Abstract
Key points
The response to neuroplasticity interventions using transcranial magnetic stimulation (TMS) is reduced in older adults, which may be due, in part, to age‐related alterations in interneuronal (I‐wave) circuitry.
The current study investigated age‐related changes in interneuronal characteristics and whether they influence motor cortical plasticity in older adults.
While I‐wave recruitment was unaffected by age, there was a shift in the temporal characteristics of the late, but not the early I‐waves.
Using I‐wave periodicity repetitive TMS (iTMS), we showed that these differences in I‐wave characteristics influence the induction of cortical plasticity in older adults.
Abstract
Previous research shows that neuroplasticity assessed using transcranial magnetic stimulation (TMS) is reduced in older adults. While this deficit is often assumed to represent altered synaptic modification processes, age‐related changes in the interneuronal circuits activated by TMS may also contribute. Here we assessed age‐related differences in the characteristics of the corticospinal indirect (I) waves and how they influence plasticity induction in primary motor cortex. Twenty young (23.7 ± 3.4 years) and 19 older adults (70.6 ± 6.0 years) participated in these studies. I‐wave recruitment was assessed by changing the direction of the current used to activate the motor cortex, whereas short‐interval intracortical facilitation (SICF) was recorded to assess facilitatory I‐wave interactions. In a separate study, I‐wave periodicity TMS (iTMS) was used to examine the effect of I‐wave latency on motor cortex plasticity. Data from the motor‐evoked potential (MEP) onset latency produced using different coil orientations suggested that there were no age‐related differences in preferential I‐wave recruitment (P = 0.6). However, older adults demonstrated significant reductions in MEP facilitation at all 3 SICF peaks (all P values < 0.05) and a delayed latency of the second and third SICF peaks (all P values < 0.05). Using I‐wave intervals that were optimal for young and older adults, these changes in the late I‐waves were shown to influence the plasticity response in older adults after iTMS. These findings suggest that temporal characteristics are delayed for the late I‐waves in older adults, and that optimising TMS interventions based on I‐wave characteristics may improve the plasticity response in older adults.
Keywords: ageing, corticospinal descending volley, transcranial magnetic stimulation
Key points
The response to neuroplasticity interventions using transcranial magnetic stimulation (TMS) is reduced in older adults, which may be due, in part, to age‐related alterations in interneuronal (I‐wave) circuitry.
The current study investigated age‐related changes in interneuronal characteristics and whether they influence motor cortical plasticity in older adults.
While I‐wave recruitment was unaffected by age, there was a shift in the temporal characteristics of the late, but not the early I‐waves.
Using I‐wave periodicity repetitive TMS (iTMS), we showed that these differences in I‐wave characteristics influence the induction of cortical plasticity in older adults.
Introduction
One of the most ubiquitous deficits associated with the ageing process is a degradation of motor function. Although the multitude of factors contributing to these deficits remain poorly understood, age‐related alterations in the ability of the brain to modify its intrinsic connectivity is likely to be an important factor. Referred to as neuroplasticity, this process is mediated in part by long‐term potentiation (LTP) or depression (LTD) of synaptic communication (Sanes & Donoghue, 2000). These processes can be studied in humans using transcranial magnetic stimulation (TMS), a non‐invasive brain stimulation (NIBS) technique able to induce and measure LTP‐ and LTD‐like changes in cortical excitability (Ziemann et al. 2008). Using this technique, a number of studies have reported that TMS‐induced neuroplastic capacity is reduced in the elderly (Müller‐Dahlhaus et al. 2008; Fathi et al. 2010; Todd et al. 2010; Freitas et al. 2011). This reduced plasticity response may limit the clinical utility of ‘priming’ TMS protocols in older adults (Opie et al. 2017a, 2017b). Despite the critical role of neuroplasticity in motor function, the mechanisms associated with deficits evident in the elderly are currently unclear.
Differences in the TMS‐induced plasticity response may be due to a number of factors, including gender, level of aerobic fitness, time of day, level of attention, synaptic history and genetics (Ridding & Ziemann, 2010). However, recent studies with TMS have shown that the ability to induce LTP‐ or LTD‐like plasticity is associated with characteristics of the descending volley (waves) generated within the corticospinal tract (Cash et al. 2009, 2013; Thickbroom 2011; Hamada et al. 2013). This multiphasic potential is composed of direct (D) waves, representing direct activation of the corticospinal neuron at or near the initial segment, and subsequent indirect (I) waves (I1–I3 occurring at ∼1.5 ms intervals) representing trans‐synaptic activation of corticospinal neurons by intracortical circuits (Di Lazzaro et al. 2012). Previous work investigating cervical epidural recordings of the TMS‐evoked descending volley has shown that several plasticity‐inducing protocols specifically modulate the activity of the interneuronal circuits responsible for generating the late I‐waves (Di Lazzaro et al. 2008a, 2008b, 2009a, 2009b). Furthermore, emerging evidence has shown that the ability of TMS to activate the late I‐waves strongly predicts the magnitude of plasticity in individual subjects (Hamada et al. 2013; Murase et al. 2015). Subsequently, physiological differences within the late I‐wave circuits are likely to contribute to variations in the TMS‐induced plasticity response. The possibility therefore exists that an age‐related change in TMS‐induced I‐wave circuitry may contribute to the reduced plasticity response commonly observed with NIBS interventions in older adults.
The aim of the current study was twofold: (1) to investigate age‐related differences in the excitability or recruitment of the interneuronal circuits responsible for I‐wave generation and (2) to explore the influence of altered I‐wave characteristics on neuroplasticity induction. I‐wave excitability was assessed using paired‐pulse TMS to examine short‐interval intracortical facilitation (SICF) in young and old adults. This protocol produces peaks of MEP facilitation when TMS pulses are paired at interstimulus intervals (ISIs) that coincide with I‐wave periodicity observed within epidural recordings (Tokimura et al. 1996; Ziemann et al. 1998a). I‐wave recruitment was assessed by altering the direction of the cortical current applied to primary motor cortex (M1) to preferentially activate different features of the descending volley (Werhahn et al. 1994; Di Lazzaro et al. 2001). Neuroplasticity induction was tested using I‐wave periodicity TMS (iTMS), which applies pairs of stimuli at intervals approximating the I‐wave frequency (Thickbroom et al. 2006; Cash et al. 2009). Given that there are age‐related differences in TMS measures of intracortical excitability and plasticity that target late I‐waves (Müller‐Dahlhaus et al. 2008; Fathi et al. 2010; Heise et al. 2013), we expected that late I‐wave excitability and recruitment would be modified in older adults, and that these changes would influence the response to iTMS.
Methods
Ethical approval
All experimentation was approved by the University of Adelaide Human Research Ethics Committee and conducted in accordance with the Declaration of Helsinki, except for registration in a database. Each subject provided written, informed consent prior to participation.
Subjects
All subjects were recruited from the university and wider community. Exclusion criteria included a history of neurological or psychiatric disease, or current use of psychoactive medication (sedatives, antipsychotics, antidepressants etc.), but nicotine use was not assessed. Hand preference and laterality were assessed using the Edinburgh Handedness Inventory (Oldfield, 1971).
