Abstract
Purpose
To determine the clinical features of optic disc progression in patients with ocular hypertension and early glaucoma.
Patients
336 eyes of 168 patients with ocular hypertension or early glaucoma
Methods
Two glaucoma specialists independently graded the baseline and most recent optic disc photographs for optic disc progression. Optic disc progression was defined as: new or increased neuroretinal rim thinning (two or more clock hours), notching (one clock hour or less of thinning of the neuroretinal rim), excavation (undermining of the neuroretinal rim or disc margin), and nerve fiber layer defect(s). They also determined the location of these changes.
Results
Ninety-two of 336 eyes (27.4%) showed optic disc progression after a median of 6.1 years. Of those with progression, excavation occurred in 89% of eyes; rim thinning occurring in 54%; and notching occurring in 16%. Fifty-six percent (56%) had two or more features of progression. The inferotemporal quadrant was the most common location for progression, but more than one location of progression occurred in at least 30% of eyes with progression.
Conclusions
Optic disc progression occurred frequently in this cohort of ocular hypertension and early glaucoma patients. When evaluating the optic disc for glaucomatous progression, eye care providers should pay particular attention to increased excavation and neuroretinal rim thinning – especially in the inferotemporal quadrant.
Keywords: optic disc progression, glaucoma, ocular hypertension, stereoscopic photography, validity
Introduction
The features of glaucomatous optic neuropathy include a vertical elongation of the optic cup [1–4] with associated decreases in the neuroretinal rim area [5, 6] and nerve fiber layer loss [7, 8]. In addition, morphologic changes of the glaucomatous optic disc correlate with characteristic patterns of visual field loss [9–12]. While eye care providers cite these features as the sine qua non of glaucoma, recent definitions have also emphasized the progressive nature of untreated glaucoma, suggesting that progressive change should also be considered as requisite for a diagnosis of glaucoma [13, 14, 15]. Clinicians may detect these progressive changes of glaucoma by documenting repeatable worsening of the visual field or progressive structural damage to the optic nerve head or retinal nerve fiber layer.
Studies have demonstrated that progressive optic disc changes occur commonly as a feature of glaucomatous progression [14–18]. Recently, several clinical trials have used progressive disc changes as an endpoint for glaucomatous progression and for the conversion from ocular hypertension to glaucoma [14, 17, 19, 20]. These studies showed that progressive disc change is an important indicator of progressive glaucomatous optic neuropathy and a key sign of the development and worsening of glaucomatous damage to the visual system [15].
Surprisingly, only a few studies describe the features of progressive optic disc changes in patients with early glaucoma or ocular hypertension [6, 21, 22]. Pederson showed increased generalized cupping as the most common feature of progressive disc changes in ocular hypertension patients. Tuulonen, who also studied ocular hypertensive patients, found an equal number of generalized and focal disc changes among patients with progressive disc changes. Odberg showed generalized enlargement of the optic cup with the majority of the cases exhibiting increased cupping in the superior- or infero- temporal quadrants. Theses studies provide valuable insight to eye care providers regarding the common features related to progressive disc changes in glaucoma. However, Pederson did not require stereo photographs (a current standard for evaluation of the optic nerve head)[21] and the studies by Tuulonen and Odberg were limited by smaller sample sizes.[6, 22]
In this study, we sought to determine the most common optic disc changes in patients with ocular hypertension or early glaucoma using serial, stereoscopic photographs of the optic disc in a large cohort study of patients. The results of this study will help eye care providers recognize early optic disc changes in their glaucoma suspect, ocular hypertensive, and glaucoma patients.
Methods
We longitudinally followed one hundred sixty-eight individuals with ocular hypertension or early glaucoma as part of the Perimetry and Psychophysics in Glaucoma study 23–25. All procedures adhered to the tenets of the Declaration of Helsinki and Legacy Health System Institutional Review Board approved the study. Participants provided written informed consent.
