Abstract
A high mean platelet volume (MPV) level has been demonstrated to predict poor clinical outcomes in patients with cardiovascular disease. However, the relationship between MPV and mortality in patients with acute cardiorenal syndrome (ACRS) is unknown. Therefore, we investigated the predictive value of MPV for in-hospital mortality of patients with ACRS who received continuous renal replacement therapy (CRRT) in this study.
We retrospectively analyzed the demographics, etiology, severity of illness, prognosis, and risk factors of ACRS patients who underwent CRRT in our hospital from January 2009 to December 2014. Patients were classified into 2 groups based on the prognosis and timing of CRRT. The receiver operating characteristic curve was used to examine the performance of MPV in predicting in-hospital mortality. Baseline characteristics, clinical, and hematological parameters at CRRT initiation were compared between the 2 groups. Factors influencing in-hospital mortality were analyzed by univariate logistic regression analysis.
The median age of patients was 74 years. Acute myocardial infarction was the most common cause of ACRS, followed by acute decompensated heart failure. The in-hospital mortality was 51.4%. Age, number of organ failure, APACHE II score, and MPV in the nonsurvivors were significantly higher than those in the survivors (P < .05). However, the cardiac function and mean arterial pressure were significantly lower in the nonsurvivors (P < .05). The prognosis of the early intervention group was better than the late-intervention group, but no significant difference was found (P > .05). The area under the curve (AUC) for in hospital mortality based on MPV was 0.735. Univariate analysis showed that age, cardiac function NYHA class, number of organ failure, APACHE II score, MAP, MPV, and use of vasopressors were associated with the prognosis of patients (P < .05).
These findings suggest that the prognosis of patients with ACRS who received CRRT was poor, and MPV might be useful as a marker for predicting the in-hospital mortality of these patients.
Keywords: acute cardiorenal syndrome, continuous renal replacement therapy, mean platelet volume
1. Introduction
Acute cardiorenal syndrome (ACRS) is characterized as the development of acute kidney injury (AKI) in the patient with acute cardiac illness such as acute decompensated heart failure (ADHF), acute coronary syndrome, cardiogenic shock, and surgery-associated low cardiac output syndrome.[1–3] ACRS is a serious condition with poor survival rates and an increasing prevalence.[4–8] Recently, 2 large studies [9,10] showed that the incidence of ACRS was approximately 20% in critically ill patients, and even mild renal impairment was also strongly related to increased risk of long-term mortality.[11] The pathophysiological mechanism of ACRS is complex, of which inflammation plays an important role in the worsening of renal function.[12,13]
Mean platelet volume (MPV) is a value calculated by the automated hematology analyzer, which reflects the average size of platelets in a blood sample. Elevation of MPV is suggestive of increasing platelet production and activation.[14–19] Furthermore, MPV can be used as a biomarker in inflammation. Some previous studies have revealed an association between high mean platelet volume (MPV) and adverse cardiovascular or noncardiovascular outcome.[20–22] However, the association of MPV level with ACRS outcome is largely unknown.
The aim of the study is to observe the characteristics, outcomes of ACRS, and evaluate the association between the MPV level and mortality in ACRS patients receiving CRRT in China. We hypothesized that the MPV has potential as a prognostic biomarker for predicting the in-hospital mortality of these patients.
2. Methods
2.1. Patient population
2.1.1. Study population
This investigation was a single-center, retrospective study based on data from all patients with AKI who underwent CRRT from January 2009 to December 2014 in our hospital. We collected the cases met with diagnostic criteria of AKI[23] and ACRS.[24] ACRS cases were selected for further analysis. Exclusion criteria were as follows: aged < 14 years; chronic renal failure; other types of cardiorenal syndrome; maintenance hemodialysis or peritoneal dialysis; length of hospital stay < 48 hours, missing serum creatinine value within 48 hours after admission; error diagnosis and incomplete data.
Patients were classified into survivors and nonsurvivors groups according to the outcome at discharge. Based on the serum urea value at CRRT initial treatment, patients were classified into the early intervention group (serum urea ≤ 25 mmol/L) and the late-intervention group (serum urea > 25 mmol/L).
