Abstract
This study assessed the functional role of WNT genes and the association between WNT signalling cascades and fibrosis in interstitial cystitis/bladder pain syndrome (IC/BPS) patients. Twenty-five patients (3 males, 22 females; mean age 59.7 ± 10.9 years), included 7 non-Hunner-type IC (NHIC), 18 Hunner-type IC (HIC), and 5 non-IC (control) groups. The expression of sonic hedgehog, WNT gene family, and genes previously reported as biomarkers for IC/BPS were examined using RT-PCR in biopsy specimens from the mucosa and submucosa layer of the bladder. WNT2B, WNT5A, WNT10A, and WNT11 functions in the urothelium were evaluated by silencing in an HBlEpC cell line. Pelvic Pain and Urgency/Frequency Patient Symptom Scale scores, O’Leary-Sant Symptom and Problem Index scores, and Visual Analogue Scores did not differ between the NHIC and HIC groups. However, HIC patients had significantly shorter symptom duration (30.9 vs 70.8 months, p = 0.046), higher daily urinary frequency (16.1 versus 8.5 times, p = 0.006), and smaller bladder capacity (208.6 versus 361.4 ml, p = 0.006) than NHIC patients. Overall WNT gene expression was lower in NHIC than HIC patients. Bladder epithelial tissues from HIC patients were characterised by the downregulation of WNT11. Silencing of WNT11, WNT2B, WNT5A, and WNT10A in HBlEpCs resulted in fibrotic changes, indicated by fibrotic morphology, increased fibrosis-related gene expression, and nuclear localisation of phosphorylated SMAD2, and increased vimentin and fibronectin levels. Downregulation of WNT11 results in fibrotic changes of bladder epithelial cells and is associated with the pathogenesis and differential diagnosis of NHIC. Decreased expression of WNT11 is a potential biomarker for predicting NHIC.
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a distressing, chronic bladder disorder characterised by urinary frequency, urgency, nocturia, and pelvic pain without bacterial infection or an identifiable pathologic cause1. The quality of life of IC/BPS patients is often poor. Only a few treatment options have been identified, and there is no cure.
Although investigated in many studies, the pathogenesis of IC/BPS still remains unclear. Previous studies have described the reduction in the glycosaminoglycan (GAG) layer in the bladder of IC/BPS patients2,3. An impaired GAG layer can result in an imbalance in urine storage, causing frequent voiding, reduced bladder capacity, and pelvic pain. The pathophysiology of IC/BPS also includes mast cell increases in the bladder4. A damaged urothelium can result in activation of mast cells, thus inducing inflammation, fibrosis, pain, vasodilation, and smooth muscle contraction5. Several theories including developmental defects in the Tamm-Horsfall protein, potassium sensitivity theory, and autoimmunity have been posited. However their detail mechanisms remain unclear6,7.
IC can be classified into Hunner-type IC (HIC) and non-Hunner-type IC (NHIC). HIC is the classic, ulcerative type of IC and is characterized by patches of red mucosa with small vessels radiating from a central pale scar, known as “Hunner’s lesions”, on cystoscopy8. HIC is currently treated using transurethral resection and coagulation (TUR-C) with good outcomes9. Conversely, NHIC is characterized by glomerulations (multiple petechial-like hemorrhages) and submucosal haemorrhages without Hunner’s lesions on cystoscopy and hydrodistention, which is used as a therapeutic option and a diagnostic tool10.
We recently reported that NHIC is histopathologically associated with severe fibrosis and increased mast cell infiltration, and that HIC is associated with severe inflammation and urothelial denudation in the whole bladder11. Importantly, the severity of bladder tissue fibrosis in IC/BPS patients was associated with increased urinary frequency and decreased bladder capacity. Furthermore, our recent pre-clinical studies demonstrated the potential of that bladder tissue fibrosis as a promising therapeutic target for IC/BPS12,13. Beneficial outcomes have been obtained following anti-fibrotic approaches using N-acetylcysteine (NAC) or mesenchymal stem cell (MSC) therapy owing to the upregulation of WNT family genes, including WNT2B, WNT5A, WNT8A, WNT8B, WNT10A, and WNT11.
