricated system. The developed system consists of an aluminum block fitted with two cartridge heaters along with a thermocouple. The system was coupled to a light source and spectrometer for a simultaneous in situ detection. Primers specific for STY2879 gene were used to amplify the nucleic acid sequence, isolated from S. typhi cells. The protocol involves 15 min of cell lysis and DNA isolation followed by 15 min for isothermal amplification and simultaneous detection. No cross-reactivity of the primers were observed at 106 CFU/mL of Escherichia coli, Vibrio cholerae, Salmonella typhimurium, Salmonella paratyphi A, Pseudomonas aeruginosa, Bacillus cereus, Lysteria monocytogenes, Clostridium botulinum, Staphylococcus aureus and Salmonella havana. In addition, the system was able to detect S. typhi of 200 CFU/mL in a concoction of 106 CFU/mL of E. coli, 106 CFU/mL of V. cholerae, and 106 CFU/mL of hepatocyte-derived cellular carcinoma HUH7 cells. The proposed rapid diagnostic system shows a promising future in the field of food and medical diagnostics.
Keywords: Salmonella typhi, Loop-mediated isothermal amplification (LAMP), Optical detection, Milk, Raw/unprocessed milk, Fruit juice, Tap water, Turbid water, Calf serum, Rapid detection
Introduction
In tropical countries, such as southern Asia, water and food substances are commonly infested with pathogens such as Escherichia coli, Vibrio cholerae, Salmonella typhimurium, Salmonella paratyphi A, Pseudomonas aeruginosa, Bacillus cereus, Lysteria monocytogenes, Clostridium botulinum, Staphylococcus aureus and Salmonella havana etc. [1–4]. Among these S. typhi is one of the harmful pathogens as suggested by Center for Disease Control and Prevention (CDC), USA. CDC has classified Salmonella as category B bioterrorism agent [5]. S. typhi infects the intestines of vertebrate species including human beings, causing non-invasive non-typhoidal salmonellosis, invasive non-typhoidal salmonellosis, and typhoid fever [6]. The essential pathogenic members of the genus Salmonella are S. typhi, S. paratyphi A and S. typhimurium of which S. typhi causes typhoid fever. According to the latest survey in 2014 by World Health Organization, 21 million cases of typhoid fever and 222,000 typhoid-related deaths occur every year [7]. Detection of clinically relevant pathogens, especially in point-of-care setup, still remains a challenge, despite of nearly three decades of sustained research in biosensing and microfabrication. Diagnostic devices demand high specificity and high sensitivity in addition to rapid detection for potential commercialization. Currently, the gold standard of detection is conventional culture method [1] followed by biochemical tests such as citrate test, urease test, motility test, triple sugar iron (TSI) test and slide agglutination test [8]. The culture method is followed in the Indian clinical scenario [9] and along with the biochemical tests, the confirmation of the presence of pathogen usually takes 72 h from collection of samples. Earlier detection and pathogen specific antibiotic will decrease the risk of evolution of drug resistance species [10].
Various research articles have been published in the detection of Salmonella and other bacterial pathogens such as (1) enzyme–linked immunosorbent assay (ELISA) [11, 12] (2) immunochromatographic (ICT) [13], (3) dot blot immunoassays [14], (4) matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) [15] (5) microarray [16] (6) lateral flow immunoassay (LFIA) using monoclonal antibody coated gold nanoparticles [17] and (7) functionalized polymeric fluorescent nanoparticles using confocal laser scanning microscope [18]. The above-mentioned methods involve either complex instrumentation or laborious protocol or higher operational time or non-portable or a combination of the challenges. Therefore, it is not suitable especially for developing economies that often face shortage of infrastructure and skilled laborers.
To increase the specificity and sensitivity of detection, nucleic acid based techniques have been researched in the past few decades. Amongst various biosensors and sensors reported in literature [19], polymerase chain reaction based detection remains as the most reliable technique and is faster than conventional culture method. However, PCR requires sophisticated instrumentation and multi-step processes to verify the presence of a particular sequence, specific to a pathogen. Alternatively, isothermal nucleic acid amplification that operates at a constant temperature, has emerged as an alternate to PCR method, owing to its low-cost infrastructure and potential opportunities for miniaturization. Unlike PCR, isothermal amplification techniques do not demand a temperature cycler and hence can function in a simpler instrumentation setup [20]. Of the several isothermal amplification techniques reported in the literature, a few notable techniques are: loop-mediated amplification (LAMP) [21, 22], recombinase polymerase amplification (RPA) [23], rolling-circle amplification (RCA) [24] and nucleic acid sequence based amplification (NASBA) [25].
The LAMP based nucleic acid amplification technique was originally developed by Notomi et al. [26]. The technique is highly sensitive and specific to target nucleic acid sequences and operates at a constant temperature (between 60 and 65 °C) [26]. Hence, the low infrastructure demanding LAMP is ideal for use in limited resource settings [21]. In this technique, a DNA polymerase [usually Bacillus stearothermophilus (Bst)] is employed along with four primers. The rate of the amplification reaction can be enhanced with additional loop primers [4]. Recently, various research groups are working on the development of new methods for detection of various pathogens from different sample matrices using LAMP. A brief description of a few select devices for the detection of S. typhi using LAMP process are described herewith. Abdullah et al. compared LAMP methods against both gold standard plate culture method as well as polymerase chain reaction (PCR) for the detection of S. typhi in blood culture broth via visualization of turbidity [21]. Abdullah et al. reported that LAMP method was ten times more sensitive than PCR method. Sayad et al. designed and fabricated a lab-on-disc where the samples were loaded at the center of the disc and upon rotation, mixed with the pre-loaded dried reagents. The presence of the Salmonella spiked in tomatoes were confirmed by the colorimetric changes in the turbidity, from orange to green. A sensitivity of 5 × 10−4 ng/μL of DNA concentration was reported [27]. Fan et al. reported LAMP assay for the rapid detection of S. typhi in simulated human blood and stool samples. The LAMP amplification was detected in Realtime Turbidimeter LA320C. The limit of detection (LOD) in simulated stool samples were found to be 200 CFU/g while, in the case of simulated blood samples the LOD was 20 CFU/mL [28]. Bozorgmehr et al. employed LAMP based gold nanoprobe method for the detection of S. typhi by surface plasmon resonance. The protocol involves extraction, amplification and detection of DNA in approximately 2 h [29]. The reported sensitivity was 20 CFU/mL. The above techniques consume a minimum of 1 h and maximum of 4.5 h or more for detection of S. typhi with LOD ranging from 20 to 2000 CFU/mL or higher. It is a matter of fact that many of the above-mentioned devices are still in the research phase and hence there is a need for a robust and a rapid detection technique. The prime focus of the current study was to create a portable device that offers rapid, specific, and sensitive detection of S. typhi in various food and water samples.