As described previously [25], we recruited patients from general eye care providers and glaucoma specialty practices from metropolitan area of Portland, Oregon. To be enrolled, all subjects must have had a diagnosis of either high-risk ocular hypertension or early glaucoma. High-risk criteria included history of untreated intraocular pressure ≥ 22 mm Hg in both eyes and at least one of the following risk factors: vertical cup-to-disk ratio ≥ 0.6 in at least one eye and/or cup-to-disk ratio asymmetry of ≥ 0.2; positive family history of glaucoma; personal history of migraine, Raynaud’s syndrome, or vasospasm; African-American ancestry; or age >70 years. In addition, both eyes of all subjects needed to have: best corrected visual acuity ≥ 20/40; spectacle refraction between +5.00 D and –5.00 D sphere; less than +/− 2.00 cylinder; and previous full-threshold or SITA-standard white-on-white perimetry (Humphrey Field Analyzer, Carl Zeiss, Meditec, Dublin CA). Exclusion criteria were any other previous or current ocular or neurologic disease, previous ocular surgery (except uncomplicated cataract surgery), or diabetes requiring medication. Visual field status (normal or abnormal) did not affect study entry, as long as the mean deviation was not worse than −6dB at the time of recruitment. Study patients were treated medically as deemed necessary by the treating physician; no patients underwent incisional surgery during the study period.
We used a simultaneous stereoscopic camera (3-Dx; Nidek Co., Ltd., Gamagori, Japan) to obtain optic disc photographs through dilated pupils at study entry (baseline) and at each yearly follow-up visit. We included both eyes of participants. We randomly labeled the optic disc photograph at baseline and the photograph at the most recent follow-up visit as “A” or “B” to mask the date of photography. We masked the reviewers to the appearance of the fellow eye. The reviewer did not have access to participant clinical information such as age and gender. Similar methods for determining progressive disc changes have been used previously[15].
Our main outcome measure was the determination of progressive disc change based on changes in optic nerve appearance. Two fellowship-trained glaucoma specialists (HN and RT) independently graded each photograph (viewed with a Stereo Viewer II, Asahi, Pentax, Tokyo, Japan) as either “normal” or ”glaucomatous optic neuropathy” based on the following characteristics: adequate clarity and stereopsis, neuroretinal rim thinning (generalized or localized), excavation, retinal nerve fiber layer defect, violation of the normal pattern of rim thickness (also known as the “ISNT” rule) [1] and cup-to-disc ratio by contour. [26]
One of the authors (SLM) used a set of optic disc stereophotographs that included examples of progressive disc changes to train the graders to recognize progressive disc changes and to standardize the nomenclature. We defined progressive disc changes as: “notching” if new or increased thinning of the neuroretinal rim occurred in 1 clock hour or less, “rim thinning” if new or increased thinning of the neuroretinal rim occurred in more than one clock hours, and, “excavation” (if there was increased depth and lateral undermining of the neuroretinal rim by the optic cup (with or without thinning of the neuroretinal rim-see “notching” and “rim thinning” above), and nerve fiber layer (NFL) defect(s) if new or increased widening of a NFL defect occurred. The Figure is a schematic describing these definitions of progressive disc change. The graders determined whether there had been any change between the two photographs, and if so, which photo was worse. The graders placed the “A” and “B” stereo photograph into separate Stereo Viewers to allow side-by-side comparisons.
Figure.
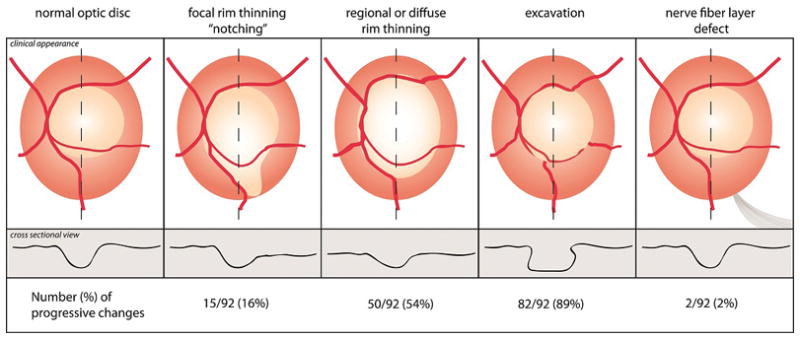
Schematic describing disc change with glaucoma. Optic discs were evaluated for the presence of rim thinning (could be focal, regional or diffuse), excavation, or a new nerve fiber layer defect compared to baseline photos. The line contours at the bottom of the image represent the changes in cross-sectional appearance (z-axis) of the optic disc.