The WNT pathway is evolutionarily conserved and plays critical roles in embryonic and neonatal development. It is typically quiescent in several adult tissues14, but is reactivated in response to injury. The WNT pathway has complex and contrasting roles by promoting regeneration and fibrosis in several fibrotic disorders, such as renal, pulmonary, cardiac, and liver fibrosis15–18.
Few studies have investigated the association of fibrosis with WNT signalling cascades in IC/BPS pathogenesis. Hence, we evaluated this association and examined the functional role of dysregulated WNT genes in the bladder tissues of human IC/BPS patients.
Results
A total of 30 patients were enrolled in the study with 25 IC patients (including 7 NHIC and 18 HIC), and 5 non-IC patients as a control. Patients were predominantly female (22 of 25 in the IC group and 4 of 5 in the control group). The mean age was 59.7 ± 10.9 years. The Pelvic Pain and Urgency/Frequency Patient Symptom Scale (PUF) scores, O’Leary-Sant Symptom and Problem Index (IC-Q) scores, and Visual Analogue Score (VAS) pain questionnaire scores did not differ between the NHIC and HIC groups. However, HIC patients demonstrated significantly shorter symptom duration, a higher urinary frequency, and smaller bladder capacity than NHIC patients (Table 1).
Table 1.
Patient characteristics.
| Total (n = 30) | NHIC (n = 7) | HIC (n = 18) | p-value | |
|---|---|---|---|---|
| Age (years) | 59.7 ± 10.9 | 65.4 ± 7.3 | 56.5 ± 11.8 | 0.064 |
| Gender (M/F) | 4 vs 26 | 1 vs 6 | 2 vs 16 | 0.929 |
| Duration of symptoms (months) | 39.6 ± 31.9 | 70.8 ± 42.9 | 30.9 ± 22.8 | 0.046 |
| PUF | 22.6 ± 6.4 | 19.8 ± 4.0 | 23.7 ± 6.9 | 0.135 |
| IC-Q | 28.3 ± 7.0 | 25.0 ± 4.6 | 29.6 ± 7.5 | 0.051 |
| VAS | 6.9 ± 2.4 | 6.8 ± 1.7 | 7.0 ± 2.7 | 0.959 |
| Voiding diary | ||||
| Urinary frequency (times/day) | 13.3 ± 6.9 | 8.5 ± 2.6 | 16.1 ± 7.3 | 0.006 |
| Urinary urgency (times/day) | 9.5 ± 7.7 | 7.0 ± 3.5 | 10.9 ± 9.5 | 0.682 |
| Urge incontinence (times/day) | 0.6 ± 1.4 | 1.1 ± 1.6 | 0.4 ± 1.3 | 0.213 |
| Maximum bladder capacity (ml) | 262.6 ± 160.6 | 361.4 ± 133.6 | 208.6 ± 163.9 | 0.006 |
Standard abbreviations: EMT, epithelial-mesenchymal transition; GAG, Glycosaminoglycans; HIC, Hunner-type IC; IC/BPS, interstitial cystitis/bladder pain syndrome; IC-Q, O’Leary-Sant Symptom and Problem Index; MSCs, mesenchymal stem cells; NAC, N-acetylcysteine; NHIC, non-Hunner-type IC; PUF, Pelvic Pain and Urgency/Frequency Patient Symptom Scale; RQ-PCR, Real-time quantitative reverse transcription polymerase chain reaction; SHH, sonic hedgehog; TUR-C, transurethral resection and coagulation; VAS, Visual Analogue Score.