In the proposed method, the presence of S. typhi was detected from food and water sample matrices such as commercially available milk, juice from packed bottles, potable water, calf serum, raw or unprocessed milk, fresh juice from carts and turbid water within 30 min (15 min for pre-conditioning/sample preparation and 15 min for detection). The device was tested in widely consumed products such as milk, and juices which are highly contaminated in low resource economies through improperly cleaned utensils and other unhygienic practices. Further, as a prelude to testing of S. typhi in blood samples, experiments were also conducted on commercially available calf serum samples. Novel GspSSD (geobacillus species) DNA polymerase was used in this work. Primers specific to gene STY2879 was detected to confirm the presence of S. typhi. Gene STY2879 codes for reverse transcriptase protein and is present in all S. typhi isolates [28]. The specificity and cross-reactivity was tested against other bacterial isolates of E. coli, V. cholerae, S. typhimurium, S. paratyphi A, P. aeruginosa, B. cereus, L. monocytogenes, C. botulinum, S. aureus, S. havana and human DNA (hepatocyte-derived cellular carcinoma HUH7 cells).
Materials and Methods
Reagents and DNA Oligonucleotides
Isothermal LAMP master mix with dye was obtained from Optigene, UK. Primers sequences specific to gene STY2879 were adopted from Fan et al. [28] and are cited below. Primer sequences were synthesized by Integrated DNA Technologies (IDT, USA). TE buffer (100X) was procured from Sigma Aldrich, USA.
F3—5′—GCCAAATTGTTTGACGAGA—3′;
B3—5′—CTGTAAGAAACTTGTCCCATAG—3′;
FIP—5′—TACTACGCCGATTGAACAAACATTGATCACATCATCCATAAACACA—3′;
BIP—5′—CTAAGCAGAGGGTTGCAAGTATTCCACATAAGCATGTCCTCC—3′.
Bacterial Culture
Salmonella typhi cells were cultured on tryptone soya broth (TSB) for 12 h at 37 °C. The cells were collected in the mid-log phase of the culture (Optical density (O.D.) ~ 0.6, approximately corresponding to 5 × 108 CFU/mL) and were diluted down to, 500, 300, 200, 100, 75 and 50 CFU/mL using 1X TE buffer.
Cell lysis and DNA Isolation
One mL of each sample was centrifuged at 12,000g for 5 min. After centrifugation, the supernatant was discarded, while the pellet was re-suspended in 100 µL 1X TE buffer and vortexed to obtain a uniform suspension. The suspension was heated at 100 °C for 5 min for cell lysis. Post heating, the suspension was centrifuged at 12,000g for 5 min to isolate cell debris from nucleic acids. Five microlitres of the supernatant were used for the LAMP assay.
LAMP Assay and Detection
A primer mix containing 5 µM of F3, 5 µM of B3, 20 µM of FIP and 20 µM of BIP was prepared. Five microliters of the pre-mixed primers were added to 15 µL of LAMP master mix (pre-mixed with the fluorescent dye), and 5 µL of the supernatant of lysed cell suspension (DNA template). LAMP master mix contains Geobacillus species DNA polymerase, thermostable inorganic pyrophosphatase, optimized reaction buffer containing Mg2Cl2, and deoxynucleotide triphosphates (Optigene, UK). LAMP amplification of nucleic acids takes place at 65 °C and the reaction was terminated by heating to 80 °C for 2 min that disintegrates the polymerase [28]. LAMP reaction was carried out using an in-house designed device. During LAMP based amplification, the intensity of fluorescence was measured using spectrometer at time intervals of 5 min and recorded using spectra suite software®. The obtained intensity was subtracted from the background intensity of white light on a vial filled with 1X TE buffer.
Design of the Device (Nucleic Acid Amplification and Detection)
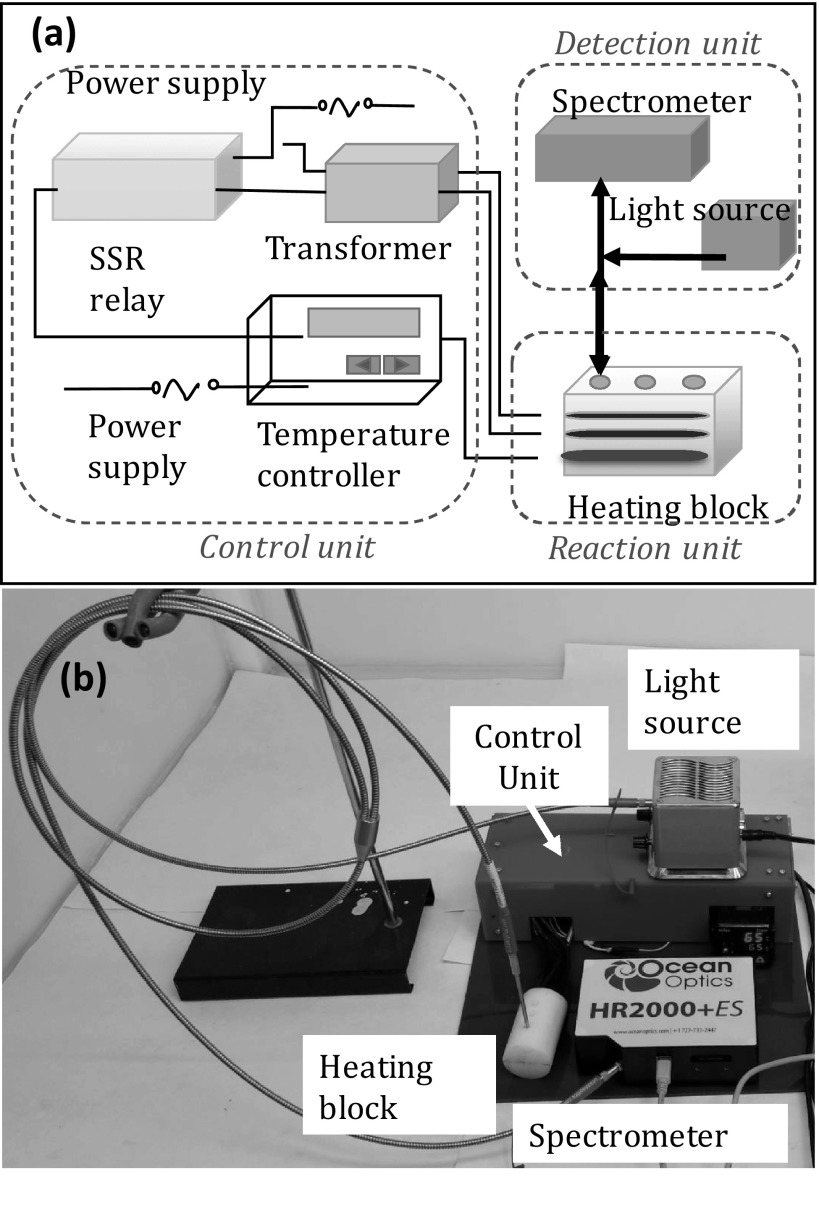
The miniature aluminum heating block was machined with multiple wells to accommodate conical PCR tubes of 0.2 mL. The depth of the well was 10 mm with a diameter of 5.5 mm. Three small horizontal holes of diameters 6 mm and length 50 mm were drilled to accommodate two cartridge type heaters (Pratik heat products Pvt. Ltd., Mumbai, India) and a thermocouple (Pixsys electronics, Italy). The thermocouple was placed just below the wells of PCR tubes with the gap of 3 mm to monitor the temperature of the wells during the reaction. The voltage generated due to thermoelectric effect was fed through an instrumentation amplifier to interpret the temperature variation. A high watt density cartridge heaters of 6 mm diameter, 40 mm length, made up of chrome nickel steel, was used for heating. These heaters can withstand the temperature up to 750 °C, operated at 12 V AC supply and are highly non-corrosive. The inner space of the heater was filled with magnesium oxide making it superior towards life expectancy. The cartridge heaters were positioned horizontally to the right and left of the wells. The heating block was covered with an opaque Teflon block to shield the tubes from ambient light. The power to the heaters and thermocouple was delivered by two 1 Ampere transformers connected in parallel. The transformer was used to convert 230 V AC to 12 V AC to facilitate the voltage supply to the heater. The thermocouple and proportional integral derivative (PID) controller were connected (Selec, Mumbai) through SSR (solid state relay) (FUTEK, USA) to control and monitor the temperature of the heating block. The PID controller was used to evaluate the error between the set point and the temperature of the heater. PID establishes control on the transformers through SSR to minimize the temperature variation using adaptive control system. SSR could switch current loads up to 40 A with 3 to 32 DC volts as control signal. For DNA amplification during LAMP reaction, the temperature was set at 65 °C for 15 min. The reaction was later terminated by denaturing the polymerase in the master mix (Optigene, UK) by heating to 80 °C for 2 min. The setup along with instrumentation is shown in Scheme 1. LAMP reactions were carried out on the heating block. Y-type optical probes were placed on the top of the vials and fixed firmly to the Teflon block. The optical fiber transmits white light from the source (Ocean optics, USA) into the vials through the transparent top cover. The fluorescence intensity from the vials during the isothermal reaction is detected via the same optic fiber, using a portable spectrometer (HR 2000 + ES, Ocean optics, USA) connected to the other leg of the Y-optical fiber. The spectrometer was equipped with CCD detector that collects the photons emitted from the vial. The spectrometer converts the optical signal to digital signal by photovoltaic effect. The data collected was structured in a graphical user interface (GUI) for simpler interpretation. The CCD detector had the sensitivity of 75 photons per count at 400 nm and 41 photons per count at 600 nm.