This study complied with medical ethics standards, and was approved by the hospital ethics committee. Informed consents were signed by all enrolled patients.
2.1.2. Data collection
Data were obtained from our hospital electronic medical record system, a computerized system used for daily patient documentation, and verified with the written records of each patient. Demographic, clinical data, severity of illness, vascular access, CRRT mode, the time of CRRT initiation, and total duration of CRRT were collected. Demographic information included age, gender and length of hospital stay. Clinical data were recorded and included primary diagnoses, comorbidities (diabetes mellitus, hypertension), use of drugs, mechanical ventilation, and outcomes. Baseline values were based on the values at admission, the initial treatment values were based on the CRRT initial treatment, and the post-treatment values were based on the post-CRRT treatment. If serum creatinine (SCr) and urea at discharge were lower than that at admission, these values were considered to be the basal level. Glomerular filtration rate (GFR) was estimated using the simplified Modification of the Diet in Renal Disease (MDRD) formula. MDRD equation: estimated GFR = 186 × (SCr level in mg/dL) −1.154 × (age in years) −0.203. The product of this formula is multiplied by a correction factor of 0.742 for women.
2.1.3. CRRT treatment
All patients underwent conventional management strategies, including fluid resuscitation, vasopressors, anti-infectives, respiratory support, nutritional support, and vital signs monitoring. CRRT was performed using the Aquarius bedside hemofiltration machine with an HF1200 hemofilter through a double-lumen 16G catheter inserted into the internal jugular or femoral vein. The mode of CRRT was continuous veno-venous hemofiltration (CVVH) or continuous veno-venous hemodiafiltration (CVVHDF). The blood flow rate was at 150 to 250 mL/min with an effluent rate of 4000 mL/h. According to the condition of patients, the duration of treatment was taken 24 hours continuous or 5 to 10 hours daily. Low molecular weight heparin or argatroban anticoagulant was used to maintain circuit patency. Heparin-free anticoagulant was used in the patients with a high risk of bleeding tendency, and the circuit was regularly rinsed with normal saline.
2.2. Statistical analysis
Continuous variables were reported as median and interquartile range (25th–75th percentile), and categorical variables were expressed as number and percentage. Continuous variables were compared using the Mann–Whitney U test. Categorical variables were compared using Fisher's exact test. The predictive ability of MPV was assessed with the area under the receiver operating characteristic curve (AUC) method. Univariate logistic regression analysis was performed to evaluate risk factors associated with the prognosis of ACRS. All tests were two-sides and P < .05 was considered statistically significant. Statistical analysis was conducted using SPSS 21.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Baseline characteristics
The selection process for the study is presented in Fig. 1. Thirty-five ACRS patients were entered in the analysis, which was the most common cause of AKI (35/223, 15.7%). Baseline characteristics are summarized in Table 1. Males and the elderly were more common. Hypertension and diabetes accounted for 48.6% and 40%, respectively. Acute myocardial infarction (AMI) was the most common cause of ACRS (54.3%), followed by ADHF (25.7%). The baseline MPV value was 11.0 fl.
Figure 1.
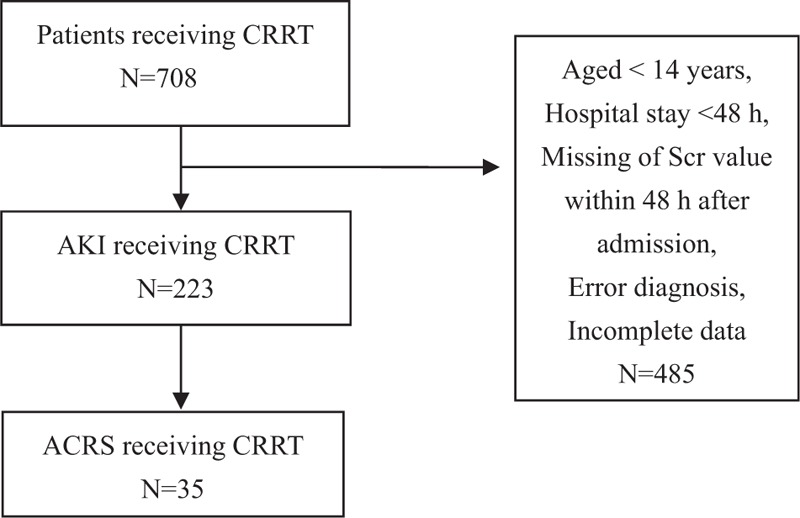
Flow of patients with ACRS admitted to our hospital from January 2009 to December 2014. CRRT = continuous renal replacement therapy; AKI = acute kidney injury; ACRS = acute cardiorenal syndrome.