For gene expression assay, we employed the bladder biopsy specimens, which were confined to mucosa and submucosa layer of the bladder. We first examined the level of gene expression associated with IC/BPS pathogenesis including inflammation (e.g., CCR2, MCP-1, NFκB), growth factors (e.g., HB-EGF, NGF), nitric oxide synthase (e.g., nNOS, iNOS, eNOS), and apoptosis (e.g., ARF). Consistent with previous histological data11, the bladder tissues of HIC patients were characterised by the upregulation of pro-inflammatory genes, such as CCR2 and NFκB (Fig. 1a), reflecting severe inflammation. The expression of HB-EGF and NGF was only slightly affected in the HIC group (Fig. 1b), despite urothelium denudation. Furthermore, in the bladder tissues of HIC patients, upregulation of ARF, which is associated with apoptosis (Fig. 1c), and nitric oxidase synthase (NOS) family genes, such as iNOS, eNOS, and nNOS (Fig. 1d), were observed although the difference was not statistically significant. Increased expression of choline O-acetyltransferase (CHAT), which is the biosynthetic enzyme for the neurotransmitter acetylcholine, was also observed (Fig. 1e). The bladder tissues of NHIC patients were characterised by downregulated expression of choline transporter (CHT, also known as solute carrier family 5 member 7; SLC5A7), (Fig. 1e) POU class 2 homeobox 1 (OCT-1), and nuclear receptor corepressor 2 (SMRT) transcription regulators (Fig. 1f). In comparison between control and IC/BPS (HIC and NHIC patients) groups, the difference in the expressions of these genes were not statistically significant (Supplementary Fig. 1). Thus, these findings demonstrate that HIC and NHIC have distinct gene expression patterns, as observed for histological profiles11.
Figure 1.
Comparison of gene expression associated with IC/BPS pathology between NHIC and HIC patient bladder tissues. (a–f) RQ-PCR analysis of the known biomarkers of IC/BPS, including those of inflammation (a), growth factors (b), apoptosis (c), nitric oxide synthase (d), acetylcholine neurotransmitter biosynthesis (e), and transcription regulators (f) in the bladder tissues of the indicated patient groups. Gene expression is presented as a percentage of GAPDH. Data are represented as a dot plot of mean ± SEM [n = 6 for non-IC; n = 11 for non-Hunner-type IC (NHIC) and n = 19 for Hunner lesions in Hunner-type IC (HIC); *p < 0.05 using one-way ANOVA analysis with a Bonferroni post-hoc test]. Non-IC, stress urinary incontinence patients.
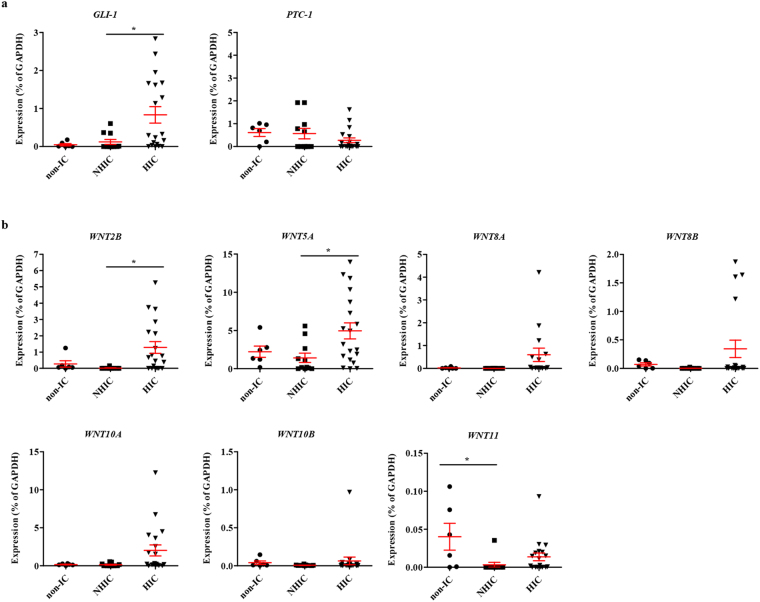
We recently reported that the SHH and WNT gene families are particularly responsible for the mechanisms underlying MSC therapy in IC/BPS animal models12,13. Thus, changes in the expression of these genes was examined in the bladder tissues of IC/BPS patients, focusing on the genes validated in pre-clinical MSC studies. Expression of the SHH pathway mediator GLI-1 was upregulated in the HIC patient group. However, the expression of PTC-1, a transmembrane receptor binding to SHH, was only slightly changed (Fig. 2a). Among the WNT family genes, WNT2B and WNT5A transcripts were increased only in the bladder tissues of the HIC patients (Fig. 2b). In particular, WNT11 expression was significantly down-regulated in the bladder tissues of IC/BPS patients (Supplementary Fig. 2), and the repression of WNT11 was characteristically observed in the NHIC patients (Fig. 2b). Overall, WNT gene expression in the bladder tissues was lower in the NHIC patients than in the HIC patients and WNT11 expression was significantly repressed in the NHIC patients (Fig. 2b).