Scheme 1.
a Schematic and b actual view of the isothermal amplification and detection assembly consisting of heating block, spectrometer, light source and control unit
Experiments: Brief Outline
Five sets of experiments were carried out. In the first set, eleven types of negative controls (NC) were experimented. In the second set of experiments, the cross-reactivity of the primers against ten common pathogenic bacterial species found in food and water substances were tested [2–4, 16], while in the third set of experiments, the specificity of 100 and 200 CFU/mL of S. typhi in a concoction of 106 CFU/mL of E. coli, 106 CFU/mL of V. cholerae, and 106 CFU/mL of HUH7 was evaluated. The fourth set of experiments were the standardization experiments. The intensities of various concentrations (500 CFU/mL down to 50 CFU/mL) of S. typhi cells suspended in 1X TE buffer were measured to understand the sensitivity of the sensor. The fifth set of experiments were carried out for S. typhi spiked in various sample matrices such as, commercially available milk, juice from packed bottles, potable water, calf serum, raw or unprocessed milk, fresh juice from carts and turbid water. Post spiking, the final concentrations of S. typhi in the sample matrices were 100 and 200 CFU/mL. All experiments were carried out in triplicates for each dilution as well as for negative control. The results are also compared with culture method.
Tests for Negative Controls
Eleven types of NCs were tested. The primary four NC are (1) NC-1: nuclease-free water without any reagents or primers or DNA, (2) NC-2: DNA (obtained from lysing of S. typhi cells—500 CFU/mL) suspended in nuclease free water without other reagents (3) NC-3: master mix without other reagents, and (4) NC-4: primers and master mix with no S. typhi DNA. NC 5, NC 6, NC 7 and NC 8 represent negative controls in which 5 µL of commercially available milk, juice from packed bottles, potable water and commercially available calf serum were used as template DNA. NC 9, NC 10 and NC 11 represent negative controls in which 5 µL of raw milk, juice and turbid water were used as template DNA.
Cross-Reactivity and Specificity with Common Pathogens/Nuclei Acids
Common pathogenic bacterial species such as E. coli O157:H7, V. cholerae 139, S. typhimurium, S. paratyphi A, P. aeruginosa, B. cereus, L. monocytogenes, C. botulinum, S. aureus and S. havana are found invariably in contaminated water and food materials along with S. typhi [30]. Hence, experiments for signal cross-reactivity were carried out using high concentration (106 CFU/mL) of each of the above-mentioned ten bacterial pathogens against the primers and master mix. In addition, real-life samples are a concoction of many bacterial organisms along with human DNA. Hence, a concoction of 106 CFU/mL of E. coli, 106 CFU/mL of V. cholerae, and 106 CFU/mL of hepatocyte derived cellular carcinoma HUH7 (National Centre for Cell Science, Pune, India) were tested spiked with 100 and 200 CFU/mL of S. typhi. Both the cross-reactivity and specificity experiments were carried out in triplicates.
Detection in Spiked Samples of Raw or Unprocessed Milk, Juice and Turbid Dirty Water
To analyze the effect of contaminants of the assay and the optical detection system, the protocol was tested against S. typhi spiked in raw samples of milk, fruit juice, and turbid dirty water. S. typhi was spiked into each of the test samples, to yield a final concentration of 200 CFU/mL. The spiked samples were tested using with and without using lysis protocol, to understand the effect of lysis protocol. The DNA was isolated via heat treatment as per the lysis protocol described earlier. Five microlitres of the supernatant were collected and used for the LAMP assay. The LAMP reaction was carried out at 65 °C for 15 min and the intensity was measured using the spectrometer.
Detection in Spiked Samples of Commercially Available Milk, Juice, Calf serum and Tap Water
To evaluate the effectiveness of both the assay and the optical detection system, the protocol was tested against S. typhi spiked samples of milk, fruit juice, tap water and calf serum. Commercially available fat-free buffalo milk and Tropicana® fruit juice from local market were obtained. Experiments were also conducted on commercially available calf serum samples (Hi-Media laboratories, Mumbai, India). Potable water at the Institute was also considered for the experiments. S. typhi was spiked exclusively into each of the test samples, to yield a final concentration of 200 CFU/mL. The DNA was isolated via lysis protocol as described earlier. Five microlitres of the supernatant were collected and used for the LAMP assay. The LAMP reaction was carried out at 65 °C for 15 min and the intensity was measured using the spectrometer.
Comparison with Conventional Culture Method
The results of proposed method were cross-validated by conventional culture method. The sample matrices including raw/unprocessed milk, fresh juice from carts, turbid water and calf serum were spiked with 200 CFU/mL of S. typhi. These spiked samples were further cultured on a Mueller–Hinton Agar plates (BioMesrieux, France) at 37 °C for 24 h. 20 µL of each spiked sample was plated on Mueller–Hinton agar plates. The formation of pale transparent colonies indicates the possible presence of S. typhi as per the standard protocol discussed elsewhere [31]. Confirmatory biochemical tests such as slide agglutination test were performed post culturing on agar plate. A slide agglutination test using Antisera (Statens serum institute, Copenhagen) was used to detect S. typhi O9, poly O and H antigens. The conventional method in total consumes a minimum of 24 h as compared to proposed method of 30 min. The experiments were carried out in triplicates.