Table 1.
Baseline characteristics.
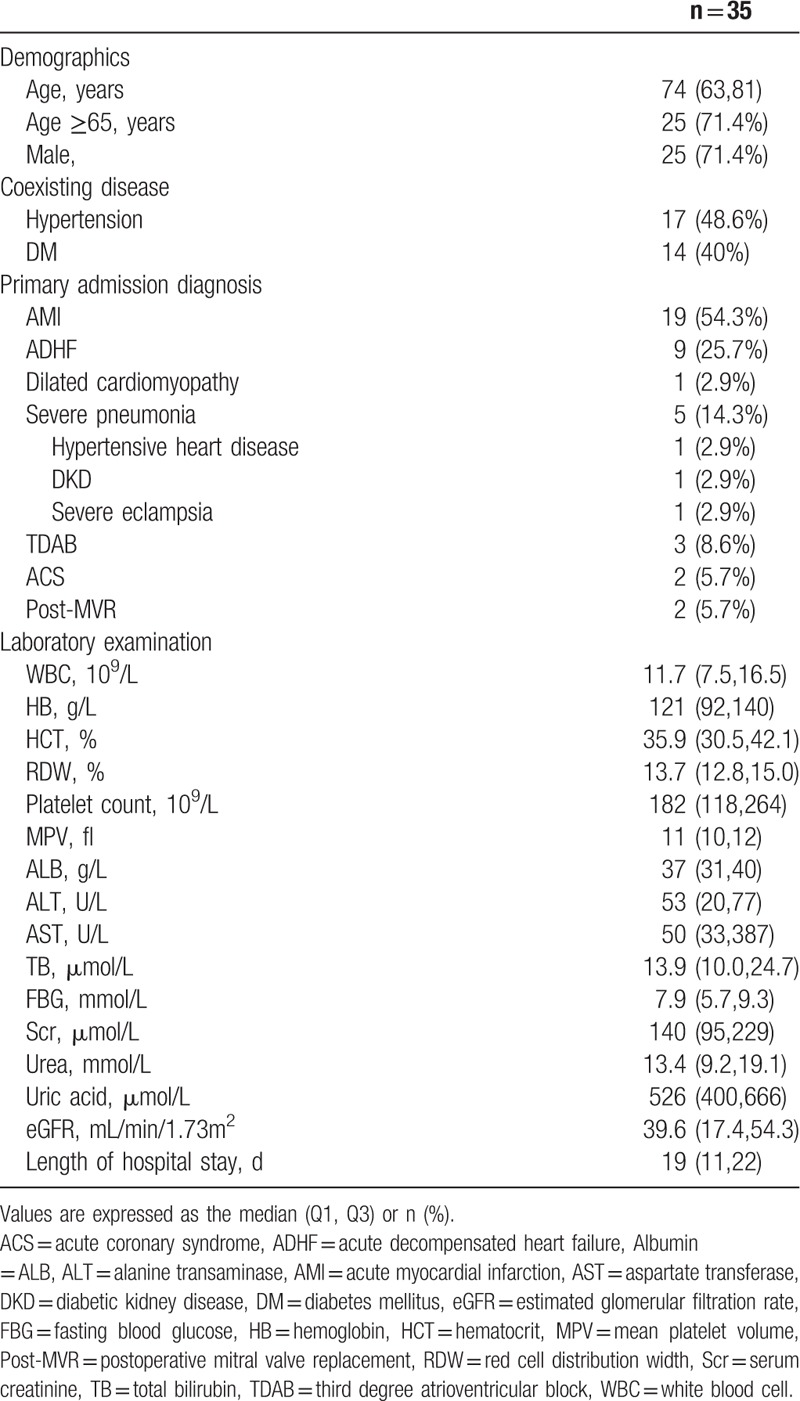
At baseline, there were significant differences in age and MPV value between survivors and nonsurvivors (P = .039 and .042, respectively). However, there were no significant differences in other variables (P > .05). Between early intervention and late-intervention group, only age was statistically different (P = .001).