Twenty young (mean age ± standard deviation: 23.7 ± 3.4 years) and 19 older adults (70.6 ± 6.0 years) were recruited for this project, which consisted of two experimental studies. Experiment 1, which involved 15 young (22.5 ± 2.9 years, 8 females) and 18 older (70.1 ± 6.0 years, 12 females) adults, consisted of a single session that assessed I‐wave recruitment and excitability. For Experiment 2, two different iTMS intervals, based on the responses obtained in Experiment 1, were examined in two separate sessions. Seven young (25.7 ± 3.4 years, 3 females) and seven older (69.9 ± 5.1 years, 4 females) adults participated in Experiment 2, eight (2 young, 6 older) of whom also participated in Experiment 1.
Experimental arrangement
For the duration of all experiments, subjects were seated in a comfortable chair with their right arm and hand relaxed on a support placed next to them. Surface electromyography (EMG) was recorded from the first dorsal interosseous (FDI) muscle of the right hand using two Ag–AgCl electrodes in a belly–tendon montage. A strap around the wrist grounded the electrodes. EMG signals were amplified (300×) and band‐pass filtered (20 Hz high pass, 1 kHz low pass) using a CED1902 signal conditioner (Cambridge Electronic Design, Cambridge, UK), and digitised at 2 kHz using a CED1401 interface (Cambridge Electronic Design). To facilitate muscle relaxation when required, real‐time EMG signals were displayed under high gain (50 μV per division) on an oscilloscope placed in front of the subject.
Transcranial magnetic stimulation (TMS)
TMS was applied to the hand area of the left M1 using a figure‐of‐eight coil with two monophasic Magstim 2002 magnetic stimulators connected via a Bistim unit (Magstim, Dyfed, UK). The coil was positioned tangentially to the scalp over the location producing an optimum response in the FDI muscle of the right hand. This location was identified during relaxation of FDI, using a posterior‐to‐anterior (PA) current flow generated by pointing the coil handle backwards and laterally (approximately 45° to the sagittal plane). This optimal spot was marked on the scalp for reference and continually checked throughout the experiment.
Experiment 1: age‐related changes in I‐wave recruitment and excitability
Two established approaches were used to examine age‐related differences in I‐wave characteristics. Assessment of I‐wave recruitment was performed by analysing MEP latencies with different coil orientations (Hamada et al. 2013). For assessment of I‐wave excitability, paired‐pulse TMS was used to examine SICF (Ziemann et al. 1998a). Both of these measures were performed in a single experimental session.
I‐wave recruitment
I‐wave recruitment was investigated by altering current flow through the motor cortex to produce descending volleys with preferential recruitment of D‐waves, and early or late I‐waves (Day et al. 1989; Werhahn et al. 1994; Hamada et al. 2013). Specifically, PA current preferentially elicits early I‐waves, whereas anterior‐to‐posterior (AP) current (coil handle 180° to PA) preferentially elicits late I‐waves and lateral‐to‐medial current (LM, coil handle 90° to the sagittal plane) preferentially elicits D‐waves. Each coil orientation was tested in a single block of trials.
As measures of I‐wave recruitment require an active muscle (see below), it was necessary to first assess maximal voluntary contraction (MVC) during index finger abduction. This allowed force levels to be normalised between individuals. During MVC assessment, the subject's right hand was positioned with the palm facing downwards. When instructed, subjects abducted the lateral surface of the index finger against a force transducer (MLP‐25, Transducer Techniques, USA) placed in‐line with the distal interphalangeal joint. Subjects were required to produce maximum force for 3 s in several repetitions, separated by 30 s rest, until the maximal force of three trials were within a 10% margin. The largest force recorded during these trials was chosen as the subject's MVC. To optimise force production, feedback was displayed on a computer monitor placed at eye level in front of the subject, and verbal encouragement was provided by the experimenter.
Active motor threshold (AMT) was defined as the minimum stimulus intensity producing an MEP response ≥ 200 μV in at least 3 out of 5 trials while the right FDI was abducted at 10% of maximum. This was assessed separately for PA (AMTPA), AP (AMTAP) and LM (AMTLM) coil orientations. To investigate the recruitment of early (PA stimulation) and late (AP stimulation) I‐waves, MEPs were recorded using stimulation intensities of 110% of AMTPA and AMTAP, respectively. For LM stimulation, a higher intensity of 150% AMTLM was used to preferentially induce D‐wave recruitment. If 150% AMTLM exceeded the maximum capacity of TMS, 100% of maximum stimulator output (MSO) was used. Within each coil orientation, 15 MEPs were recorded while the FDI muscle was abducted at 10% MVC. The difference in onset latencies between PA and LM (PA‐LM), and between AP and LM (AP‐LM) stimulation were calculated as measures of early and late I‐wave recruitment efficiency (Hamada et al. 2013).
Short‐interval intracortical facilitation (SICF)
SICF was investigated in the resting FDI muscle using an established paired‐pulse TMS protocol, which produces peaks in the surface EMG recording that are compatible with I‐wave latencies recorded from the epidural space (Ziemann et al. 1998a). A subthreshold conditioning stimulus was applied at short ISIs (0.9–5.5 ms in 0.2 ms steps, totalling 24 intervals) following a suprathreshold test stimulus. The intensity of the test stimulus was set to elicit a peak‐to‐peak MEP amplitude of approximately 1 mV when given alone (MEP1mV), whereas the conditioning stimulus intensity was set at 90% of resting motor threshold (RMT). RMT was defined as the minimum stimulus intensity producing an MEP response ≥50 μV in at least 3 out of 5 trials while the right FDI was completely relaxed. To identify MEP1mV, the stimulus intensity was set 5% MSO above RMT and then adjusted in 1% MSO steps until the MEP response was approximately 1 mV in amplitude. A block of 15 trials was then recorded at this intensity, and the average calculated. If the average amplitude deviated from 1 mV by more than ±0.3 mV, this process was repeated. For the SICF protocol, 12 trials were recorded for each condition (300 stimuli in total). In order to maintain subject attention during SICF assessment, this protocol was applied as 6 blocks of 50 stimuli, with 2 repeats of each condition applied in a pseudorandomised order within each block.
Experiment 2: age‐related differences in I‐wave periodicity TMS (iTMS)
This experiment investigated whether age‐related differences in late I‐waves influence plasticity induction with iTMS. The advantage of this technique over other non‐invasive brain stimulation protocols is that it directly targets the interneuron networks by increasing the efficacy of trans‐synaptic events that lead to the generation of I‐waves (Thickbroom et al. 2006).
I‐wave periodicity TMS (iTMS)
For both sessions, iTMS consisted of 180 paired stimuli that were applied every 5 s for a total intervention time of 15 min (Cash et al. 2009). This protocol has been shown to produce a robust potentiation of MEP amplitude that is thought to represent an LTP‐like change in corticospinal excitability (Thickbroom et al. 2006). The intensity of the first stimulus was set to produce MEP1mV, whereas the intensity of the second stimulus was set at 90% RMT. ISIs corresponding to the average latency of the third SICF peak in young (Young Peak, 4.1 ms) and older (Old Peak, 4.9 ms) adults (identified in Experiment 1) were applied in separate sessions, with the order of sessions randomised between subjects. All sessions within a subject were conducted at least 1 week apart and at the same time of day. Furthermore, to avoid the confounding influence of diurnal variations in cortisol on neuroplasticity induction (Sale et al. 2007; Sale et al. 2008), all iTMS interventions were conducted after 11.00 h. Changes in corticospinal excitability were assessed by recording MEP1mV prior to iTMS, and then every 10 min for 30 min following iTMS, with 20 trials recorded at each time point.