The graders also determined whether a disc hemorrhage, acquired pit of the optic nerve, or change in beta parapapillary atrophy (PPA) occurred between photos. [27–31] However, these latter factors were not considered criteria for progressive disc changes, rather only recorded for subsequent analysis.
In addition, graders used one or more sectoral locations (inferonasal, superotemporal, etc.) for excavation and rim thinning and one or more clock-hour designations for focal notching, NFL defect, disc hemorrhage, and optic nerve pits. The reviewers mediated disagreements about progression, type of progression, and location of progressive features, by re-examining the photographs together to reach consensus; Cohen’s Kappa value was used to describe the level of intra-observer reproducibility. If consensus could not be reached, then an additional masked grader (GAC or SLM) made a final adjudication. Furthermore, progressive disc changes were only deemed to have occurred if the photo that was called worse was from the follow-up visit. Eyes were considered as “false progression” if graders determined change had occurred but in the “wrong” temporal direction (i.e. the appearance in baseline photograph was graded as worse than the follow-up).
Two glaucoma experts (GAC, SLM) selected 10 examples of progressive disc changes from the medical records of their clinical practices that were separate from the study cohort. These were chosen sequentially, without selecting more obvious or more subtle examples. These stereo photographs of 10 cases of disc progression was labeled using the same ‘A’ and ‘B’ labeling scheme, and then randomly inserted into the study set to serve as a test of sensitivity. Thus, sensitivity was defined as the proportion of these 10 eyes that the graders (RT, HN) identified as progressing in the correct temporal order. We also included an additional subset of 10 cases from the larger study cohort in which the pair of stereo photographs had been obtained on the same day (multiple exposures are routinely obtained at each yearly visit). These 10 cases, though redundant, were randomly assigned a unique ID# and inserted into the study set as a test of specificity (i.e. rate of correctly identifying no change). Thus specificity was defined as the proportion of these 10 eyes that the graders determined to have remained stable (no change between A and B time points). Finally, we duplicated the photo pair for 10 eyes and reassigning each pair with a second unique ID number and determined the intraobserver reproducibility as the proportion of these ten eyes which received the same grade each time. The graders were not aware that these 30 cases were not part of the study cohort or that these cases were being used to determine sensitivity, specificity and reproducibility. This set of photographs for determining reliability was different from the training set.
Results
Subjects
The study consisted of both eyes of 96 women (57%) and 72 men (43%) with a self-described racial composition of 162 (96%) white, 1 African-American, 2 Asian, 2 Hispanic, and 1 Native American. The age range at study entry was 34 to 87 years (mean ± SD: 58.1 ± 11.1). The mean number of years between baseline and most recent follow up photo was 5.5 +/− 1.7(SD) years (range 2.0 – 7.9) with a median of 6.1 years. The graders determined that 58% (195) of the 336 study eyes were glaucomatous at baseline based on optic disc appearance.
Progressive glaucomatous optic neuropathy
Ninety-two of 336 (27.4%) eyes in 67 patients were found to have progressive disc changes; 44 were right eyes and 48 were left eyes. This corresponds to 40.0% (67/168) of patients having progressive disc changes in one or both eyes (bilaterally in 25 study subjects and unilaterally in 42 subjects). Table 1 shows that increased excavation was more common than any other feature of progressive disc changes (p<0.001). Increased rim thinning occurred second most commonly. Fifty-two of the 92 eyes (56%) with progression were noted to have more than one feature of progressive disc changes, such as increased excavation and increased rim thinning in the same eye. Sixteen eyes (17%) had three or more signs of progressive disc changes.