Figure 2.
Downregulation of SHH or WNT family genes in the bladder tissue of NHIC patients. (a,b) RQ-PCR analysis of the genes involved in SHH (a) and WNT (b) pathways in the bladder tissues of the indicated patient groups. Gene expression is presented as percentage of GAPDH. Data are represented as a dot plot of mean ± SEM [n = 6 for non-IC; n = 11 for NHIC and 19 for HIC patient groups; *p < 0.05 by one-way ANOVA analysis with a Bonferroni post-hoc test].
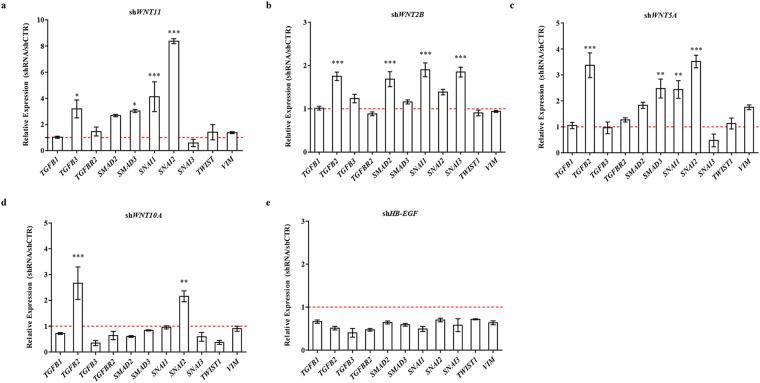
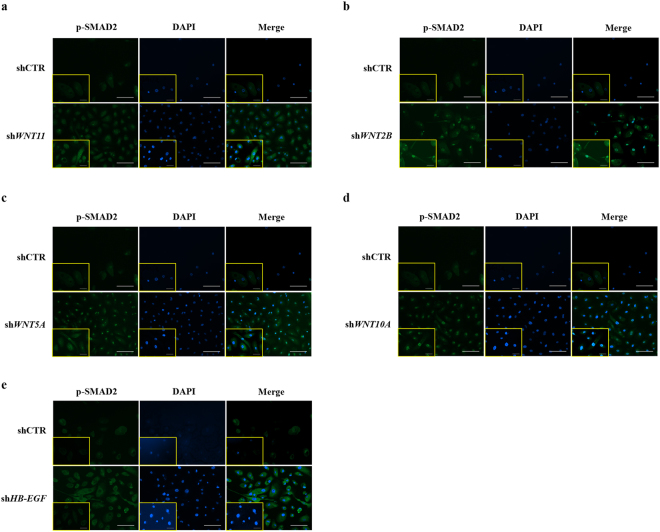
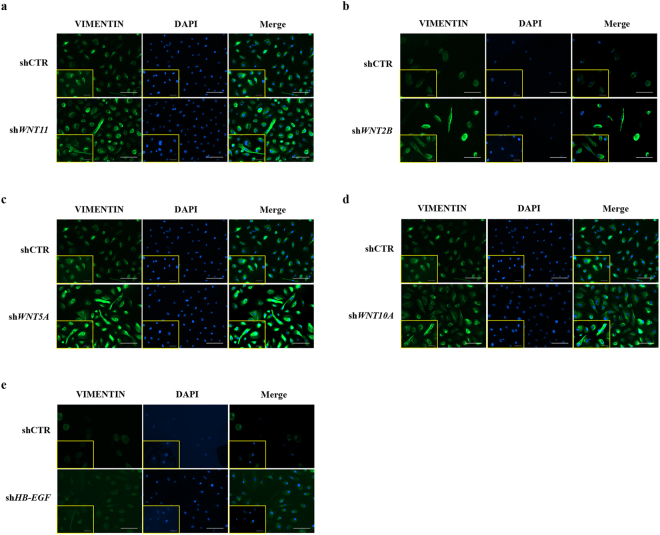
We further characterised the potential effects of WNT pathway dysregulation by infecting HBlEpC with lentivirus containing specific short hairpin (sh)RNA, thus silencing every WNT family gene that was altered in IC/BPS patients (Fig. 3a). For comparison, knockdown of HB-EGF, a growth factor responsible for urothelium integrity, was also performed. Silencing of WNT11 induced fibrosis which was microscopically evident (Fig. 3b). The induction of fibrotic change was also observed by knock-down of WNT2B, WNT5A, and WNT10A, which were significantly up-regulated in the bladder tissues of HIC patients (Fig. 3c–e). Conversely, these fibrotic changes were barely observed in HB-EGF knockdown in HBlEpC cells (Fig. 3f). Consistent with these results, silencing of these WNT genes, unlike HB-EGF, activated the genes associated with transforming growth factor-beta (TGF-β) signalling, such as TGFB2, SMAD2, and SMAD3 and epithelial-mesenchymal transition (EMT), such as SNAI1, SNAI2, TWIST, and vimentin (VIM) (Fig. 4)19. Activation of TGF-β signalling in these WNT silenced HBlEpC cells was further validated by immunostaining for nuclear localisation of phosphorylated SMAD2 (Fig. 5a–d) and increased level of vimentin, a cytoskeletal protein (Fig. 6a–d). Conversely, silencing of HB-EGF only slightly affected TGF-β signalling (Figs 5e and 6e). Accordingly, downregulation of these WNT genes resulted in an increased expression of fibronectin, an extracellular matrix protein (Fig. 7). The activation of TGF-β signalling and increased proteins associated with fibrosis in the WNT knock-downed HBlEpC cells were further validated by Western blot assay (Fig. 8). Taken together, these in vitro functional studies indicated that mis-regulation of a subset of WNT genes including WNT11 could trigger fibrotic changes in the epithelial cells of the bladder.
Figure 3.