Data analysis
Analysis of EMG data was completed manually via visual inspection of offline recordings. For measures in resting muscle, traces showing muscle activity ≥20 μV peak‐to‐peak amplitude in the 100 ms prior to TMS application were excluded from the analysis. MEP amplitudes were measured peak‐to‐peak and expressed in millivolts (mV).
The MEP onset latency for different coil orientations was assessed via a semi‐automated process using a custom‐written script within the Signal program (v 4.11, Cambridge Electronic Design). For each trial, onset was defined as the point at which the rectified EMG signal following the TMS artefact exceeded the mean pre‐stimulus rectified EMG amplitude (calculated over the 100 ms prior to TMS application) plus 2 standard deviations. MEP onset latencies were averaged over individual trials within each subject and coil orientation.
For SICF measures within each subject, individual conditioned MEP trials within each ISI were expressed as a percentage of the mean test alone MEP amplitude. To objectively identify and quantify SICF peaks in individual subjects, we used a 3 Gaussian model utilised by previous SICF studies (Delvendahl et al. 2014; Cirillo et al. 2015). This procedure provided greater sensitivity to estimate latency (onset and peak) and duration of each SICF peak. Data derived from individual subject SICF curves were fitted with a model consisting of the sum of 3 Gaussian curves (Thickbroom, 2011; Delvendahl et al. 2014), accounting for changes in the baseline level of facilitation. For each peak i with a given latency ti, amplitude Ai, width (Gaussian sigma) σi and overall baseline y0 and small‐ISI baseline y 0, L, all peaks were modelled as:
where y is the peak amplitude and t is the ISI. To achieve this, a 1000‐iteration bootstrapping procedure using the MATLAB bootci function was utilised. On each iteration, a dataset was created by sampling individual normalised MEPs (see above) with replacement. A curve fit was then performed using a trust region reflective least‐squares fit algorithm (Coleman & Li, 1996). Parameter estimates for each subject were chosen as the mean of the 1000 fits, and 95% confidence intervals (CIs) were computed across this sample. Peaks where amplitudes had CIs not inclusive of 0 were deemed significant. The frequency of each peak was also calculated based on the peak latency identified by the model.
Changes in corticospinal excitability following iTMS were quantified by expressing individual MEP amplitudes recorded at each post intervention time point as a percentage of the mean MEP amplitude recorded at baseline.
Statistical analysis
Student's unpaired t tests were used to compare age, handedness, RMT and the intensity required to produce MEP1mV between age groups (young, older). Two‐way repeated measures analysis of variance (ANOVARM) was used to compare AMT, MEP onset latency and MEP amplitude between coil orientations (PA, AP and LM) and age groups. Two‐way ANOVARM was also used to compare MEP latency difference between conditions (PA‐LM, AP‐LM) and age groups. In addition, two‐way ANOVARM was used to investigate the effect of peak (first, second and third) and age group on characteristics of the SICF peaks derived from the 3 Gaussian model (i.e., duration, onset, latency and frequency). Sphericity was assessed using Mauchly's test, with the degrees of freedom adjusted using the Greenhouse‐Geisser adjustment when necessary. All main effects and interactions were investigated using Bonferroni post hoc tests. Linear mixed model analysis was used to compare the amplitude of the test alone MEP between groups, whereas linear mixed model analysis with repeated measures was used to investigate the fixed effects of ISI (0.9–5.5 ms) and age group on SICF measures for all subjects tested. Linear mixed model analysis with repeated measures was also used to investigate the fixed effects of age group, time (10, 20 and 30 min) and ISI (Young Peak, Old Peak) on the response to iTMS. Changes in MEP amplitude following iTMS were also compared to baseline (i.e. 100%) using one‐sample t tests. For all linear mixed models, subject was included as a random effect, and significant main effects and interactions were further investigated with Bonferroni corrected custom contrasts. Unless otherwise stated, data are presented as means ± SEM and significance was set at P < 0.05.
Results
Subject characteristics are shown in Table 1. As expected, age was significantly greater in older subjects compared with young (P = 0.001). However, there was no significant difference between groups for handedness scores (P > 0.9), RMT (P = 0.8) or MEP1mV (P > 0.9). AMT was not different between age groups (F 1,29 = 0.6, P = 0.4), but varied between coil orientations (F 2,58 = 59.2, P < 0.0001) and there was an interaction between factors (F 2,58 = 4.9, P = 0.01). For both young and older adults, AMTPA was significantly reduced relative to both AMTAP (P < 0.001) and AMTLM (P < 0.001). In addition, AMTAP was also reduced relative to AMTLM in older (P < 0.001), but not young (P = 0.3) adults. Comparisons between age groups showed that AMTLM was significantly higher in older adults (P = 0.04).
Table 1.
Subject characteristics for Experiment 1
| Young (n = 15) | Old (n = 18) | |
|---|---|---|
| Age (years) | 22.5 ± 0.7 | 70.6 ± 1.4c |
| Handedness (L.Q) | 0.85 ± 0.07 | 0.85 ± 0.05 |
| RMT (% MSO) | 45.8 ± 2.4 | 47.2 ± 2.4 |
| MEP1mV (mV) | 1.44 ± 0.19 | 1.42 ± 0.17 |
| AMT (% MSO) | ||
| AMTPA | 38.5 ± 2.4 | 37.6 ± 2.3 |
| AMTAP | 49.2 ± 2.7a | 48.2 ± 2.6a |
| AMTLM | 54.0 ± 3.1a | 63.3 ± 3.0b,c |
P < 0.05 compared to PA, b P < 0.05 compared to PA and AP, c P < 0.05 between groups.
Experiment 1: I wave recruitment and facilitation
MEP onset latencies
Due to subject discomfort during high intensity (i.e. 100% MSO) stimulation, it was not possible to record MEPs using the LM coil orientation in three older adults. Subsequently, analysis of MEP onset latencies was performed using a reduced cohort of 15 older subjects, whereas all 15 young subjects were included. The amplitude of the MEP produced by each coil orientation is shown in Table 2. MEPs varied between coil orientations (F 1.06,29.4 = 49.2, P < 0.0001) and age groups (F 1,28 = 6.4, P = 0.02) and there was an interaction between factors (F 1.06,29.4 = 14.3, P = 0.001). Post hoc comparisons between groups showed no difference in amplitude for PA (P = 0.7) and AP (P = 0.5) stimulation, whereas MEPs following LM stimulation were significantly reduced in older subjects (P = 0.002). The MEP onset latency produced by each coil orientation in young and older adults is shown in Fig. 1 A. Latencies varied between coil orientations (F 1.6,45.7 = 123.7, P < 0.0001) and age groups (F 1,28 = 5.5, P = 0.03), but there was no interaction between factors (F 1.6,45.7 = 0.5, P = 0.6). Post hoc comparisons showed that MEP onset following LM stimulation was reduced relative to both PA and AP stimulation in all subjects, whereas MEP onset during PA stimulation was also reduced relative to AP stimulation in all subjects (all P values < 0.0001). In addition, MEP onset latency was delayed by approximately 1.6 ms in older adults (P = 0.03). MEP latency differences for PA‐LM and AP‐LM (i.e. efficacy of I‐wave recruitment) are shown for individual subjects in Fig. 1 B and group data are shown in Fig. 1 C. As expected, all subjects demonstrated a longer AP‐LM latency compared with a PA‐LM latency. For group data, there was a significant effect of coil orientation (F 1,28 = 81.2, P < 0.0001), with the AP‐LM difference being significantly greater than PA‐LM difference. However, this did not vary between age groups (F 1,28 = 0.09, P = 0.8) and there was no interaction between factors (F 1,28 = 1.1, P = 0.3).