3.2. Clinical characteristics at the CRRT initial treatment
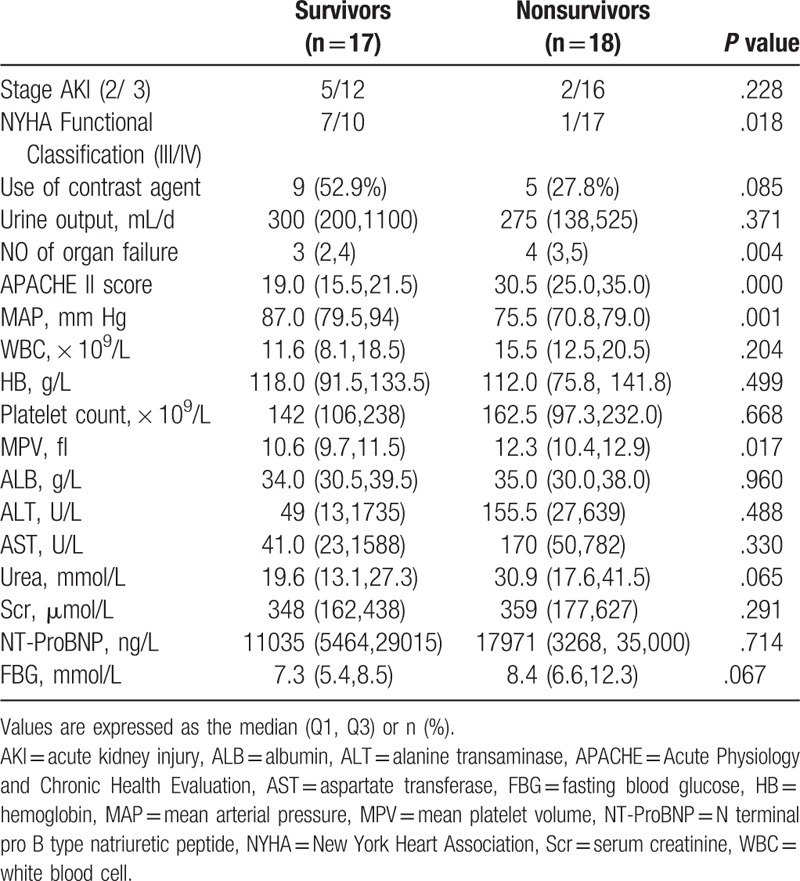
The comparison between survivors and nonsurvivors are shown in Table 2. Number of organ failure, APACHE II score and MPV in the nonsurvivors were significantly higher than those in the survivors (P < .05). However, the cardiac function class and mean arterial pressure were significantly lower in the nonsurvivors (P < .05).
Table 2.
Clinical characteristics at the CRRT initial treatment between survivors and nonsurvivors.