Morphological changes following silencing of WNT family genes in HBlEpC. (a) RQ-PCR analysis of the indicated genes in HBlEpC primary bladder epithelial cells at 4 days after infection with lentivirus containing each shRNA construct. Data normalised to the scramble control group (shCTR) are represented as mean ± SEM, n = 4, ***p < 0.001 using one-way ANOVA analysis. (b–f) Representative image of HBlEpC at the indicated days after infection with lentivirus containing shWNT11 (b), shWNT2B (c), shWNT5A (d), shWNT10A (e), and shHB-EGF (f) or scramble control (shCTR) construct (magnification x100, scale bar = 100 μm).
Figure 4.
Upregulation of fibrosis-related genes by knockdown of WNT family genes. (a–e) RQ-PCR analysis of genes involved in TGFβ signalling (e.g., TGFB1, TGFB2, TGFB3, TGFBR2, SMAD2, and SMAD3) and EMT (e.g., SANI1, SNAI2, SNAI3, TWIST1, and VIM) in HBlEpC cells at 7 days after infection with lentivirus containing shWNT11 (a), shWNT2B (b), shWNT5A (c), shWNT10A (d), and shHB-EGF (e) constructs. A scramble construct was used as the control (shCTR). Gene expression is presented as expression relative to the shCTR and is represented as mean ± SEM, n = 4, *p < 0.05, **p < 0.01, ***p < 0.001 using one-way ANOVA analysis.
Figure 5.
Nuclear localization of phosphorylated SMAD2 protein induced by downregulation of WNT family genes. (a–e) Immunofluorescent staining for nuclear localization of phosphorylated SMAD2 protein (green) in HBlEpC cells at 4 days after infection with lentivirus containing shWNT11 (a), shWNT2B (b), shWNT5A (c), shWNT10A (d), and shHB-EGF (e) constructs (magnification x200, scale bar = 100 μm). The higher magnification images (magnification x400, scale bar = 50 μm) are inserted in lower left corner in each image. Nuclei were stained with DAPI (blue). A scramble shRNA construct was used as the control.
Figure 6.
Induction of vimentin intermediate filament protein by silencing of WNT family genes. (a–e) Immunofluorescent staining of vimentin (green), an intermediate filament protein induced by the EMT process in HBlEpC cells at 4 days after infection with lentivirus containing shWNT11 (a), shWNT2B (b), shWNT5A (c), shWNT10A (d), and shHB-EGF (e) constructs (magnification x200, scale bar = 100 μm). The higher magnification images (magnification x400, scale bar = 50 μm) are inserted in lower left corner in each image. Nuclei were stained with DAPI (blue). Note that low vimentin expression was observed following downregulation of HB-EGF unlike that following downregulation of WNT family genes. A scramble shRNA construct was used as the control.