Table 2.
MEP amplitude for each coil orientation
| Young | Old | |
|---|---|---|
| PA | 1.1 ± 0.6 | 1.2 ± 0.7 |
| AP | 1.1 ± 0.6 | 1.2 ± 0.6 |
| LM | 7.2 ± 4.4 | 3.1 ± 2.0a |
P < 0.05 between groups.
Figure 1. Age‐related changes in I‐wave recruitment efficiency.
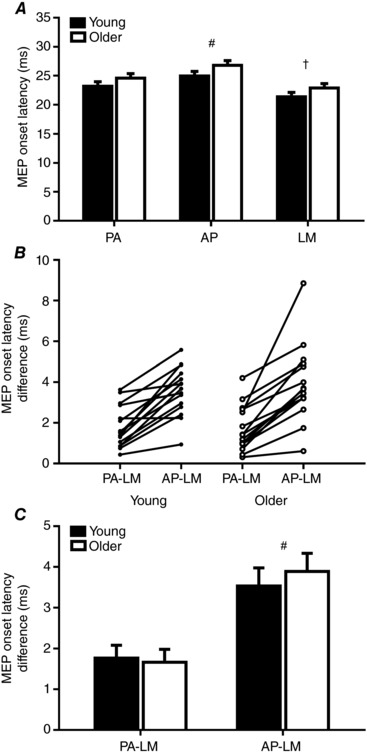
Data show the MEP onset latency recorded following PA, AP and LM stimulation (A), as well as individual (B) and group (C) PA‐LM and AP‐LM differences in onset latency in young (filled bars) and older (open bars) adults. Error bars show standard error of the mean. # P < 0.05 when compared to PA/PA‐LM; † P < 0.05 when compared to PA and AP.
SICF
Representative SICF data are shown for a single young (18 years) and older (76 years) subject in Fig. 2 A. These subjects demonstrated comparable RMT (young, 39% MSO; older, 42% MSO) and MEP1mV (young, 1.5 mV; older, 1.2 mV) values. The traces shown were generated at the latencies associated with maximum facilitation in each subject; this was comparable between subjects for peak 1 (∼1.3 ms) and 2 (∼2.9 ms), whereas peak 3 was delayed in the older subject (young, ∼4.3 ms; older, ∼5.3 ms). In the young subject, facilitation of the first, second and third MEP peak was, respectively, 254%, 218% and 190% of the test alone MEP, whereas in the older subject MEP peaks were, respectively, 171%, 141% and 166% of the test alone MEP.
Figure 2. Age‐related changes in facilitatory I‐wave interactions.
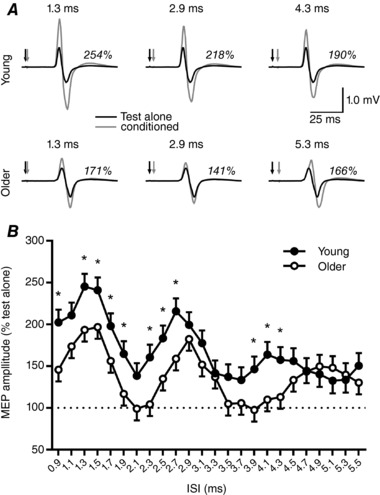
A, representative SICF data in a single young (top row) and single older (bottom row) subject having comparable baseline characteristics and MEP1mV (see Results). Black lines show the test alone response, grey lines show the conditioned response, black arrows indicate the test stimulus and grey arrows indicate the conditioning stimulus. Italicised numbers show the facilitation observed at each MEP peak. B, group data comparing the response to SICF in all young (filled circles) and older (open circles) subjects. The dashed horizontal line shows the amplitude of the test alone MEP and error bars show standard error of the mean. * P < 0.05 between groups.
The response of all tested subjects to the SICF protocol is compared between young and older subjects for each ISI (0.9–5.5 ms in 0.2 ms steps) in Fig. 2 B. While these data were not different between age groups (F 1,31 = 3.4, P = 0.08), the response varied between ISIs (F 23,4910 = 19.2, P < 0.0001) and there was an interaction between factors (F 23,4910 = 2.8, P < 0.0001). Post hoc comparisons between groups showed that young subjects had significantly greater MEP facilitation at ISIs of 0.9, 1.3–1.9, 2.3–2.7 and 3.9–4.3 ms (all P values < 0.05; Fig. 2 B). Large differences between groups were also apparent for 1.1 and 2.1 ms ISIs, although these values failed to reach a conventional level of significance (both P values < 0.1).
As a result of the curve fitting, 13/15 young and 11/18 older adults demonstrated three MEP peaks and were included in the group analysis of modelled data. An example of the curves fitted to the data of the representative subjects from Fig. 2 is shown in Fig. 3 A and B. Temporal characteristics of the three SICF peaks, quantified using the fitted curves (see Methods), are compared between age groups in Fig. 3 C–F. For all included subjects, peak duration was not different between peaks (F 1.4,30.6 = 2.0, P = 0.2) or groups (F 1,22 = 0.2, P = 0.6) and there was no interaction between factors (F 1.4,30.6 = 2.2, P = 0.1; Fig. 3 C). Peak onset was greater for later peaks (F 1.5,33.8 = 1412.8, P < 0.0001), was delayed in older subjects (F 1,22 = 26.9, P < 0.0001) and there was an interaction between factors (F 1.5,33.8 = 8.1, P < 0.0001; Fig. 3 D). Post hoc comparisons between groups showed no differences in the onset of peak 1 (P = 0.3), whereas older subjects showed a significantly delayed onset for peaks 2 (P = 0.04) and 3 (P < 0.0001). Peak latency was also increased for later peaks (F 2,44 = 1239.8, P < 0.0001), was delayed in older subjects (F 1,22 = 14.2, P = 0.001) and there was an interaction between factors (F 2,44 = 11.1, P = 0.0001; Fig. 3 E). Post hoc comparisons between groups showed no difference in latency for peaks 1 (P > 0.9) and 2 (P = 0.2), whereas peak 3 was significantly delayed in older subjects (P = 0.0002). Peak frequency was reduced for later peaks (F 2,44 = 12.7, P < 0.0001) and was decreased in older subjects (F 1,22 = 13.6, P = 0.001), but there was no interaction between factors (F 2,44 = 1.6, P = 0.2; Fig. 3 F).
Figure 3. MEP peak characteristics from a 3 Gaussian model.
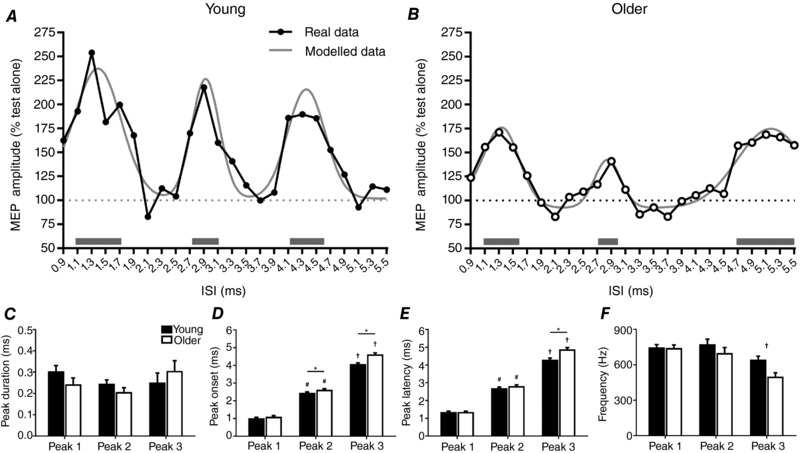
Top panels show examples of the curves fitted to data from an individual young (A) and older (B) subject, with horizontal grey bars highlighting the location and duration of each MEP peak, derived from the modelled data. Bottom panels show group averages for the duration (C), onset (D), latency (E) and frequency (F) of the 3 MEP peaks in young (filled bars) and older (open bars) adults. Only subjects demonstrating 3 significant peaks were included in the analysis (13/15 young, 11/18 older). Error bars show standard error of the mean. # P < 0.05 compared to peak 1; † P < 0.05 compared to peaks 1 and 2; * P < 0.05 between groups.