Table 1.
| Type of change | Number of eyes (%) | |
|---|---|---|
| Excavation | 82 | (89) |
| Rim thinning (> 2 clock hours) | 50 | (54) |
| Notching | 15 | (16) |
| New optic disc hemorrhage* | 8 | (8.6) |
| Increased beta peripapillary atrophy* | 5 | (5) |
| Nerve fiber layer defect | 2 | (2) |
| New optic nerve pit* | 1 | (1) |
not criterion for progressive glaucomatous optic neuropathy.
Increased Excavation
As noted above, increased excavation was the most commonly described feature of optic nerve progression; noted in 89% of eyes with progressive disc changes (n=82/92). Increased excavation most frequently occurred in the inferotemporal quadrant (76%), followed by the superotemporal quadrant (54%). The superonasal (17%) and inferonasal (8.5%) quadrants were less commonly affected (Table 2). These percentages add to more than 100% because 38% of eyes (n=31) had increased excavation in more than one quadrant. Twenty-seven of these 31 eyes (87%) had increased excavation in at least one of the temporal quadrants (supero- and inferotemporal quadrants). Five of the 82 eyes with increased excavation (6%) exhibited increased excavation in all four quadrants.
Table 2.
Location of excavation or rim thinning in eyes with progressive disc change
| Type of Change | Number of eyes | Supero-nasal | Supero-temporal | Infero-nasal | Infero-temporal | >1 quadrant |
|---|---|---|---|---|---|---|
| Number of eyes (%) in each quadrant | ||||||
| Excavation | n = 82 | 14 (17) | 44 (54) | 7 (8.5) | 62 (76) | 31 (38) |
| Rim thinning | n = 50 | 11 (22) | 26 (52) | 5 (10) | 38 (76) | 18 (36) |
Increased Rim Thinning
Increased rim thinning was the second-most commonly feature of optic nerve progression; occurring in 50 of the 92 eyes (54%) with progressive disc changes; 46 eyes exhibiting 1–3 quadrants of rim thinning and 4 eyes with generalized rim thinning. The locations of increased rim thinning were similar to the excavation findings, with the inferotemporal quadrant most commonly affected (76% of eyes with rim thinning). The superotemporal (52%), superonasal (22%), and inferonasal (10%) quadrants were affected less frequently (Table 2). Eighteen of the fifty eyes (36%) had rim thinning in more than one quadrant. Ten of those eighteen eyes (56%) exhibited rim thinning affecting the temporal quadrants only.
Other features of progression
New or increased notching was noted in 15 eyes (16%); in 14 of the 15 (93%) eyes, the notch was located in the superior or inferior three clock-hours (11–1 o’clock and 5–7 o’clock). New or increased retinal nerve fiber layer defects were noted in 2 eyes and both were located in the inferotemporal region (5 o’clock meridian for left eye and 7 o’clock for the right eye).
Other changes (not required for progression)
We noted new disc hemorrhages in 8 eyes (9%); six of these were located in the inferior or inferotemporal quadrant whereas the other two were located either superonasally or superotemporally. We noted one new acquired pit of the optic nerve and it was located at the 12 o’clock meridian. We found increased beta parapapillary atrophy in 5 eyes (5%).
Sensitivity, Specificity, and Reproducibility of grading stereo disc photography
Initial agreement between the graders was 71% (Cohen’s kappa 0.4). They were able to reach consensus on 95% of eyes after re-examining the photographs together. Adjudication by a third grader was required for 16 of the 336 eyes (5.3%). Twenty eyes were classified as having “false progression” (i.e. the baseline image was judged to be worse than the follow up image). The sensitivity, specificity, and reproducibility of determining progressive disc changes were each 80% (8/10) (95% confidence interval 54–100%).