Results and Discussion
Optimization of Parameters for LAMP Assay and Rapid Detection Using Optical System
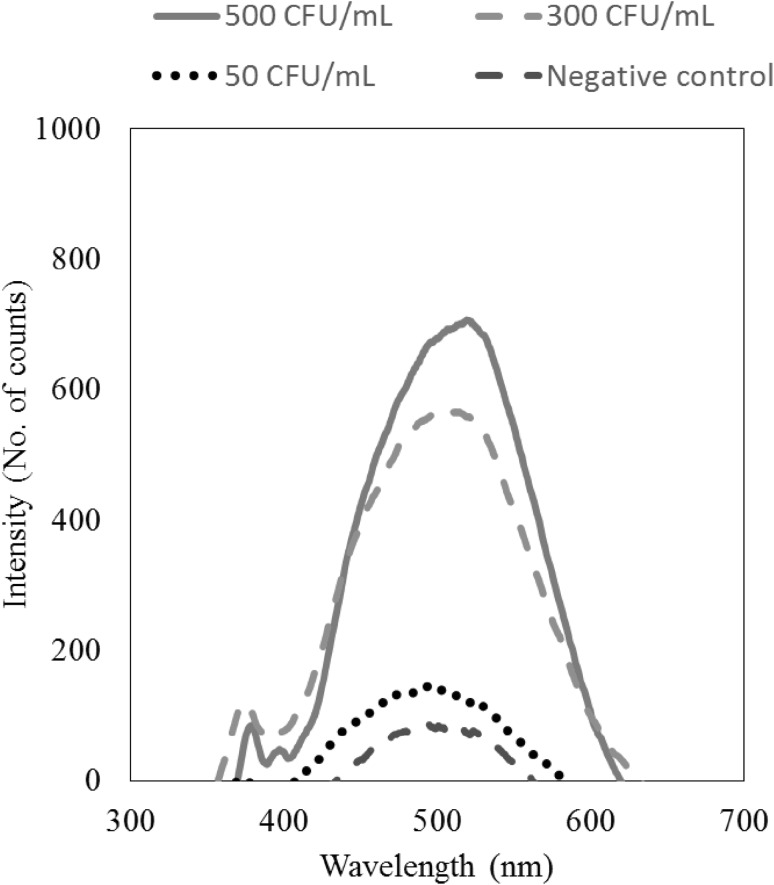
Initial LAMP experiments were carried out to 30 min to standardize the reaction volumes and concentration of the primers followed by optimization of detection time. As the focus of the work was to achieve rapid detection, the shortest time was preferred. The intensity counts were recorded at different time intervals from 5 to 30 min over 400 to 700 nm wavelength. The intensity obtained from negative control was also recorded. The intensity increases slowly at 10 min from the start of the LAMP reaction and continues to increase with time. During the recording of intensity, the maximum intensity was observed at 520 nm. After 15 min of reaction time, the intensities of positive and negative control were distinct from each other at 520 nm wavelength as shown in Fig. 1. The intensity of 300 CFU/mL and 500 CFU/mL were ten times higher than the intensity of negative control. However, the intensity of 50 CFU/mL was not clearly distinguishable from the negative control.
Fig. 1.
Intensity of signals between 400 and 720 nm for various bacterial concentrations. The maximum intensity was achieved at 520 nm
Tests for Negative Controls
The specificity of the device performance was evaluated by eleven types of negative controls as shown in Fig. 2a. The intensity (in counts) of all type of negative controls in order of NC-1 to NC-11 were: 13.2 ± 3.94, 4.06 ± 2.67, 32.53 ± 4.58, 62.40 ± 30.27, 30.34 ± 2.58, 39.93 ± 2.87, 25.37 ± 3.38, 44.00 ± 12.00, 28.17 ± 15.28, 37.68 ± 5.70 and 45.67 ± 4.65. It was compared with the intensity corresponding to 500 CFU/mL of S. typhi with master mix and primers. All intensity measurements correspond to readings at 520 nm at 15 min. The maximum count from the negative controls was 62.40 while that from 500 CFU/mL S. typhi was 700.
Fig. 2.
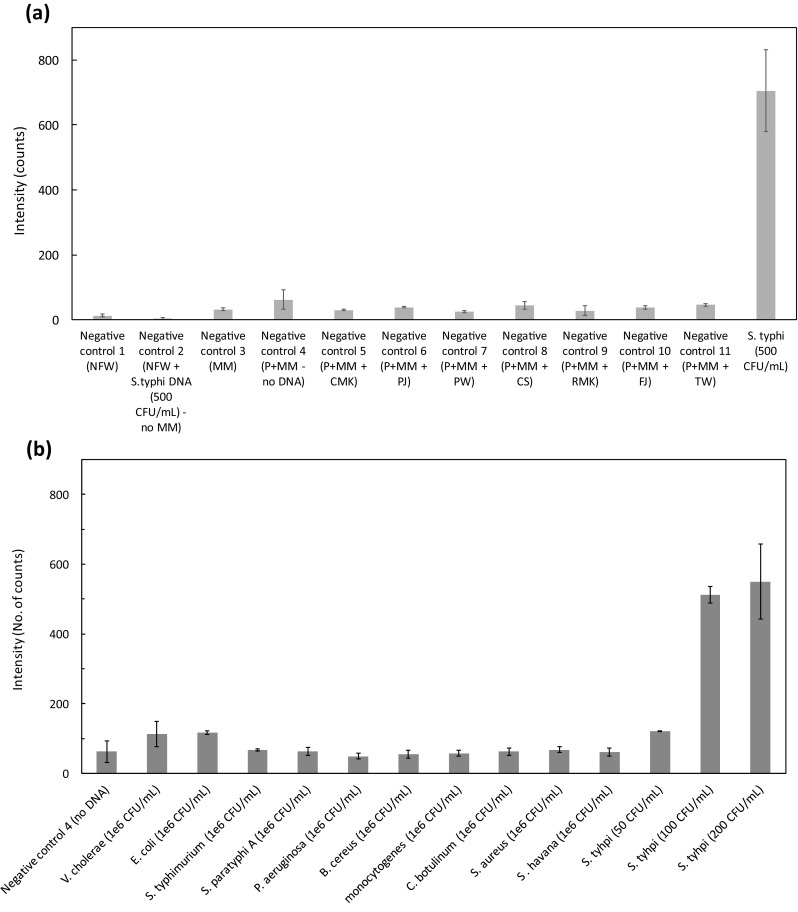
a. Response at 15 min using different types of negative controls vs positive control sample. NFW nuclease free water, P primers, MM master mix, CMK commercially available milk, PJ packed juice, PW potable water, CS calf serum, RMK raw milk, FJ fresh juice, TW turbid water. b. Check for cross reactivity of primers in the real-time LAMP assay against E. coli, V. cholerae 139, S. typhimurium, S. paratyphi A, P. aeruginosa, B. cereus, L. monocytogenes, C. botulinum, S. aureus and S. havana. The negative control includes primers, master mix and nuclease free water (NC-4)
Cross-Reactivity Tests for Primers
The cross-reactivity of the primers for STY2879 gene was tested against high concentrations (106 CFU/mL) of E. coli O157:H7, V. cholerae 139, S. typhimurium, S. paratyphi A, P. aeruginosa, B. cereus, L. monocytogenes, C. botulinum, S. aureus and S. havana cells. Five microlitres of the supernatant of the lysed solution (DNA template) was used for LAMP reaction. The intensity was measured as described earlier (15 min at 520 nm). Figure 2b represents the intensity measured for the cross-reactivity reactions of the cell lines mentioned above. Even at high concentrations of the order of 106 CFU/mL, there was very low intensity observed for the pathogenic bacterial species when compared against 100 CFU/mL or higher S. typhi. The intensity in counts of E. coli O157:H7, V. cholerae 139, S. typhimurium, S. paratyphi A, P. aeruginosa, B. cereus, L. monocytogenes, C. botulinum, S. aureus and S. havana were 116.59 ± 5.8, 113.22 ± 35.86, 66.66 ± 3.24, 63.39 ± 11.72, 49.11 ± 7.98, 54.76 ± 11.84, 57.44 ± 8.51, 62.84 ± 9.95, 67.85 ± 8.29 and 61.62 ± 11.31 respectively. These intensities were found to be much lower than the intensity of 100 and 200 CFU/mL of S. typhi (512.24 ± 23.78 counts and 550 ± 106.94 counts) used as a positive control.