Experiment 2: I‐wave periodicity TMS
Averaged SICF data from Experiment 1 showed that peak 3 latency in young subjects occurred at 4.1 ms, whereas it occurred at 4.9 ms in older adults (Fig. 2 B). Both of these intervals were subsequently used in the iTMS protocol, and are hereafter referred to as Young Peak ISI and Older Peak ISI, respectively.
Changes in MEP amplitude following iTMS are compared between ISIs, age groups and time points in Fig. 4. While MEP amplitude did not vary over time (F 2,1411 = 1.1, P = 0.3), it was different between age groups (F 1,12 = 8.0, P = 0.02) and ISIs (F 1,477 = 4.5, P = 0.04). Furthermore, there was a significant interaction between all factors (F 2,1474 = 6.0, P = 0.003; Fig. 4). For young subjects, post hoc comparisons showed that MEP facilitation following iTMS was not different between ISIs at any time point (all P values > 0.1). For older subjects, there was no difference between ISIs at the 10 min time point after iTMS (P = 0.7), but MEP amplitude was significantly greater with the Older Peak ISI than the Young Peak ISI at both 20 min (P = 0.03) and 30 min (P < 0.0001). Comparisons between age groups found that MEP facilitation of the young group was significantly greater than the older group at both 10 min and 20 min time points, irrespective of ISI (all P values < 0.05). However, for the 30 min time point, although the response of young subjects was significantly greater than that of older subjects for the Young Peak ISI (P = 0.003), there was no difference between groups for the Older Peak ISI (P > 0.9). Results of one‐sample t tests showed that all data were significantly different when compared to 100% (i.e. baseline; all P values < 0.05).
Figure 4. Age‐related differences in the response to iTMS using optimised and non‐optimised ISIs.
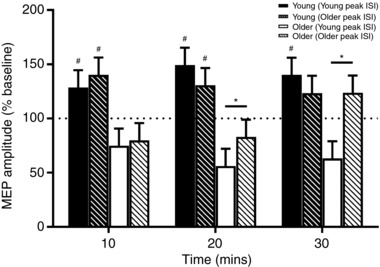
Data show the average response of 7 young (filled bars) and 7 older (open bars) subjects to iTMS applied at interstimulus intervals corresponding to the third SICF peak of each group (young, filled bars; older, hashed bars). Peak latency was identified in Experiment 1. The dotted horizontal line represents baseline MEP amplitude and error bars show standard error of the mean. # P < 0.05 when compared to the same iTMS interval in older subjects; * P < 0.05.
Discussion
The current study investigated age‐related changes in I‐wave characteristics and their contribution to TMS‐induced plasticity in young and older adults. Using single pulse TMS in different coil orientations, we found that I‐wave recruitment was similar between young and older adults. However, paired‐pulse TMS‐induced I‐waves assessed though SICF showed an age‐related shift in the temporal characteristics of the late facilitatory peaks to longer latencies, in addition to an age‐related reduction in the facilitatory interaction between I‐waves. A second experiment using iTMS showed that these altered temporal dynamics influenced the TMS‐induced motor cortex plasticity response in older adults.
I‐wave recruitment is maintained in older adults
When MEP onset latency was compared between age groups, a delay of ∼1.6 ms was identified in older adults. Given that this difference was consistent across coil orientations (Fig. 1 A), this delay most likely represents a reduction in conduction velocity along the corticospinal pathway that is commonly observed in the elderly (Taylor, 1984; Bouche et al. 1993; Metter et al. 1998). Despite this discrepancy, both PA‐LM and AP‐LM differences, which are measures of I‐wave recruitment efficiency (Hamada et al. 2013) that are independent of corticospinal conduction velocity, were similar between groups. In both young and old subjects, the shortest MEP onset latency was produced by LM stimulation, with the response to PA stimulation occurring ∼1.7 ms later, whereas the response to AP stimulation was delayed by ∼3.5–4 ms relative to LM (Figs 1 B and C). These values are consistent with a number of previous studies in young subjects (Sakai et al. 1997; Ni et al. 2011; Hamada et al. 2013; Cirillo & Byblow, 2016) and probably represent D‐wave recruitment following LM, early I‐wave recruitment following PA and late I‐wave recruitment following AP in both subject groups. Taken together, these findings suggest that the preferential recruitment of interneuronal circuits activated when varying the direction of the cortical current induced by TMS (i.e. early vs. late I‐waves) are similar between young and older adults.
The finding that there is no age‐related change in I‐wave recruitment (PA‐LM and AP‐LM differences) provides new information about the preferential activation of I‐waves with TMS in older adults. However, it is important to highlight that the technique utilised here (involving changing the coil orientation to alter the current direction in the cortex) cannot differentiate between individual components of the corticospinal volley. It therefore remains possible that the ageing process may result in more subtle alterations to the descending volley that may not be identified using this methodological approach. Alternative methods to more precisely characterise age‐related changes in the descending volley may be needed to confirm this finding, such as epidural recordings of the descending volley in the spinal cord, or intramuscular recordings to detect single motor unit latencies during voluntary muscle activation, both of which are more invasive and technically demanding than the approach used in this study.
I‐wave facilitation is altered in older adults
Application of SICF in young subjects resulted in three peaks of MEP facilitation at ISIs of approximately 1.4, 2.7 and 4.3 ms, values that closely match findings from previous studies that used identical stimulus parameters (Ziemann et al. 1998a; Cirillo & Perez, 2015). These facilitatory effects are thought to be cortical in origin (Tokimura et al. 1996; Ziemann et al. 1998a; Di Lazzaro et al. 1999), and to represent interactions between the excitatory postsynaptic potentials (EPSPs) produced by application of conditioning and test stimulation within the interneuronal circuits responsible for I‐wave generation (Ziemann & Rothwell, 2000; Hanajima et al. 2002). Additionally, given that the SICF troughs occur at a periodicity that exceeds what could be expected following decay of the corticospinal EPSP, it is also likely that these peaks of facilitation are truncated by activation of intracortical inhibitory interneurons (Wagle‐Shukla et al. 2009).