Figure 7.
Increased fibronectin extracellular matrix protein by knockdown of WNT family genes. (a–d) Immunofluorescent staining of fibronectin (green), an extracellular matrix protein in HBlEpC cells, at 7 days after infection with lentivirus containing shWNT11 (a), shWNT2B (b), shWNT5A (c), and shWNT10A (d) constructs (magnification x200, scale bar = 100 μm). The higher magnification images (magnification x400, scale bar = 50 μm) are inserted in the lower left corner in each image. Nuclei were stained with DAPI (blue). A scramble shRNA construct was used as the control.
Figure 8.
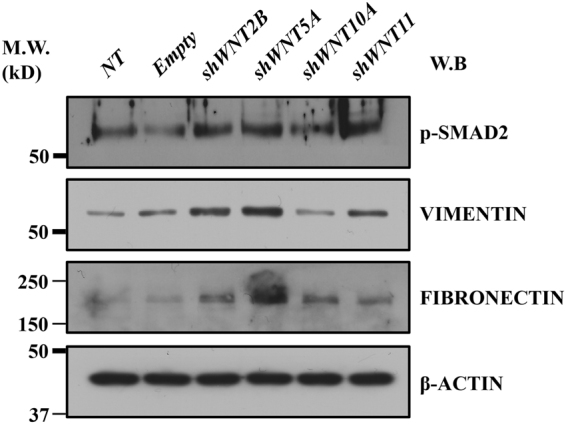
Western blot analysis of TGFβ activation and fibrosis by knockdown of WNT family genes. Western blot was used to detect the phosphorylated SMAD2 protein (p-SMAD2), vimentin, and fibronectin proteins in HBlEpC cells at 7 days after infection with lentivirus containing shWNT2B, shWNT5A, shWNT10A, and shWNT11 constructs. For control, HBlEpC cells without infection (not-treated; NT) or infected with empty lentivirus (Empty) were used. β-ACTIN was used as a loading control. Molecular weight (M.W.) marker sizes (kD) are shown on the left.
Discussion
Here, we observed that downregulation of WNT11 is associated with bladder tissue fibrosis in NHIC patients than in HIC patients. Moreover, silencing of WNT family genes in bladder epithelial cells induced fibrotic changes. Higher WNT2B and WNT5A expression was observed in HIC patients than in NHIC patients. WNT11 expression was characteristically down-regulated in NHIC patients. To our knowledge, this is the first study to investigate the relationship between the WNT signalling pathway and bladder tissue fibrosis in IC/BPS patients.
IC is classified into HIC and NHIC. However, whether they belong to the same disease entity is still controversial. In practice, the phenotypes of both types of IC differ. A diagnosis of HIC can be established based on the presence of Hunner’s lesions. However, such lesions are absent in NHIC patients. Therefore, urine or tissue biomarkers may be helpful for the diagnosis of IC. Previously, we observed that NHIC is characterised by severe fibrosis11. Thus, we attempted to identify genes that are associated with fibrosis in NHIC patients.
The WNT gene functions in embryonic development, and acts as an oncogene when aberrantly triggered20. In our previous study, we revealed that WNT signalling genes were downregulated in the IC rat model13. The WNT signalling pathway is known to modulate tissue fibrosis and associated EMT processes21. Thus, we further investigated the association of fibrosis with WNT signalling cascades in IC/BPS patients.
In this study, WNT11 expression was remarkably repressed in NHIC patients. In our previous study, we observed that NHIC was characterised by severe fibrosis and increased mast cell infiltration, while HIC was characterised by severe inflammation and urothelial denudation in the whole bladder11. These results indicate that the downregulation of WNT11 enhances EMT activation and bladder tissue fibrosis, which is mainly observed in an NHIC bladder. Thus, we propose that inhibition of the WNT11 would be novel pathogenesis and differential diagnosis of NHIC.