Specificity analysis
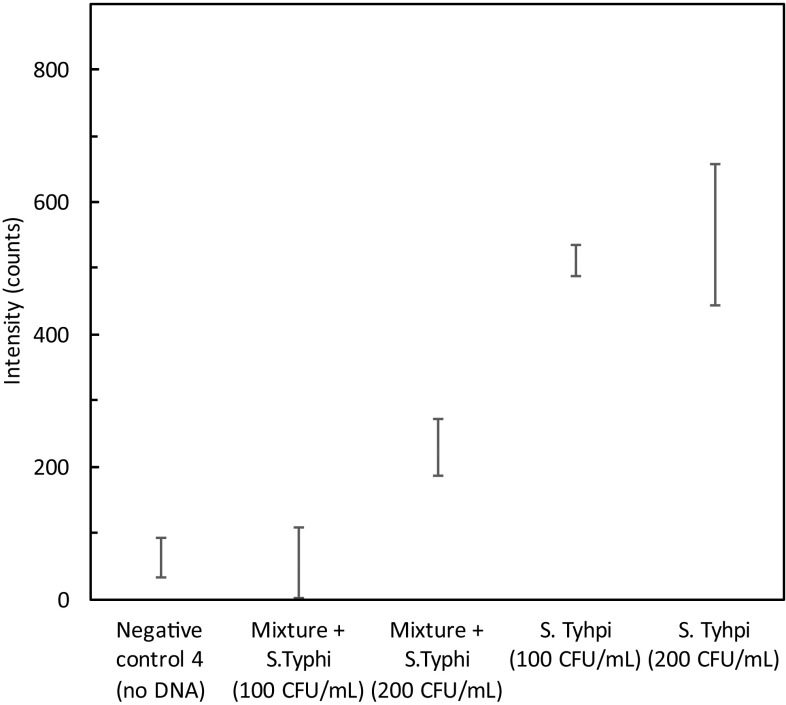
The specificity of the primers for STY2879 gene was tested by spiking S. typhi cell lines with other cells including E. coli O157:H7, V. cholerae 139 and hepatocyte-derived cellular carcinoma HUH7 cells. In the concoction, the concentration of S. typhi cells was 200 CFU/mL while, that of other cells were 1 × 106 CFU/mL. Same lysis protocol was followed to the mixture. Figure 3 shows the specificity test results in a concoction of other cellular DNA. The intensity of 100 CFU/mL (55.63 ± 32.37 counts) of S. typhi in a mixture coincides with that of the negative control (62.40 ± 30.27 counts). However, 200 CFU/mL of S. typhi in a mixture yielded 229.50 ± 42.32 counts. When compared with the values attained in the standardization curve, some attenuation was observed. This could be attributed to the presence of other cellular DNA in high concentrations (~ 106 CFU/mL) compared to the target DNA (200 CFU/mL) thereby decreasing the efficiency of amplification of target sequence. It can, therefore, be concluded that even in the presence of high concentration of non-target DNA, the LAMP assay could amplify the low concentration (200 CFU/mL) of target DNA.
Fig. 3.
Specificity study in presence of a concoction of 106 CFU/mL of E. coli, 106 CFU/mL of V. cholerae, 106 CFU/mL of HUH7, S. typhi (100 and 200 CFU/mL), negative control-4(No DNA). Intensity was measured at 15 min response time
Standardization of Sensing Parameters
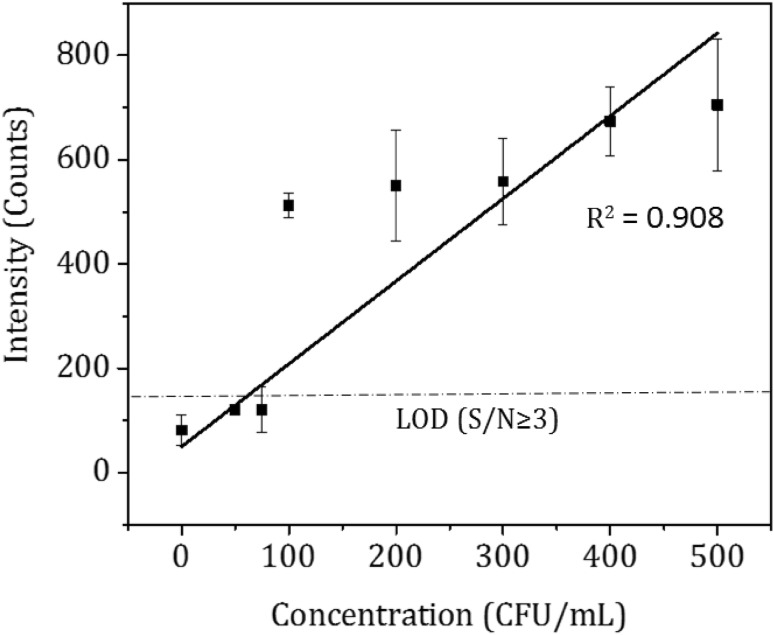
As the results between positive and negative controls were distinct, the lysis and detection protocol was extended to the evaluation of various concentrations of S. typhi from 50 to 500 CFU/mL. The sensor response in terms of intensity counts obtained is plotted as a function of S. typhi concentrations as seen in Fig. 4. The intensity corresponding to negative control 4 from Fig. 2a (is marked as 0 CFU/mL in Fig. 4), was approximately 62 counts. From Fig. 4, the lowest distinguishable concentration against negative control was 100 CFU/mL corresponding to 500 counts. The limit of detection (LOD) was calculated as 3 times the sensor reading of negative control (3 × 62 counts = 186 counts). Hence based on the S/N ≥ 3, [32–34] the LOD of the proposed method was 100 CFU/mL as shown in Fig. 2b. The R2 value of calibration curve was 0.908. The equation of the calibration curve is X = (Y − 50)/1.585 represents linear equation obtained by curve fitting where X represents the concentration (CFU/mL) and Y represents the intensity. The slope of the calibration curve was calculated as 1.585 counts per CFU/mL that represents the sensitivity of the sensor. Using the equation of the standard curve, the unknown concentration of cells suspended in 1X TE buffer can be determined.