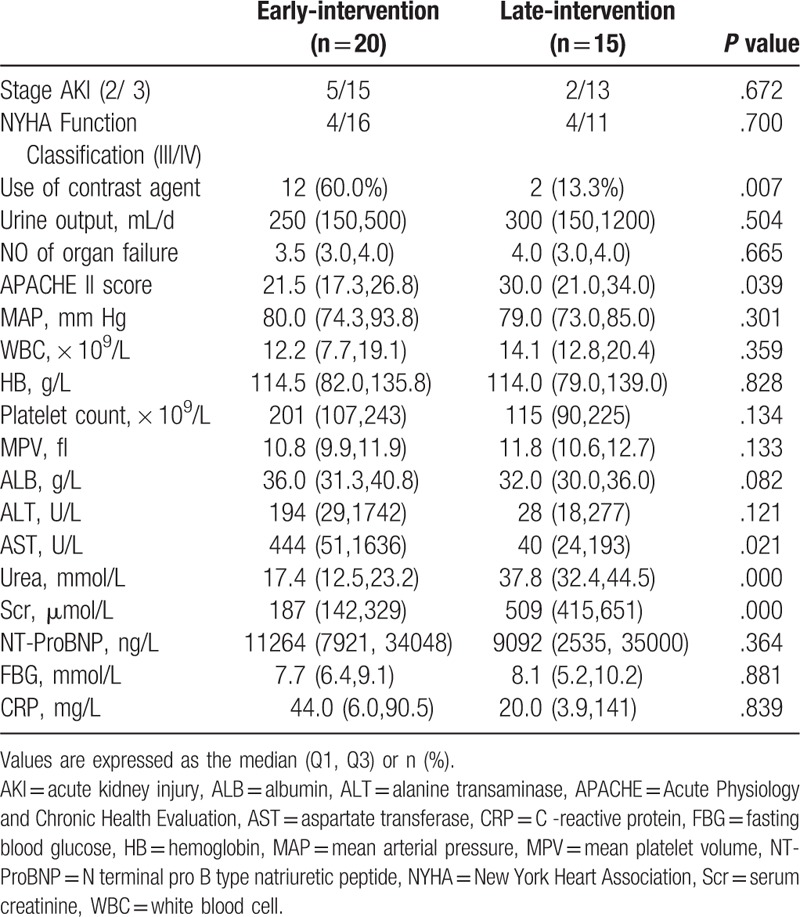
In a comparison between the early-intervention group and the late-intervention group, there were significant differences in the use of contrast agent, APACHE II score and serum AST, (P <.05), but no significant differences were found in other variables (P > .05) (Table 3).
Table 3.
Clinical characteristics at the CRRT initial treatment between Early-intervention and Late-intervention group.
3.3. Clinical outcomes
The in-hospital mortality was 51.4% (18/35) in this study. The mortality of the late-intervention group was 66.7%, which was higher than that of the early intervention group (40%), but the difference was not statistically significant (P = .118). No matter in different prognostic groups or in different treatment timing groups, there were no significant differences in the routine blood tests (WBC, HB, MPV, etc.) after finishing CRRT treatment (P > .05).
3.4. MPV for predicting in-hospital mortality in ACRS patients receiving CRRT
To examine the predictive value of MPV for in-hospital mortality, ROC curve analysis was performed. As shown in Fig. 2, the AUC value of MPV as predictor of in-hospital mortality was 0.735 (P < .05).
Figure 2.
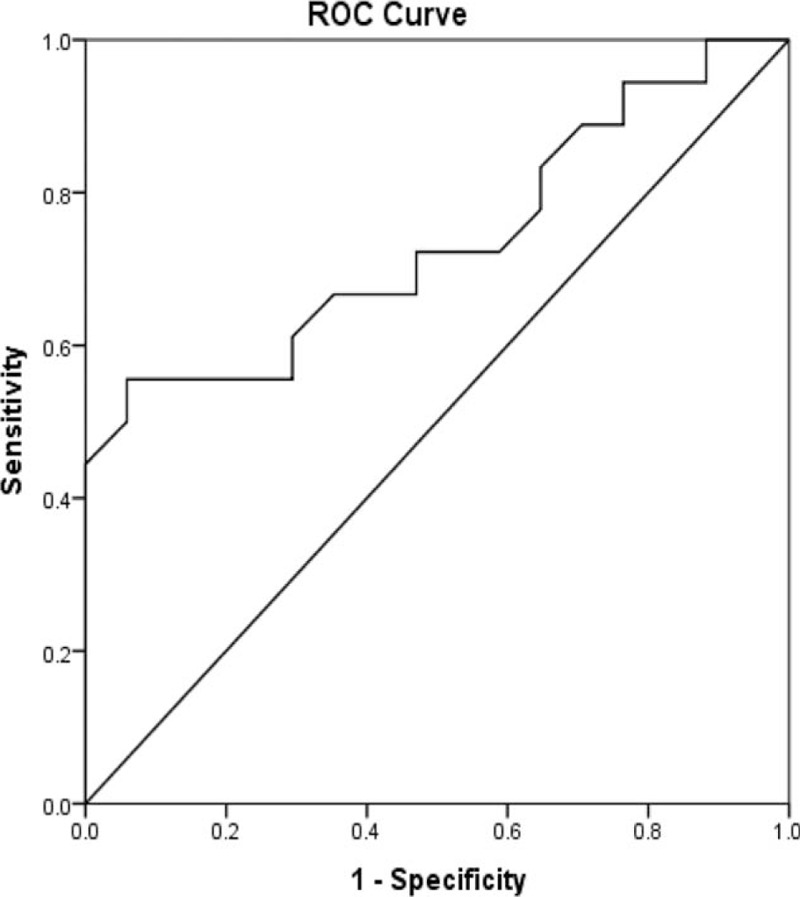
Receiver operating characteristic (ROC) curves of MPV for ACRS. ACRS = acute cardiorenal syndrome.