Although the latency of the first peak in older subjects was identical to the young group, peaks 2 and 3 both showed a delayed onset, whereas peak 3 also showed a delayed latency in the older group (Fig. 3 D and E). In addition, peak frequency was also reduced in the older subjects for the later peaks (Fig. 3 F). While SICF has been previously investigated in older adults (Clark et al. 2011), this study was unable to adequately address age‐related changes in the timing of optimum facilitation because only a single ISI was recorded for each peak (1.5, 2.5 and 4.5 ms), which was identified based on previous data from young subjects. Subsequently, our more precise assessment using a range of discrete (0.2 ms) intervals suggest that the ageing process results in a shift in the temporal dynamics of the late, but not early, SICF peaks to longer latencies. This longer latency cannot be due to the slowing of conduction along central (corticospinal) or peripheral nerves, because the MEP responses from paired‐pulse TMS are expressed relative to a single TMS pulse, removing any effect of conduction velocity along the output pathway. Therefore, any age‐related differences in latency between SICF peaks could only be ascribed to differences in conduction velocity within the cortical interneuron networks that are responsible for generating the I‐waves. If a generalised conduction delay in older adults was responsible for this effect, the difference in latency between groups would be cumulative for successive peaks (e.g. peak 2 difference would be twice that of peak 1, peak 3 difference would be three times that of peak 1). However, this was not observed using the modelled data in our study (peak 1 difference: 0 ms, peak 2 difference: 0.12 ms, peak 3 difference: 0.58 ms; see Fig. 3 E). We therefore suggest that factors other than an age‐related delay in conduction velocity of the interneurons generating the I‐waves are contributing to this effect, with the interneuron circuits responsible for generating late I‐waves demonstrating a particular impairment in older adults. Furthermore, these observations support suggestions that the early and late SICF peaks likely involve different mechanisms of action (Wagle‐Shukla et al. 2009; Shirota et al. 2010; Cirillo et al. 2015).
While the cause of this temporal shift is currently unclear, a number of factors may have contributed. For example, prolonged activation of the inhibitory circuits responsible for generating the SICF troughs could explain our observations. This may initially appear contradictory to a number of studies reporting that the ageing process does not affect GABAergic inhibition at short ISIs (i.e. short‐interval intracortical inhibition, SICI; Wassermann, 2002; Oliviero et al. 2006; Rogasch et al. 2009; Smith et al. 2009; Cirillo et al. 2010, 2011). However, the inhibitory circuits mediating SICI, and those mediating the SICF troughs, are likely to be independent (Wagle‐Shukla et al. 2009; Shirota et al. 2010). Alternatively, an age‐related change in the effect of stimulus intensity on peak onset (i.e. increased intensity results in reduced latency; Ziemann et al. 1998a; Delvendahl et al. 2014) could also be suggested, with older adults requiring greater intensities to produce facilitation at latencies comparable to young adults. However, this seems unlikely given that all peaks are modified by changes in stimulus intensity, whereas only the timing of the late SICF peaks was altered in older adults. It may also be possible that alterations at the subcortical level influenced our results, as recent studies have reported that factors within the spinal cord may contribute to the late but not early SICF peaks (Cirillo & Perez, 2015; Cirillo et al. 2015).
When examining the amplitude of the SICF peaks for all subjects, there was reduced facilitation of MEP amplitude at three broad ranges of ISIs in older adults (1.3–1.9 ms, 2.3–2.7 ms, 3.9–4.3 ms) that closely resemble I‐wave latencies (Fig. 2 B), suggesting an age‐related reduction in facilitatory interactions between I‐waves. A single previous study using three ISIs (1.5, 2.5 and 4.5 ms) to assess age‐related changes in SICF reported that older adults demonstrated greater facilitation at 1.5 ms, reduced facilitation at 2.5 ms and no change at 4.5 ms (Clark et al. 2011). Our data support this previous finding for the two longer ISIs. In particular, the reduced facilitation at 2.5 ms in older adults is positively correlated with manual dexterity (Clark et al. 2011). Furthermore, modulation of SICF with an ISI of 2.5 ms during grasp preparation has also been shown to predict muscle activity used during the task (Cattaneo et al. 2005). These findings suggest that the second SICF peak may have some functional relevance. In addition, we also show no age‐related difference in SICF at 4.5 ms. However, our data show that this is due to a temporal shift in the third SICF peak to longer latencies in older adults, and demonstrates that it can be misleading to compare individual SICF peaks between different groups using a single ISI. Nonetheless, our finding of reduced SICF at 1.5 ms in older adults is in contrast to the increased SICF observed previously (Clark et al. 2011). It is currently unclear how to reconcile these divergent findings for the first SICF peak, but our data show a similar peak onset, latency and duration for young and old adults, suggesting that a lack of ISI precision does not account for this discrepancy.
A number of factors may have contributed to the reduced I‐wave facilitation that we observed in older subjects. For example, the amplitude of the TMS‐induced corticospinal EPSP has been previously reported to be reduced in older adults (Eisen et al. 1996), which could result in reduced summation between I‐waves. Alternatively, age‐related changes in signal propagation (Taylor, 1984) could result in a dispersed (more variable) descending volley that produces less efficient EPSP summation, leading to reduced recruitment of spinal motoneurons and a smaller MEP. However, one potential outcome of this would be a broadening of the SICF peaks, which was not observed here. Furthermore, given that facilitation increases in response to higher intensity conditioning stimuli (Wagle‐Shukla et al. 2009), it may be possible that SICF recruitment gain is reduced in older adults. However, we only assessed SICF at one conditioning intensity (90% RMT), and a range of conditioning intensities would be needed to confirm this notion. Finally, as SICF peaks are heavily influenced by GABAergic inhibition (Ziemann et al. 1998b; Wagle‐Shukla et al. 2009; Shirota et al. 2010), reductions in peak amplitude may reflect an age‐related increase in intracortical inhibitory circuits that are distinct from those mediating SICI.
Age‐related I‐wave alterations influence plasticity induction in older adults
A growing literature suggests that the ability of TMS to induce neuroplastic changes in cortical excitability is reduced in the elderly (Müller‐Dahlhaus et al. 2008; Fathi et al. 2010; Todd et al. 2010; Freitas et al. 2011). The prevailing view has been that this reduced plasticity in older adults is due to age‐related declines in the intrinsic mechanisms of synaptic plasticity (e.g. Müller‐Dahlhaus et al. 2008; Todd et al. 2010). However, emerging evidence now suggests that the way in which TMS recruits I‐waves in the corticospinal tract predicts the ability of NIBS interventions to induce LTP‐ or LTD‐like plasticity (Cash et al. 2009, 2013; Thickbroom, 2011; Hamada et al. 2013). Consequently, the age‐related changes in paired‐pulse TMS measures of I‐wave interactions reported here may provide an alternative explanation for the declines in NIBS‐induced plasticity commonly observed in the elderly. In particular, the altered periodicity of the late SICF peaks could suggest an age‐related shift in the preferred temporal dynamics of the late I‐waves. Given that the timing of the neuronal activation required to induce neuroplastic change is tightly regulated (Dan & Poo, 2004), such a shift may be sufficient to ensure that the frequency of activation that is effective in young subjects is not optimal in older subjects.
As a test of this hypothesis, we used iTMS to strengthen late I‐wave circuits in young and older adults. In both subject groups, we used an ISI that corresponded to the third peak of the SICF curve in young (4.1 ms) and older subjects (4.9 ms). Based on previous studies that have optimised the ISI for iTMS (Sewerin et al. 2011; Long et al. 2017), the expectation was that MEP amplitude would be facilitated at both intervals for young subjects, because they both produce facilitation in the SICF curve. In contrast, older adults would exhibit potentiation of MEP amplitude only at the longer ISI (4.9 ms), because the shorter ISI was located in a SICF trough where no facilitation was evident (see Fig. 2 B). The outcomes from our iTMS experiment support this hypothesis. There was MEP facilitation in young adults at both ISIs tested, whereas MEP facilitation was only seen in older subjects when the iTMS interval was adjusted to compensate for the delayed dynamics of the third SICF peak (Fig. 4). Furthermore, the short ISI that was optimal for young subjects (Young Peak, 4.1 ms) produced MEP facilitation in the young group but MEP depression in older adults. Therefore, TMS‐induced plasticity deficits with advancing age may, in part, be attributed to suboptimal interventional parameters in older adults. Subsequently, our findings suggest that interventions that are optimised for I‐wave activation are likely to be more effective at inducing motor cortex plasticity in older adults.