Fig. 4.
Calibration curve: Intensity versus various bacterial concentrations; Real time LAMP assay for 0 to 500 CFU/mL at 15 min response time
Detection in Spiked Samples of Commercially Available Milk, Packed Juice, Calf Serum and Potable Tap Water
The sensitivity of LAMP assay was evaluated by spiking the S. typhi cells (100 and 200 CFU/mL) in milk, juice, tap water and calf serum. DNA was isolated using heat treatment method. Five microliters of the supernatant from the lysed suspension were used for the LAMP reaction. LAMP assay was performed at 65 °C and intensity were measured using optical detection system at 520 nm and 15 min. Figure 5a shows the results of detection of S. typhi in the spiked samples. S. typhi of 200 CFU/mL concentration was clearly detected from all the four types of samples compared to 100 CFU/mL. The intensity of the negative controls for milk (30 ± 3 counts), juice (39 ± 3 counts), potable tap water (25 ± 3 counts) and calf serum (44 ± 12 counts) without spiked S. typhi cells was quite low as compared to spiked cells. The intensity of the spiked 200 CFU/mL S. typhi cells for milk, juice, tap water and calf serum were 282.91 ± 31.41, 203.29 ± 89.09, 292.13 ± 20.80, and 249.10 ± 4.69 counts respectively. Hence, the technique was able to detect S. typhi in the real sample. The same device can also be used for detecting other bacterial pathogens when used with corresponding primers in real samples. The primers for a specific gene can be designed using LAMP designer® or Primer explorer V4®.
Fig. 5.
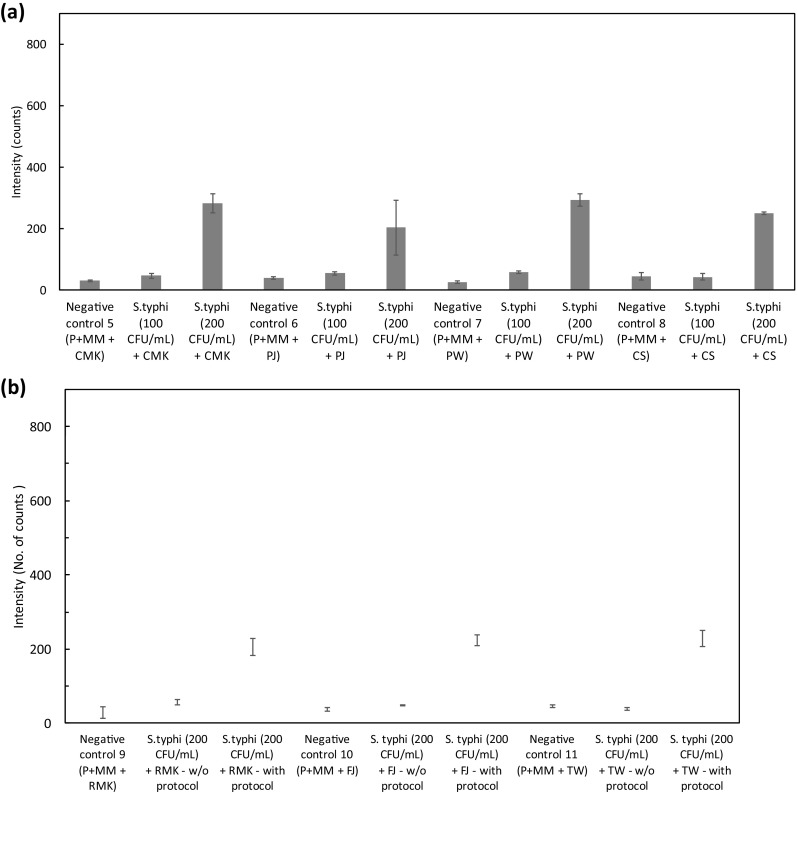
a. Detection in spiked samples of commercially available milk, fruit juice, tap water and calf serum with 100, 200 CFU/ml of S. typhi. P primers, MM master mix, CMK commercially available milk; PJ packed juice, PW potable water, CS calf serum. b. Detection in spiked samples of raw milk, juice and turbid dirty water with 200 CFU/mL with and without using lysis protocol. P primers, MM master mix, RMK raw milk, FJ fresh juice, TW turbid water
Detection in Spiked Samples of Raw Milk, Juice and Turbid Dirty Water
Salmonella . typhi spiked in raw milk, juice and turbid dirty water was used with and without lysis protocol to see the effect of contaminants on LAMP assay. Figure 5b shows the results of detection of S. typhi in the spiked samples. S. typhi of 200 CFU/mL concentration was clearly detected from all the four types of samples. The intensity of the negative controls for raw milk (28.17 ± 15.28 counts), juice (37.68 ± 5.70 counts), and turbid dirty water (45.67 ± 4.65 counts) without spiked S. typhi cells was quite low as compared to spiked cells. The intensity (counts) of the S. typhi spiked raw milk, juice, and turbid dirty water without using lysis protocol were 56.66 ± 7.13, 48.16 ± 1.87, and 39.16 ± 4.01 counts respectively while the counts using lysis protocol were 205.91 ± 23.32, 224.58 ± 14.94, and 228.28 ± 21.01 counts respectively. Hence, the technique was able to detect S. typhi in the raw samples.
Comparison with Conventional Culture Method
Assessment of spiked samples such as raw milk, juice, turbid water and calf serum by proposed method and cross-validation with conventional method were performed. In the conventional method, the spiked samples were cultured on Mueller–Hinton Agar and incubated for 24 h. S. typhi was confirmed by formation of pale transparent colonies on agar plates and visible clumps using slide agglutination test. The minimum time taken for the conventional method was 24 h. Hence, it can be concluded that our proposed method is quite efficient at prediction given its reduced time for diagnosis and at lower LOD. The results of conventional and proposed method are shown in Table 1.
Table 1.