3.5. Analysis of related risk factors associated with the prognosis
Univariate logistic regression analysis of related risk factors was performed. The results demonstrated that age, cardiac function NYHA class, number of organ failure, APACHE II score, MAP, MPV, and use of vasopressors were associated with the prognosis of patients (P < .05).
4. Discussion
In the present study, we compared the clinical characteristics of patients with ACRS who underwent CRRT in different prognostic as well intervention timing groups, and investigated the predictive value of MPV for in-hospital mortality of these patients between 2009 and 2014 in our hospital. The results indicated that MPV might be useful in predicting short-term mortality in patients with ACRS.
Acute cardiorenal syndrome, also known as cardiorenal syndrome type 1, is a common clinical condition, but its epidemiology is still unclear.[24] Previous studies have shown that the incidence of AKI in ADHF and acute coronary syndromes was 20–45% and 9% to 20%, respectively,[5,6,25,26] while in cardiogenic shock, up to 70% of patients might develop AKI.[26] Similarly, we found that ACRS has become the most common cause of AKI, especially among males and the elderly. AMI was the first cause of ACRS patients treated with CRRT (54.3%) in the study. Furthermore, patients with ACRS had increased risk of AMI.[27] Because the etiology of ACRS varies widely, the prognosis is not the same. Recently, Prins and colleagues [28] retrospectively analyzed 37 ACRS patients with ADHF who underwent CRRT. In their study, the hospital mortality was 62%, which was higher than our reported 51.4%. Although CRRT plays an important role in the treatment of patients with ACRS,[29] the timing of CRRT initiation was controversial due to the difficulty in defining early and late initiation.[30–32] Compared with the early intervention group, the mortality rate in the late-intervention group was higher (66.7% vs 40.0%) in this study, however, the difference was not statistically significant (P = .118). Therefore, the relationship between the timing of CRRT and prognosis of ACRS still need to be further explored.
MPV is a well-established marker of platelet size and has been known to be a marker of platelet activation.[33,34] Also, platelet activation is a hallmark feature in inflammation. Large platelets contain more prothrombotic cytokines and these factors have a variety of effects in endothelial function and inflammation associated with cardiovascular disease. In fact, various studies have shown the association between MPV and cardiovascular disease.[35] Additionally, MPV was also associated with non-cardiovascular disease such as AKI. In a recent study including 349 patients with AKI, the result indicated that MPV was an independent predictor of mortality.[36] In the present study, we found that the platelet count was lower in the late-intervention group. Meantime, the MPV level in the nonsurvivors was 12.3 fl, which was significantly higher than that in the survivors (10.6 fl). We think this may be associated with higher platelet activation and more severe inflammation in the non-survivor group. The AUC of MPV for predicting in-hospital mortality was 0.735. In addition, univariate analysis showed that MPV along with other factors such as cardiac function classification, APACHE II score were associated with adverse outcomes. Therefore, our findings suggest elevated MPV may be useful in predicting hospital mortality of ACRS patients.
There are several limitations in the study. First, MPV values were not continuously monitored, which made the significance of the study limited. Moreover, the CRRT timing (early or late) may affect the value of MPV. Second, some patients lacked pre-admission data (e.g., blood routine, renal function), which might lead to inaccurate baseline data, although it was not uncommon in clinical practice.[37,38] Third, this was a small sample size, observational study. The cut-off value of MPV could not be obtained, and it was difficult to draw the conclusion of higher MPV and adverse outcome.