While optimised iTMS was able to produce MEP facilitation in older adults, this response only became apparent at the 30 min time point, whereas facilitation was immediate in young subjects. A similar delay has also been observed following anodal transcranial direct current stimulation in older adults (Fujiyama et al. 2014). The reason for this delay in the plasticity response is currently unknown, but may be due to an age‐related deterioration of the cellular processes that contribute to LTP in the ageing brain (Barnes, 2003). However, when the iTMS interval was optimised for young and older adults, the magnitude of MEP facilitation was similar between the two groups at the 30 min time point. This suggests that the plastic capacity of the motor cortex may be preserved in the elderly, but the cellular process that contribute to the plasticity response may be less efficient, resulting in the delayed facilitation. An alternative possibility for the delay in older adults may result from using the SICF peak that was averaged over all subjects, with evidence suggesting that it may be possible to further optimise the response when using I‐wave intervals from each subject (Sewerin et al. 2011). To better understand the role of interneuron networks in any age‐related differences in the capacity, time course and maintenance of the plasticity response in the ageing brain, future work investigating individualised ISIs and longer follow‐out periods will be an important extension of this study.
In conclusion, the current study investigated age‐related changes in I‐wave characteristics and whether they contribute to the impaired plasticity response in older adults. Our results suggest that while I‐wave recruitment is maintained with age, older adults demonstrate reduced facilitatory interactions between I‐waves, in addition to a temporal shift of the late I‐waves to longer latencies. Furthermore, the response to a plasticity intervention based on late I‐wave activation in young subjects was reduced in older subjects. However, a more effective response was recorded in older subjects when the intervention was adjusted to suit their delayed late I‐wave characteristics. Subsequently, the interneuronal circuits activated by TMS, particularly those responsible for generating late I‐waves, appear to be altered by the ageing process, and these changes could contribute to the TMS‐induced plasticity deficits often reported in older adults. These findings suggest that it is possible to improve the efficacy of plasticity inducing protocols in older adults by modifying neuroplastic interventions to accommodate age‐related changes in cortical interneuronal activation.
Additional information
Competing interests
The authors declare no competing interests
Author contributions
All experiments were performed at the University of Adelaide, South Australia, Australia. G.M.O., J.C. and J.G.S. conceived and designed experiments; G.M.O. performed experiments; G.M.O., J.C. and J.S. analysed the data; G.M.O. drafted the manuscript; J.C. and J.G.S. critically revised the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This research was supported under Australian Research Council's Discovery Projects funding scheme (project number DP150100930).
Biography
George Opie received his PhD from the University of Adelaide in 2015, for which he studied how the ageing process modifies intracortical inhibitory circuits within the primary motor cortex. He is currently a postdoctoral researcher at the University of Adelaide, focusing on understanding age‐related changes in motor cortical neuroplasticity, and how to improve the response to motor training in the elderly.
2

This is an Editor's Choice article from the 1 July 2018 issue.
References
- Barnes CA (2003). Long‐term potentiation and the ageing brain. Philos Trans R Soc Lond B Biol Sci 358, 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche P, Cattelin F, Saint‐Jean O, Leger J, Queslati S, Guez D, Moulonguet A, Brault Y, Aquino J & Simunek P (1993). Clinical and electrophysiological study of the peripheral nervous system in the elderly. J Neurol 240, 263–268. [DOI] [PubMed] [Google Scholar]
- Cash R, Benwell N, Murray K, Mastaglia F & Thickbroom G (2009). Neuromodulation by paired‐pulse TMS at an I‐wave interval facilitates multiple I‐waves. Exp Brain Res 193, 1–7. [DOI] [PubMed] [Google Scholar]
- Cash RF, Mastaglia FL & Thickbroom GW (2013). Evidence for high‐fidelity timing‐dependent synaptic plasticity of human motor cortex. J Neurophysiol 109, 106–112. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Voss M, Brochier T, Prabhu G, Wolpert DM & Lemon RN (2005). A cortico‐cortical mechanism mediating object‐driven grasp in humans. Proc Natl Acad Sci U S A 102, 898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo J & Byblow WD (2016). Threshold tracking primary motor cortex inhibition: the influence of current direction. Eur J Neurosci 44, 2614–2621. [DOI] [PubMed] [Google Scholar]
- Cirillo J, Calabro FJ & Perez MA (2015). Impaired organization of paired‐pulse TMS‐induced I‐waves after human spinal cord injury. Cereb Cortex 26, 2167–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo J & Perez MA (2015). Subcortical contribution to late TMS‐induced I‐waves in intact humans. Front Integr Neurosci 9, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo J, Rogasch NC & Semmler JG (2010). Hemispheric differences in use‐dependent corticomotor plasticity in young and old adults. Exp Brain Res 205, 57–68. [DOI] [PubMed] [Google Scholar]
- Cirillo J, Todd G & Semmler JG (2011). Corticomotor excitability and plasticity following complex visuomotor training in young and old adults. Eur J Neurosci 34, 1847–1856. [DOI] [PubMed] [Google Scholar]
- Clark J, Loftus A & Hammond G ( 2011). Age‐related changes in short‐interval intracortical facilitation and dexterity. Neuroreport 22, 499–503. [DOI] [PubMed] [Google Scholar]
- Coleman TF & Li Y (1996). An interior trust region approach for nonlinear minimization subject to bounds. SIAM J Optim 6, 418–445. [Google Scholar]
- Dan Y & M‐m Poo (2004). Spike timing‐dependent plasticity of neural circuits. Neuron 44, 23–30. [DOI] [PubMed] [Google Scholar]
- Day BL, Dressler D, Denoordhout AM, Marsden CD, Nakashima K, Rothwell JC & Thompson PD (1989). Electric and magnetic stimulation of human motor cortex – surface EMG and single motor unit responses. J Physiol 412, 449–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvendahl I, Lindemann H, Jung NH, Pechmann A, Siebner HR & Mall V (2014). Influence of waveform and current direction on short‐interval intracortical facilitation: a paired‐pulse TMS study. Brain Stimul 7, 49–58. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Pilato F, Profice P, Oliviero A, Mazzone P, Insola A, Capone F, Ranieri F & Tonali P (2009a). Associative motor cortex plasticity: direct evidence in humans. Cereb Cortex 19, 2326–2330. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Profice P, Pilato F, Oliviero A, Mazzone P, Di Iorio R, Capone F, Ranieri F & Florio L (2009b). LTD‐like plasticity induced by paired associative stimulation: direct evidence in humans Exp Brain Res 194, 661–664. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Insola A, Mazzone P, Profice P, Tonali P & Rothwell JC (2001). The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res 138, 268–273. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Meglio M & Tonali P (2008a). The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol 586, 3871–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Tonali PA & Rothwell JC (2008b). Low‐frequency repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol 586, 4481–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A & Pilato F (2012). I‐wave origin and modulation. Brain Stimul 5, 512–525. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell J, Oliviero A, Profice P, Insola A, Mazzone P & Tonali P (1999). Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp Brain Res 129, 494–499. [DOI] [PubMed] [Google Scholar]