Results from conventional and proposed method for spiked samples in TE buffer, raw juice, milk, turbid water and serum
| S. No. | S. typhi CFU/mL (buffer/samples) | Bacterial culture for S.typhi | Slide agglutination test | Confirmation of S. typhi by conventional method | Confirmation of S. typhi by proposed method | Concurrence of results between proposed method and conventional |
|---|---|---|---|---|---|---|
| 1 | NC-4 | Negative | Negative | Negative | Negative | Yes |
| 2 | 50 (buffer) | Negative | Negative | Negative | Negative | Yes |
| 3 | 100 (buffer) | Negative | Negative | Positive | Positive | No |
| 4 | 200 (buffer) | Positive | Positive | Positive | Positive | Yes |
| 5 | 300 (buffer) | Positive | Positive | Positive | Positive | Yes |
| 6 | 400 (buffer) | Positive | Positive | Positive | Positive | Yes |
| 7 | 500 (buffer) | Positive | Positive | Positive | Positive | Yes |
| 8 | NC-8 | Negative | Negative | Negative | Negative | Yes |
| 9 | 200 (serum) | Positive | Positive | Positive | Positive | Yes |
| 10 | NC-9 | Negative | Negative | Negative | Negative | Yes |
| 11 | 200 (raw milk) | Positive | Positive | Positive | Positive | Yes |
| 12 | NC-10 | Negative | Negative | Negative | Negative | Yes |
| 13 | 200 (fresh juice) | Positive | Positive | Positive | Positive | Yes |
| 14 | NC-11 | Negative | Negative | Negative | Negative | Yes |
| 15 | 200 (turbid water) | Positive | Positive | Positive | Positive | Yes |
Comparison of Recent Devices for Detection of S. typhi
The recent methods developed post 2014 for the detection of S. typhi are given in Table 2. The detection methods have been experimented in various sample matrices. From the Table, it is also evident that the total operational time (sample preparation and detection) range from 1¼ to 4½ h, while proposed method can achieve its target in 30 min. Also, some of the earlier methods demand high cost equipment with maintenance effort and cost.
Table 2.
S. typhi detection by various recent devices and methods
| Sample matrix | Sample preparation time | Detection time | LOD | Mechanism | References |
|---|---|---|---|---|---|
| Contaminated water | 3 h | 500 CFU/mL | LAMP amplification and detection by Confocal laser scanning microscope | [18] | |
| Tryptic soy broth media | 35 min | 2 h | 20 CFU/mL | LAMP amplification and detection by surface plasmon resonance | [29] |
| Blood and stool | 16 min | 1 h | 20 CFU/ml and 200 CFU/g | LAMP amplification and detection by realtime turbidimeter LA320C | [28] |
| Nutrient broth and blood culture bottle | 25 min | 1 h | 20 CFU/reaction | LAMP amplification and detection by colorimeter | [21] |
| Other methods | |||||
| Water | 4.5 h in total | 2000 CFU/mL | Surface aminated polycarbonate membrane enhanced ELISA | [11] | |
| Proposed method | |||||
| Water, fruit juice, serum, milk | 15 min | 15 min | 200 CFU/mL | LAMP amplification and detection by in-house method | Proposed method |
Conclusion
In this work, we have demonstrated a rapid detection of S. typhi with a portable system employing a LAMP assay. The reaction was performed in a simple, and rapid manner. The LOD is 100 CFU/mL in TE buffer. However, in commercially available milk, packaged juice, calf serum, potable tap water, raw or unprocessed milk, fresh juice, and turbid water, the LOD was 200 CFU/mL. After design changes and miniaturization, this device has the potential to be catered to low resource settings as well as a field device. This portable device simultaneously runs three reactions. The device can be modified to include multiple vials with a slight increase in power requirement. Additionally, more than one pathogen can be detected by incorporating multiple colorimetric dyes, which is the intended future scope of work. This detection device can be an advantage for especially food and medical applications where rapid, in situ testing is necessary for rapid detection of potential sources of contamination and infection.
Acknowledgements
The authors would also like to acknowledge Professor Arti Kapil of All India Institute of Medical Sciences for valuable inputs.
Funding
The authors would like to acknowledge the funding agencies for their continued support: Department of Science and Technology (YSS/2014/000880, and IDP/MED/05/2014), Indo-German Science and Technology Centre (IGSTC/Call 2014/Sound4All/24/2015-16), Naval research board (NRB/4003/PG/359), BIRAC, Department of Biotechnology (BIRAC/BT/AIR0275/PACE-12/17).
Compliance with Ethical Standards
Conflicts of interest
AK declares that she has no conflict of interest. RD declares that she has no conflict of interest. MRN declares that he has no conflict of interest. RE declares that he has no conflict of interest. DP declares that she has no conflict of interest. SJ declares that he has no conflict of interest. DK declares that he has no conflict of interest.
Ethical Approval
The study does not involve any human samples and there is no ethical clearance required for this work.
Contributor Information
Sandeep Jha, Phone: +91-11-2659-1119, Email: sandeepkjha@gmail.com.
Dinesh Kalyanasundaram, Phone: +91-11-26597344, Email: dineshk.iitdelhi@gmail.com, Email: dineshk@cbme.iitd.ac.in.
References
- 1.Kalia VC, Kumar P. Genome wide search for biomarkers to diagnose Yersinia infections. Indian J Microbiol. 2015;55:366–374. doi: 10.1007/s12088-015-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranjbar R, Mortazavi SM, Mehrabi Tavana A, Sarshar M, Najafi A, Zanjani RS. Simultaneous molecular detection of Salmonella enterica Serovars Typhi, Enteritidis, Infantis, and Typhimurium, Iran. J Public Health. 2017;46:103–111. [PMC free article] [PubMed] [Google Scholar]
- 3.Seo JH, Park BH, Oh SJ, Choi G, Kim DH, Lee EY, Seo TS. Development of a high-throughput centrifugal loop-mediated isothermal amplification microdevice for multiplex foodborne pathogenic bacteria detection. Sensors Actuators B Chem. 2017;246:146–153. doi: 10.1016/j.snb.2017.02.051. [DOI] [Google Scholar]
- 4.Wachiralurpan S, Sriyapai T, Areekit S, Kaewphinit T, Sriyapai P, Santiwatanakul S, Chansiri K. Development of a rapid screening test for Listeria monocytogenes in raw chicken meat using loop-mediated isothermal amplification (LAMP) and lateral flow dipstick (LFD) Food Anal Methods. 2017;10:3763–3772. doi: 10.1007/s12161-017-0949-4. [DOI] [Google Scholar]
- 5.Karwa M, Currie B, Kvetan V. Bioterrorism: preparing for the impossible or the improbable. Crit Care Med. 2005;33:S75–95. doi: 10.1097/01.CCM.0000151070.56915.22. [DOI] [PubMed] [Google Scholar]
- 6.D’Agostino M, Robles S, Hansen F, Ntafis V, Ikonomopoulos J, Kokkinos P, Alvarez-Ordonez A, Jordan K, Delibato E, Kukier E, et al. Validation of a loop-mediated amplification/ISO 6579-based method for analysing soya meal for the presence of Salmonella enterica. Food Anal Methods. 2016;9:2979–2985. doi: 10.1007/s12161-016-0602-7. [DOI] [Google Scholar]