In our functional study using HBlEpC, we selected WNT2B, WNT5A, and WNT11, as their expression is lower in NHIC patients, and selected WNT10A as its expression is lower in the NHIC group than in the HIC group, although there was no clinical significance. HB-EGF, a growth factor responsible for urothelial integrity is a potential biomarker for the diagnosis of IC/BPS. Further, its expression is higher in NHIC patients than in HIC and non-IC patients. Hence, it was selected for comparison22. The knockdown of all selected WNT genes induced fibrotic changes in EMT-related morphology and activated the TGF-β pathway (Figs 4–8). However, these fibrotic changes were barely observed in HB-EGF-silenced HBlEpCs. These in vitro functional studies support our hypothesis that downregulation of WNT genes triggers fibrotic changes in bladder epithelial cells, thus playing a pivotal role in the pathogenesis of NHIC.
Studies on IC/BPS have been published for a century, but pathophysiology of IC/BPS is still complex and multifactorial. Numerous reports have implicated mast cell activation, GAG layer defects, developmental defects in the Tamm-Horsfall protein, and the autoimmune theory in the pathophysiology of IC/BPS. However, consensus is lacking2–4,7,23. Studies have proposed antiproliferative factor, epidermal growth factor, GAGs, bladder nitric oxide, and multiple urine proteins and serum cytokines as potential biomarkers of IC/BPS24. However, no definite biomarker has been identified for the differential diagnosis of IC/BPS. Furthermore, to our knowledge few studies on biomarkers of NHIC have been conducted, whereas urinary CXCL10 has been recently reported as a promising biomarker and the Bladder Permeability Defect Risk Score (BP-RS) using crowdsourcing has been developed (IP4IC study) in HIC patients25,26. Of note, no study has reported that downregulation of WNT pathway leads to fibrotic changes of HBlEpCs, and severe fibrosis observed in NHIC is strongly associated with decreased levels of WNT-related gene expression.
In this study, HIC patients had significantly more episodes of urinary frequency (16.1 versus 8.5 times/day, p = 0.006) and lower maximal bladder capacity (208.6 ml versus 361.4 ml, p = 0.006) than NHIC patients. Duration of symptom was significantly shorter (30.9 vs 70.8 months, p = 0.046) in HIC patients. This is probably due to the severity in symptoms, which might have provoked patients to seek earlier medical treatment leading to earlier diagnosis. Conversely, as the symptoms are less severe in NHIC, diagnosis and treatment could be delayed. Therefore, it is important to identify biomarkers that can help diagnose NHIC quickly.
Patients can be easily diagnosed with HIC owing to characteristic symptoms, such as bladder pain and/or urinary urgency/frequency, and Hunner’s lesions observed on cystoscopy. However, if Hunner’s lesions is not observed on cystoscopy and obvious glomerulation or submucosal haemorrhage is not observed during hydrodistention, a diagnosis of NHIC will be difficult to establish. Thus, histopathologic and gene expression studies of a bladder mucosal biopsy sample can aid in the differential diagnosis of IC/BPS by identifying fibrotic changes and WNT-related gene expression levels. Fibrotic changes could be a potential therapeutic target for NHIC. Indeed, in the rat models in our previous study, direct administration of MSCs into the submucosal layer of the bladder significantly restored voiding function and ameliorated tissue fibrosis12,13. These preclinical results indicate that fibrosis of the bladder epithelium may be treatable and require additional clinical trials using stem cells for confirmation. The use of antifibrotic agents, such as NAC, which was effective in an IC rat model, may be helpful for treating fibrosis in NHIC patients27.
This study has several limitations. First, the biopsy specimens in this study were confined to the mucosa and submucosa layer of the bladder and in vitro functional assays of WNT genes were investigated using only primary bladder epithelial cells. In this regard, the results represent the pathogenesis, which could be responsible mainly for the urothelium, but not the whole bladder. Further studies employing the animal models with the alternation of the WNT genes in entire bladder tissues could be required to understand the precise role of these WNT genes on the pathogenesis of IC/BPS. Second, this study involves a tissue biomarker and not a urine biomarker, and although urine-based studies are difficult, a urine biomarker is ideal because urine collection is more convenient and noninvasive than a bladder mucosal biopsy. Third, the sample size of each group was heterogeneous. The number of patients was relatively smaller in the NHIC and control groups than in the HIC group; hence, it was difficult to obtain significant differences between each group. A prospective study involving a higher number of participants is required in the future. Nevertheless, novel findings have been obtained here via the WNT signalling pathway, which have not been shown in previous IC/BPS studies.