In conclusion, our study demonstrated that ACRS was a strong predictor of adverse events with an incompletely understood pathophysiology. CRRT therapy played an important role in saving the critically ill patients with ACRS. MPV was an inexpensive and rapid method of examination, which might be a useful predictor of ACRS prognosis. Our conclusion still needs to be confirmed by prospective multicenter randomized controlled trials.
Acknowledgments
We thank the staff of the Medical Records Room and Biochemical Room for their assistance. We thank Xiaoqing Pan and Yifei Wan (Department of Bioinformatics, Medical College of Wisconsin) for his help in statistical analysis, we thank Michelle Robert (Department of Physiology, Medical College of Wisconsin) for his help in English editing and we thank our patients and their families for their participation.
Author contributions
JL: data curation and writing-original draft. XS, YL: data curation. DC, FW: statistical analysis. XW, YF: validation. TX: project administration. GJ, NW: study design and supervision.
Data curation: Xiaohua Sheng, Yongguang Li.
Formal analysis: Dongsheng Cheng, Feng Wang.
Project administration: Tao Xu.
Supervision: Niansong Wang, Guihua Jian.
Validation: Xiaoxia Wang, Ying Fan.
Writing – original draft: Junhui Li.
Writing – review & editing: Junhui Li.
Footnotes
Abbreviations: ACRS = acute cardiorenal syndrome, APACHE = acute physiology and chronic health evaluation, CRRT = continuous renal replacement therapy, MPV = mean platelet volume, ROC = receiver operating characteristic.
JL and XS contributed equally to this work.
This study was supported by a grant from the National Natural Science Foundation of China (No.81270824).
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Acute Dialysis Quality Initiative (ADQI) consensus group. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J 2010;31:703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Eren Z1, Ozveren O, Buvukoner E, et al. A single-centre study of acute cardiorenal syndrome: incidence, risk factors and consequences. Cardiorenal Med 2012;2:168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ronco C, Cicoira M, McCullough P. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol 2012;60:1031–42. [DOI] [PubMed] [Google Scholar]
- [4].Damman K, Valente MA, Voors AA, et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Hear 2014;J 35:455–69. [DOI] [PubMed] [Google Scholar]
- [5].Virzì GM, Day S, de Cal M, et al. Heart-kidney crosstalk and role of humoral signaling in critical illness. Crit Care 2014;18:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cruz DN. Cardiorenal syndrome in critical care: the acute cardiorenal and renocardiac syndromes. Adv Chronic Kidney Dis 2013;20:56–66. [DOI] [PubMed] [Google Scholar]
- [7].Pimienta González R, Couto Comba P, Rodríguez Esteban M, et al. Incidence, mortality and positive predictive value of type 1 cardiorenal syndrome in acute coronary syndrome. PLoS One 2016;11:e0167166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim WH, Park JY, Ok SH, et al. Association between the neutrophil/lymphocyte ratio and acute kidney injury after cardiovascular surgery: a retrospective observational study. Medicine (Baltimore) 2015;94:e1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol 2011;57:1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol 2012;59:246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Parikh CR, Coca SG, Wang Y, et al. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med 2008;168:987–95. [DOI] [PubMed] [Google Scholar]
- [12].Ortega-Hernández J, Springall R, Sánchez-Muñoz F. et al Acute coronary syndrome and acute kidney injury: role of inflammation in worsening renal function. BMC Cardiovasc Disord 2017;26:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hsiao P-G, Hsieh C-A, Yeh C-F, et al. Early prediction of acute kidney injury in patients with acute myocardial injury. J Crit Care 2012;27:525.e1–7. [DOI] [PubMed] [Google Scholar]
- [14].Vanderschueren S, De Weerdt A, Malbrain M, et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med 2000;28:1871–6. [DOI] [PubMed] [Google Scholar]