- 7.World Health Organization, Typhoid, World Heal. Organ (2015). http://www.who.int/immunization/diseases/typhoid/en/. Accessed Aug 5 2017
- 8.Fabiani L, Pucci E, Delibato E, Volpe G, Piermarini S, De Medici D, Capuano F, Palleschi G. ELIME assay vs real-time PCR and conventional culture method for an effective detection of salmonella in fresh leafy green vegetables. Talanta. 2017;166:321–327. doi: 10.1016/j.talanta.2017.01.071. [DOI] [PubMed] [Google Scholar]
- 9.Kumar M, Dahiya S, Sharma P, Sharma S, Singh TP, Kapil A, Kaur P. Structure based in silico analysis of quinolone resistance in clinical isolates of Salmonella Typhi from India. PLoS ONE. 2015;10:e0126560. doi: 10.1371/journal.pone.0126560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S. Antimicrobial-resistant pathogens associated with healthcare- associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;3:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 11.Jain S, Chattopadhyay S, Jackeray R, Abid CKVZ, Kohli GS, Singh H. Highly sensitive detection of Salmonella typhi using surface aminated polycarbonate membrane enhanced-ELISA. Biosens Bioelectron. 2012;31:37–43. doi: 10.1016/j.bios.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Ahari H, Kakoolaki S, Anvar SAA. Detection of Salmonella typhi using four developed kits of ELISA for cleaning in place purification. Int J Environ Sci Technol. 2017 [Google Scholar]
- 13.Preechakasedkit P, Pinwattana K, Dungchai W, Siangproh W. Biosensors and bioelectronics development of a one-step immunochromatographic strip test using gold nanoparticles for the rapid detection of Salmonella typhi in human serum. Biosens Bioelectron. 2012;31:562–566. doi: 10.1016/j.bios.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Hsu H. Development of enzyme linked, tissue blot and dot blot immunoassays for plant virus detection. In: Burns R, editor. Plant Pathology: technology and protocols. Totowa: Humana Press; 2009. pp. 15–25. [DOI] [PubMed] [Google Scholar]
- 15.Kalia VC, Kumar R, Kumar P, Koul S. A genome-wide profiling strategy as an aid for searching unique identification biomarkers for Streptococcus. Indian J Microbiol. 2016;56:46–58. doi: 10.1007/s12088-015-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kekre A, Bhushan A, Kumar P, Kalia VC. Genome wide analysis for searching novel markers to rapidly identify Clostridium strains. Indian J Microbiol. 2015;55:250–257. doi: 10.1007/s12088-015-0535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh J, Sharma S, Nara S. Nanogold based lateral flow assay for the detection of Salmonella typhi in environmental water samples. Anal Methods. 2015;7:9281–9288. doi: 10.1039/C5AY02271A. [DOI] [Google Scholar]
- 18.Jain S, Chattopadhyay S, Jackeray R, Abid Z, Singh H. Detection of Salmonella typhi utilizing bioconjugated fluorescent polymeric nanoparticles. J Nanoparticle Res. 2016;18:111. doi: 10.1007/s11051-016-3414-1. [DOI] [Google Scholar]
- 19.Lazcka O, Del Campo FJ, Muñoz FX. Pathogen detection: a perspective of traditional methods and biosensors. Biosens Bioelectron. 2007;22:1205–1217. doi: 10.1016/j.bios.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Notomi T, Mori Y, Tomita N, Kanda H. Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol. 2015;53:1–5. doi: 10.1007/s12275-015-4656-9. [DOI] [PubMed] [Google Scholar]
- 21.Abdullah J, Saffie N, Sjasri FAR, Husin A, Abdul-Rahman Z, Ismail A, Aziah I, Mohamed M. Rapid detection of Salmonella Typhi by loop-mediated isothermal amplification (LAMP) method. Braz J Microbiol. 2014;45:1385–1391. doi: 10.1590/S1517-83822014000400032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kokkinos PA, Ziros PG, Bellou M, Vantarakis A. Loop-mediated isothermal amplification (LAMP) for the detection of salmonella in food. Food Anal Methods. 2014;7:512–526. doi: 10.1007/s12161-013-9748-8. [DOI] [Google Scholar]
- 23.Babu B, Washburn BK, Miller SH, Poduch K, Sarigul T, Knox GW, Ochoa-Corona FM, Paret ML. A rapid assay for detection of Rose rosette virus using reverse transcription-recombinase polymerase amplification using multiple gene targets. J Virol Methods. 2017;240:78–84. doi: 10.1016/j.jviromet.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y, Zou S, Cao X. A simple dendrimer-aptamer based microfluidic platform for E. coli O157:H7 detection and signal intensification by rolling circle amplification. Sensors Actuators B Chem. 2017;251:976–984. doi: 10.1016/j.snb.2017.05.146. [DOI] [Google Scholar]
- 25.Hønsvall BK, Robertson LJ. Real-time nucleic acid sequence-based amplification (NASBA) assay targeting MIC1 for detection of Cryptosporidium parvum and Cryptosporidium hominis oocysts. Exp Parasitol. 2017;172:61–67. doi: 10.1016/j.exppara.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sayad AA, Ibrahim F, Uddin SM, Pei KX, Mohktar MS, Madou M, Thong KL. A microfluidic lab-on-a-disc integrated loop mediated isothermal amplification for foodborne pathogen detection. Sensors Actuators B Chem. 2016;227:600–609. doi: 10.1016/j.snb.2015.10.116. [DOI] [Google Scholar]
- 28.Fan F, Du P, Kan B, Yan M. The development and evaluation of a loop-mediated isothermal amplification method for the rapid detection of Salmonella enterica serovar Typhi. PLoS ONE. 2015 doi: 10.1371/journal.pone.0124507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozorgmehr A, Yazdanparast R, Hamidreza M. Non-crosslinking gold nanoprobe-LAMP for simple, colorimetric, and specific detection of Salmonella typhi. J. Nanoparticle Res. 2016;18:351. doi: 10.1007/s11051-016-3657-x. [DOI] [Google Scholar]
- 30.Jin D-Z, Xu X-J, Chen S-H, Wen S-Y, Ma X-E, Zhang Z, Lin F, Wang S-Q. Detection and identification of enterohemorrhagic Escherichia coli O157:H7 and Vibrio cholerae O139 using oligonucleotide microarray., Infect. Agent. Cancer. 2007;2:23. doi: 10.1186/1750-9378-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DL K, PH G (2007) Manual of clinical microbiology, 9th ed. American Society for Microbiology Press
- 32.Soni A, Jha SK. A paper strip based non-invasive glucose biosensor for salivary analysis. Biosens Bioelectron. 2015;67:763–768. doi: 10.1016/j.bios.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 33.Lin Y, Lu F, Tu Y, Ren Z. Glucose biosensors based on carbon nanotube nanoelectrode ensembles. Nano Lett. 2004;4:191–195. doi: 10.1021/nl0347233. [DOI] [Google Scholar]
- 34.Cai B, Huang L, Zhang H, Sun Z, Zhang Z, Zhang G-J. Gold nanoparticles-decorated graphene field-effect transistor biosensor for femtomolar MicroRNA detection. Biosens Bioelectron. 2015;74:329–334. doi: 10.1016/j.bios.2015.06.068. [DOI] [PubMed] [Google Scholar]