Discussion
Progressive disc changes in our patient population most frequently manifest as increasing excavation or rim thinning, especially in the inferotemporal quadrant. Our results showed acceptable sensitivity, specificity, and reproducibility for detecting progressive disc changes in patients with ocular hypertension and early glaucoma. Since optic disc changes have been recently been shown to be highly predictive of future visual field loss in patients with ocular hypertension and glaucoma[15, 27], the current findings may be important for clinicians to help them recognize early changes to the optic disc from glaucoma. Large, well-controlled clinical studies such as the Ocular Hypertension Treatment Study and the European Glaucoma Prevention Study also validate the importance of closely monitoring the optic disc for signs of early progression [14,17]. With early detection of progressive disc changes from glaucoma, clinicians may aggressively adjust therapy in their patients to avoid subsequent visual field loss and future worsening of vision-associated quality of life.
Rate of progression
Tuulonen et al6 showed progressive disc changes in 38% of ocular hypertensive eyes (23 of 61 eyes) over 10 years. Another study by Odberg22 showed progressive disc changes in 41% of ocular hypertensive eyes (19 of 46 eyes) over 7.5 years and Pederson21, et al described a lower rate of 7% over 15 years (18 eyes of 259 patients). The current study demonstrated 27.6% of eyes with progressive disc changes over 5 years.
If one assumes a constant, linear cumulative risk over a fifteen year period[33], the risk of progressive disc changes is 57% in Tuulonen, 82% in Odberg, 7% in Pederson, and 83% in the current study over a 15 year period. These differences in the rate of progressive disc changes may be related to differences in the evaluation of stereoscopic photographs and in each study’s definition of “high risk” ocular hypertension or glaucoma [17]. For example, Pederson et al did not require color stereoscopic photographs for their analysis. Also, our study included early glaucoma patients, which may have a higher risk of progressive disc changes when compared to ocular hypertension patients. Regardless of the actual rate of glaucomatous loss, progressive disc changes occur frequently in patients with ocular hypertension and early glaucoma. Eye care providers should pay special attention to the optic disc when examining ocular hypertension and glaucoma patients.
Mode of progression
Excavation was the most common feature of progressive disc changes. Excavation describes an undermining of the optic disc as the cup expands from loss of neural tissue and the laminar connective tissues undergo posterior bowing in the z-axis.[34] These structural changes may predispose some eyes to more overt forms of progression such as rim thinning or notching. Eye care providers may recognize new or increased excavation by noticing a shift in the location of the optic disc vessels and this may be the most easily detectable change when compared to other forms of progressive disc changes such as nerve fiber defects and increased rim thinning. This may explain why excavation was the most common form of progressive disc changes. Changes in parallax between baseline and repeat imaging could potentially account for apparent increases z-axis changes.
These z-axis changes usually require a stereoscopic image of the optic disc to appreciate. Therefore, eye care providers may overlook and under-appreciate excavation without stereophotography. Also, we believe that one should compare optic disc photographs obtained with simultaneous stereophotography side-by-side with two separate stereo viewers. This was performed in the current study, and allows one to move back and forth between photographs to detect subtle differences. This may have increased the detection rate of progressive disc changes in the current study.
The other most common form of progressive disc changes in the current study was rim thinning. Rim thinning occurs when neural tissue is lost in the x- and/or y-axis. Similar to excavation, one may most easily detect rim thinning when comparing optic disc photographs side-by-side with two separate stereo viewers. This allows one to detect changes in rim thickness. Our results are in agreement with recent longitudinal studies evaluating glaucoma progression, which find rim thinning to be a main criterion for progressive disc changes. [14, 16, 34–37]
Studies have shown poor correlation of confocal scanning laser ophthalmoscopy and clinicians estimates of disc parameters from optic disc photographs [26]. This may be explained because topographic measurements of the optic disc are most variable in locations associated with vessels making excavation difficult to detect using CSLO, while in optic disc photos this may be one of the easiest features of progressive disc changes to detect. Further studies are needed to determine why optic disc photographs of progressive disc changes do not correlate well with objective structural testing such as CSLO.
Location of progressive changes
Our study shows the inferotemporal quadrant as the most frequently damaged location in early glaucomatous optic neuropathy. This finding is in agreement with clinical observation and cross-sectional studies and corresponds to regional histologic differences in the trabecular beams and laminar pores within the optic nerve head [1, 6, 21, 39].