- Eisen A, EntezariTaher M & Stewart H (1996). Cortical projections to spinal motoneurons: Changes with aging and amyotrophic lateral sclerosis. Neurology 46, 1396–1404. [DOI] [PubMed] [Google Scholar]
- Fathi D, Ueki Y, Mima T, Koganemaru S, Nagamine T, Tawfik A & Fukuyama H (2010). Effects of aging on the human motor cortical plasticity studied by paired associative stimulation. Clin Neurophysiol 121, 90–93. [DOI] [PubMed] [Google Scholar]
- Freitas C, Perez J, Knobel M, Tormos JM, Oberman LM, Eldaief M, Bashir S, Vernet M, Peña‐Gómez C & Pascual‐Leone A (2011). Changes in cortical plasticity across the lifespan. Front Aging Neurosci 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama H, Hyde J, Hinder MR, Kim S‐J, McCormack GH, Vickers JC & Summers JJ (2014). Delayed plastic responses to anodal tDCS in older adults. Front Aging Neurosci 6, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M & Rothwell JC (2013). The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex 23, 1593–1605. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, Furubayashi T, Uesugi H, Iwata NK & Kanazawa I (2002). Mechanisms of intracortical I‐wave facilitation elicited with paired‐pulse magnetic stimulation in humans. J Physiol 538, 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise K‐F, Zimerman M, Hoppe J, Gerloff C, Wegscheider K & Hummel FC (2013). The aging motor system as a model for plastic changes of GABA‐mediated intracortical inhibition and their behavioral relevance. J Neurosci 33, 9039–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J, Federico P & Perez MA (2017). A novel cortical target to enhance hand motor output in humans with spinal cord injury. Brain 140, 1619–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metter E, Conwit R, Metter B, Pacheco T & Tobin J (1998). The relationship of peripheral motor nerve conduction velocity to age‐associated loss of grip strength. Aging Clin Exp Res 10, 471–478. [DOI] [PubMed] [Google Scholar]
- Müller‐Dahlhaus JFM, Orekhov Y, Liu Y & Ziemann U (2008). Interindividual variability and age‐dependency of motor cortical plasticity induced by paired associative stimulation. Exp Brain Res 187, 467–475. [DOI] [PubMed] [Google Scholar]
- Murase N, Cengiz B & Rothwell JC (2015). Inter‐individual variation in the after‐effect of paired associative stimulation can be predicted from short‐interval intracortical inhibition with the threshold tracking method. Brain Stimul 8, 105–113. [DOI] [PubMed] [Google Scholar]
- Ni Z, Charab S, Gunraj C, Nelson AJ, Udupa K, Yeh I‐J & Chen R (2011). Transcranial magnetic stimulation in different current directions activates separate cortical circuits. J Neurophysiol 105, 749–756. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F & Di Lazzaro V (2006). Effects of aging on motor cortex excitability. Neurosci Res 55, 74–77. [DOI] [PubMed] [Google Scholar]
- Opie GM, Post AK, Ridding MC, Ziemann U & Semmler JG (2017a). Modulating motor cortical neuroplasticity with priming paired associative stimulation in young and old adults. Clin Neurophysiol 128, 763–769. [DOI] [PubMed] [Google Scholar]
- Opie GM, Vosnakis E, Ridding MC, Ziemann U & Semmler JG (2017b). Priming theta burst stimulation enhances motor cortex plasticity in young but not old adults. Brain Stimul 10, 298–304. [DOI] [PubMed] [Google Scholar]
- Ridding MC & Ziemann U (2010). Determinants of the induction of cortical plasticity by non‐invasive brain stimulation in healthy subjects. J Physiol 588, 2291–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch NC, Dartnall TJ, Cirillo J, Nordstrom MA & Semmler JG (2009). Corticomotor plasticity and learning of a ballistic thumb training task are diminished in older adults. J Appl Physiol 107, 1874–1883. [DOI] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T & Kanazawa I (1997). Preferential activation of different I waves by transcranial magnetic stimulation with a figure‐of‐eight‐shaped coil. Exp Brain Res 113, 24–32. [DOI] [PubMed] [Google Scholar]
- Sale MV, Ridding MC & Nordstrom MA (2007). Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res 181, 615–626. [DOI] [PubMed] [Google Scholar]
- Sale MV, Ridding MC & Nordstrom MA (2008). Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci 28, 8285–8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN & Donoghue JP (2000). Plasticity and primary motor cortex. Annu Rev Neurosci 23, 393–415. [DOI] [PubMed] [Google Scholar]
- Sewerin S, Taubert M, Vollmann H, Conde V, Villringer A & Ragert P (2011). Enhancing the effect of repetitive I‐wave paired‐pulse TMS (iTMS) by adjusting for the individual I‐wave periodicity. BMC Neurosci 12, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota Y, Hamada M, Terao Y, Matsumoto H, Ohminami S, Furubayashi T, Nakatani‐Enomoto S, Ugawa Y & Hanajima R (2010). Influence of short‐interval intracortical inhibition on short‐interval intracortical facilitation in human primary motor cortex. J Neurophysiol 104, 1382–1391. [DOI] [PubMed] [Google Scholar]
- Smith AE, Ridding MC, Higgins RD, Wittert GA & Pitcher JB (2009). Age‐related changes in short‐latency motor cortex inhibition. Exp Brain Res 198, 489–500. [DOI] [PubMed] [Google Scholar]
- Taylor PK (1984). Non‐linear effects of age on nerve conduction in adults. J Neurol Sci 66, 223–234. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW (2011). A model of the contribution of late I‐waves to alpha‐motoneuronal activation: implications for paired‐pulse TMS. Brain Stimul 4, 77–83. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Edwards DJ & Mastaglia FL (2006). Repetitive paired‐pulse TMS at I‐wave periodicity markedly increases corticospinal excitability: a new technique for modulating synaptic plasticity. Clin Neurophysiol 117, 61–66. [DOI] [PubMed] [Google Scholar]
- Todd G, Kimber TE, Ridding MC & Semmler JG (2010). Reduced motor cortex plasticity following inhibitory rTMS in older adults. Clin Neurophysiol 121, 441–447. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Ridding M, Tokimura Y, Amassian V & Rothwell JC (1996). Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol 101, 263–272. [DOI] [PubMed] [Google Scholar]
- Wagle‐Shukla A, Ni Z, Gunraj CA, Bahl N & Chen R (2009). Effects of short interval intracortical inhibition and intracortical facilitation on short interval intracortical facilitation in human primary motor cortex. J Physiol 587, 5665–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM (2002). Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol 113, 1165–1171. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JKY, Meyer BU, Priori A, Rothwell JC, Day BL & Thompson PD (1994). The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol 93, 138–146. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual‐Leone A, Byblow WD, Berardelli A, Siebner HR, Classen J, Cohen LG & Rothwell JC (2008). Consensus: Motor cortex plasticity protocols. Brain Stimul 1, 164–182. [DOI] [PubMed] [Google Scholar]
- Ziemann U & Rothwell JC (2000). I‐waves in motor cortex. J Clin Neurophysiol 17, 397–405. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J & Paulus W (1998a). Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol 511, 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wischer S, Hildebrandt J & Paulus W (1998b). Pharmacological control of facilitatory I‐wave interaction in the human motor cortex. A paired transcranial magnetic stimulation study. Electroencephalogr Clin Neurophysiol 109, 321–330. [DOI] [PubMed] [Google Scholar]