- [15].Zampieri FG, Ranzani OT, Sabatoski V, et al. An increase in mean platelet volume after admission is associated with higher mortality in critically ill patients. Ann Intensive Care 2014;4:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim CH, Kim SJ, Lee MJ, et al. An increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shock. PLoS One 2015;10:e0119437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Venkata C, Kashyap R, Farmer JC, et al. Thrombocytopenia in adult patients with sepsis: incidence, risk factors, and its association with clinical outcome. J Intensive Care 2013;1:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Laine O, Joutsi-Korhonen L, Lassila R, et al. Elevated thrombopoietin and platelet indices confirm active thrombopoiesis but fail to predict clinical severity of puumala hantavirus infection. Medicine (Baltimore) 2016;95:e5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gasparyan AY, Ayvazyan L, Mikhailidis DP, et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 2012;17:47–58. [DOI] [PubMed] [Google Scholar]
- [20].Shah B, Oberweis B, Tummala L, et al. Mean platelet volume and long-term mortality in patients undergoing percutaneous coronary intervene. Am J Cardiol 2013;111:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shah B, Valdes V, Nardi MA, et al. Mean platelet volume reproducibility and association with platelet activity and anti-platelet therapy. Platelets 2014;25:188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tajarernmuang P, Phrommintikul A, Limsukon A, et al. The role of mean platelet volume as a predictor of mortality in critically ill patients: a systematic review and meta-analysis. Crit Care Res Pract 2016;2016:4370834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney int 2012;2(suppl):1–38. [Google Scholar]
- [24].Ronco C, House AA, Haapio M. Cardiorenal syndrome: refining the definition of a complex symbiosis gone wrong. Intensive Care Med 2008;34:957–62. [DOI] [PubMed] [Google Scholar]
- [25].Bagshaw SM, Cruz DN, Aspromonte N, et al. Acute Dialysis Quality Initiative Consensus Group. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI consensus conference. Nephrol Dial Transpl 2010;25:1406–16. [DOI] [PubMed] [Google Scholar]
- [26].Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF registry. J Am Coll Cardiol 2007;50:768–77. [DOI] [PubMed] [Google Scholar]
- [27].Mavrakanas TA, Khattak A, Singh K, et al. Epidemiology and natural history of the cardiorenal syndromes in a cohort with echocardiography. Clin J Am Soc Nephrol 2017;12:1624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Prins KW, Wille KM, Tallaj JA, et al. Assessing continuous renal replacement therapy as a rescue strategy in cardiorenal syndrome 1. Clin Kidney J 2015;8:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ronco C, Ricci Z, Backer DD, et al. Renal replacement therapy in acute kidney injury: controversy and consensus. Crit Care 2015;19:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li J, Wang N, Wang F, et al. Acute renal failure in hospitalized patients in China: a prospective study. Ren Fail 2009;31:431–7. [DOI] [PubMed] [Google Scholar]
- [32].Kim IY, Kim JH, Lee DW, et al. Fluid overload and survival in critically ill patients with acute kidney injury receiving continuous renal replacement therapy. PLoS One 2017;12:e0172137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bath PM, Bullworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrnolysi 1996;7:157–61. [PubMed] [Google Scholar]
- [34].Shah B, Sha D, Xie D, et al. The relationship between diabetes, metabolic syndrome, and platelet activity as measured by mean plateletvolume: the National Health and Nutrition Examination Survey. Diabetes Care 2012;35:1074–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Slavka G, Perkmann T, Haslacher H, et al. Mean platelet volume may represent a predictive parameter for overall vascular mortality and ischemic heart disease. Arterioscler Thromb Vasc Bio 2011;31:1215–8. [DOI] [PubMed] [Google Scholar]
- [36].Han JS, Park KS, Lee MJ, et al. Mean platelet volume is a prognostic factor in patients with acute kidney injury requiring continuous renal replacement therapy. J Crit Care 2014;29:1016–21. [DOI] [PubMed] [Google Scholar]
- [37].De Rosa S, Samoni S, Ronco C. Creatinine-based definitions: from baseline creatinine to serum creatinine adjustment in intensive care. Crit Care 2016;20:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li J, Li Y, Sheng X, et al. Combination of mean platelet volume/platelet count ratio and the APACHE II score better predicts the short-term outcome in patients with acute kidney injury receiving continuous renal replacement therapy. Kidney Blood Press Res 2018;43:479–89. [DOI] [PubMed] [Google Scholar]