Although the majority of subjects in this study demonstrated focal features of progressive disc changes located in the supero- and inferotemporal quadrants, at least one-third of eyes with increased excavation exhibited progression in multiple locations – also focused primarily in the supero- and inferotemporal quadrants. Tuulonen [6] similarly reported 44% of ocular hypertensives exhibiting change in several locations, termed diffuse structural loss. Pederson [21] reported a higher proportion of eyes exhibiting diffuse or generalized changes. Odberg [22] does not comment on location or extent of increased cupping in his study. Future studies may examine whether progressive disc changes occurs in one location at a time or progresses simultaneously at multiple locations.
Our study evaluated the ability of masked graders to determine progressive disc changes. Our percentage of initial agreement between graders is comparable to published agreement rates [40] and our reproducibility (80%) correlates well with other longitudinal studies using stereoscopic optic nerve photos to monitor glaucomatous optic disc changes [17, 41–43]. Although this approach showed good sensitivity, specificity and reliability, the subjective nature of evaluating progressive disc changes makes a true “gold standard” difficult to establish.
Our study includes several limitations. Since this study includes patients with ocular hypertension or early glaucoma, our findings may not apply to patients with moderate or advanced glaucomatous optic neuropathy as changes in these optic discs may be more difficult to appreciate if there is little remaining neuroretinal rim. We did not differentiate patients with normal tension glaucoma which may demonstrate different patterns of disc progression. Our study population were mostly White; and our results need to be confirmed in African-American, Native American, and Latino populations. In addition, several modalities are now currently available for objective structural testing of the optic disc and retinal nerve fiber layer analysis which may uncover additional changes not detected with stereophotographs; each should be carefully evaluated to determine the validity and reproducibility [44]. Finally, our kappa values and diagnostic precision were moderate, which would create variability for determining optic disc progression. However, these values were better or similar to previous studies.[22, 45–46] Overall, optic disc examination remains an important method to determine progressive disc changes.
Further analyses examining whether both eyes of a particular individual progress in the same way; correlation of stereophotography to objective quantitative imaging and visual field testing; and risk factors for progressive disc changes would be informative and we hope to evaluate these questions in the near future.
Acknowledgments
Support: NIH- EY0155501
NIH-EY03424
Legacy Good Samaritan Foundation
Footnotes
Financial Interests: No authors have any financial/conflicting interests to disclose
References
- 1.Jonas JB, Fernandez MC, Sturmer J. Pattern of glaucomatous neuroretinal rim loss. Ophthalmology. 1993;100:63–8. doi: 10.1016/s0161-6420(13)31694-7. [DOI] [PubMed] [Google Scholar]
- 2.Jonas JB, Gusek GC, Naumann GO. Optic disc morphometry in chronic primary open-angle glaucoma. II Correlation of the intrapapillary morphometric data to visual field indices. Graefes Arc Clinl Exp Ophthalmol. 1988;226:531–8. doi: 10.1007/BF02169200. [DOI] [PubMed] [Google Scholar]
- 3.Weisman RL, et al. Vertical elongation of the optic cup in glaucoma. Trans Am Acad Ophthalmol Otolaryngol. 1973;77:OP157–61. [PubMed] [Google Scholar]
- 4.Kirsch RE, Anderson DR. Clinical recognition of glaucomatous cupping. Am J Ophthalmol. 1973;95:442–54. doi: 10.1016/0002-9394(73)91153-7. [DOI] [PubMed] [Google Scholar]
- 5.Airaksinen PJ, Drance SM, Schulzer M. Neuroretinal rim area in early glaucoma. Am J Ophthalmol. 1985;99:1–4. doi: 10.1016/s0002-9394(14)75856-8. [DOI] [PubMed] [Google Scholar]
- 6.Tuulonen A, Airaksinen PJ. Initial glaucomatous optic disk and retinal nerve fiber layer abnormalities and their progression. Am J Ophthalmol. 1991;111:485–90. doi: 10.1016/s0002-9394(14)72385-2. [DOI] [PubMed] [Google Scholar]
- 7.Emdadi A, et al. Patterns of optic disk damage in patients with early focal visual field loss. Am J Ophthalmol. 1998;126:763–71. doi: 10.1016/s0002-9394(98)00281-5. [DOI] [PubMed] [Google Scholar]
- 8.Spaeth GL, Hitchings RA, Sivalingam E. The optic disc in glaucoma: pathogenetic correlation of five patterns of cupping in chronic open-angle glaucoma. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976;81:217–23. [PubMed] [Google Scholar]
- 9.Cioffi GA, et al. Structural-functional relationships of the optic nerve in glaucoma. J Glaucoma. 2000;9:3–4. doi: 10.1097/00061198-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Demirel S, Fortune B, Fan J, et al. Predicting progressive glaucomatous optic neuropathy using baseline standard automated perimetry data. Invest Ophthalmol Vis Sci. 2009;50:674–80. doi: 10.1167/iovs.08-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drance SM. The disc and the field in glaucoma. Ophthalmol. 1978;85:209–14. doi: 10.1016/s0161-6420(78)35670-0. [DOI] [PubMed] [Google Scholar]
- 12.Hart WM, et al. Quantitative visual field and optic disc correlates early in glaucoma. Arch Ophthalmol. 1978;96:2209–11. doi: 10.1001/archopht.1978.03910060511007. [DOI] [PubMed] [Google Scholar]
- 13.Van Buskirk EM, Cioffi GA. Glaucomatous optic neuropathy. Am J Ophthalmol. 1992;113:447–52. doi: 10.1016/s0002-9394(14)76171-9. [DOI] [PubMed] [Google Scholar]
- 14.Gordon MO, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 15.Medeiros FA, et al. Prediction of Functional Loss in Glaucoma From Progressive Optic Disc Damage. Arch Ophthalmol. 2009;127:1250–1256. doi: 10.1001/archophthalmol.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miglior S, et al. Results of the European Glaucoma Prevention Study. Ophthalmol. 2005;112:366–75. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 18.Greenfield DS, Weinreb RN. Role of optic nerve imaging in glaucoma clinical practice and clinical trials. Am J Ophthalmol. 2008;145:598–603. doi: 10.1016/j.ajo.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heijl A, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 20.Kamal DS, et al. Detection of optic disc change with the Heidelberg retina tomograph before confirmed visual field change in ocular hypertensives converting to early glaucoma. Br J Ophthalmol. 1999;83:290–4. doi: 10.1136/bjo.83.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pederson JE, Anderson DR. The mode of progressive disc cupping in ocular hypertension and glaucoma. Arch Ophthalmol. 1980;98:490–5. doi: 10.1001/archopht.1980.01020030486010. [DOI] [PubMed] [Google Scholar]
- 22.Odberg T, Riise D. Early diagnosis of glaucoma. The value of successive stereophotography of the optic disc. Acta Ophthalmol. 1985;63:257–63. doi: 10.1111/j.1755-3768.1985.tb06801.x. [DOI] [PubMed] [Google Scholar]
- 23.Demirel S. Predicting progressive glaucomatous optic neuropathy using baseline standard automated perimetry data. Invest Ophthalmol Vis Sci. 2009;50:674–80. doi: 10.1167/iovs.08-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansberger SL. Community visual field screening: prevalence of follow-up and factors associated with follow-up of participants with abnormal frequency doubling perimetry technology results. Ophthalmic Epidemiol. 2007;14:134–40. doi: 10.1080/09286580601174060. [DOI] [PubMed] [Google Scholar]
- 25.Fortune B, et al. Comparing multifocal VEP and standard automated perimetry in high-risk ocular hypertension and early glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1173–80. doi: 10.1167/iovs.06-0561. [DOI] [PubMed] [Google Scholar]
- 26.Zangwill L, et al. Agreement between clinicians and a confocal scanning laser ophthalmoscope in estimating cup/disk ratios. Am J Ophthalmol. 1995;119:415–21. doi: 10.1016/s0002-9394(14)71226-7. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan BC, Nicolela MT, Artes PH. Incidence and rates of visual field progression after longitudinally measured optic disc change in glaucoma. Ophthalmol. 2009;116:2110–8. doi: 10.1016/j.ophtha.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 28.Radius RL, Maumenee AE, Green WR. Pit-like changes of the optic nerve head in open-angle glaucoma. Br J Ophthalmol. 1978;62:389–93. doi: 10.1136/bjo.62.6.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begg IS, Drance SM, Sweeney VP. Haemorrhage on the disc--a sign of acute ischaemic optic neuropathy in chronic simple glaucoma. Canadian Journal of Ophthalmology. 1970;5(4):321–30. [PubMed] [Google Scholar]
- 30.Drance SM, Begg IS. Sector haemorrhage--a probable acute ischaemic disc change in chronic simple glaucoma. Can J Ophthalmol. 1970;5:137–41. [PubMed] [Google Scholar]
- 31.Budde WM, Jonas JB. Enlargement of parapapillary atrophy in follow-up of chronic open-angle glaucoma. Am J Ophthalmol. 2004;137:646–54. doi: 10.1016/j.ajo.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Jonas JB, Budde WM, Panda-Jonas S. Ophthalmoscopic evaluation of the parapapillary region of the optic nerve head. Klin Oczna. 2004;106:279–89. [PubMed] [Google Scholar]
- 33.Smith SD, Katz J, Quigley HA. Analysis of progressive change in automated visual fields in glaucoma. Invest Ophthalmol Vis Sci. 1996;37:1419–28. [PubMed] [Google Scholar]
- 34.Yang H, et al. 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: lamina cribrosa and peripapillary scleral position and thickness. Invest Ophthalmol Vis Sci. 2007;48:4597–607. doi: 10.1167/iovs.07-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medeiros FA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123:1351–60. doi: 10.1001/archopht.123.10.1351. [DOI] [PubMed] [Google Scholar]
- 36.Heijl A, et al. Measuring visual field progression in the Early Manifest Glaucoma Trial. Acta Ophthalmol Scand. 2003;81:286–93. doi: 10.1034/j.1600-0420.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 37.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–97. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 38.Medeiros FA, et al. Long-term intraocular pressure fluctuations and risk of conversion from ocular hypertension to glaucoma. Ophthalmology. 2008;115:934–40. doi: 10.1016/j.ophtha.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeyen TG, Caprioli J. Progression of disc and field damage in early glaucoma. Arch Ophthalmol. 1993;111:62–5. doi: 10.1001/archopht.1993.01090010066028. [DOI] [PubMed] [Google Scholar]
- 40.Medeiros FA, et al. Use of progressive glaucomatous optic disk change as the reference standard for evaluation of diagnostic tests in glaucoma. Am J Ophthalmol. 2005;139:1010–8. doi: 10.1016/j.ajo.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Artes PH, Chauhan BC. Longitudinal changes in the visual field and optic disc in glaucoma. Prog Ret Eye Res. 2005;24:333–54. doi: 10.1016/j.preteyeres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Kwon YH, et al. Rate of optic disc cup progression in treated primary open-angle glaucoma. J Glaucoma. 2003;12:409–16. doi: 10.1097/00061198-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Coleman AL, et al. Interobserver and intraobserver variability in the detection of glaucomatous progression of the optic disc. J Glaucoma. 1996;5:384–9. [PubMed] [Google Scholar]
- 44.O’Leary N. Glaucomatous progression in series of stereoscopic photographs and Heidleberg retinal tomographic images. Arch Ophthalmol. 2010;128:560–8. doi: 10.1001/archophthalmol.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jampel HD, et al. Agreement among glaucoma specialists in assessing progressive disc changes from photographs in open-angle glaucoma patients. Am J Ophthalmol. 2009;147:39–44. doi: 10.1016/j.ajo.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reus NJ. Clinical assessment of Stereoscopic Optic Disc Photographs for Glaucoma: The European Optic Disc Assessment Trial. Ophthalmol. 2010;117:717–23. doi: 10.1016/j.ophtha.2009.09.026. [DOI] [PubMed] [Google Scholar]