In conclusion, downregulated WNT genes resulted in fibrotic changes in bladder epithelial cells and is significantly associated with the pathogenesis and differential diagnosis of NHIC. Decreased expression WNT genes could have diagnostic value as a biomarker for predicting NHIC. Additional clinical trials using stem cells or antifibrotic agents could be helpful for treating IC/BPS patients.
Methods
Study approval
This prospective study was approved by the Institutional Review Board of Asan Medical Center and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients prior to enrollment in the study.
Human samples
Diagnosis of IC/BPS was based on the American Urological Association criteria11. Patients’ baseline symptoms were assessed with the IC-Q, PUF, and VAS pain questionnaire. Inclusion criteria was ≥13 points on the PUF, ≥12 points on the IC-Q with ≥2 points on the pain and nocturia categories, and ≥4 points on the VAS. Thorough history taking, physical examination, urine culture, urine cytology, cystoscopy, and abdominopelvic computed tomography (CT) were performed at the outpatient clinic to specify the subtypes of IC, and to exclude patients with active urinary tract infections, urological malignancies, urolithiasis, neurologic diseases and pathologic pelvic conditions, such as malignancies or endometriosis. For the control group (non-IC), five patients receiving a sling operation for stress urinary incontinence with preoperative microscopic haematuria were enrolled.
Based on the presence of Hunner’s lesion on preoperative cystoscopy, we divided patients into NHIC (negative H-lesion) and HIC (positive H-lesion) group. NHIC patients were treated with hydrodistension of the bladder and HIC patients were treated with TUR-C. In the NHIC group, bladder biopsy was performed after hydrodistension. In the control group, biopsy was performed after the sling operation, while identifying the reason for microscopic haematuria and injury in bladder wall or urethra. In both groups, we randomly targeted three sites on the posterior wall, both lateral walls of the bladder and cold cup biopsy was done with biopsy forceps. In HIC patients, Hunner’s lesion was targeted and specimens were obtained with endoscopic electrosurgical loop. Unlike routine TUR of bladder tumour, resection depth was confined to mucosa and submucosa layer of the bladder. Muscularis propria was not included in TUR specimen. Similarly, cold cup biopsy specimens included mucosa and submucosa layer of the bladder.
Real-time quantitative reverse transcription polymerase chain reaction (RQ-PCR)
Expression of the WNT pathway genes and the pathology of IC/BPS were examined using RQ-PCR, as previously described28,29. Briefly, bladder tissues were freshly obtained from patients and total RNA was extracted using the RNeasy Mini Kit (QIAGEN, Valencia, CA). The RNA was treated with DNase I (QIAGEN) to remove contaminated genomic DNA. Total RNA (400 ng) was reverse-transcribed using TaqMan Reverse-Transcription Reagents (Applied Biosystems, Waltham, MA), according to the manufacturer’s instructions. Target gene expression was quantified via RQ-PCR with the HOT FIREPol® EvaGreen® qPCR Mix Plus (Solis BioDyne, Tartu, Estonia) on the PikoReal™ Real-Time PCR System (Thermo Fisher Scientific, Pittsburgh, PA). The threshold cycle (Ct) and the cycle number at which the fluorescence of the amplified gene reaches a fixed threshold were subsequently determined, and the relative expression level of the target genes was quantified via the 2−ΔΔCt method using glyceralydehyde-3-phosphate dehydrogenase mRNA levels as an endogenous control and the mRNA levels of the indicated groups as calibrators. All primers used for RQ-PCR analysis are listed in Table 2.
Table 2.
Sequences of the primers used in this study.
| Gene | Symbol | Forward Primer | Reverse primer |
|---|---|---|---|
| C-C motif chemokine receptor 2 | CCR2 | TACGCTCCATCGCTGTCATCTC | GCGAAGCACTGAAACACTCGAA |
| C-C motif chemokine ligand 2 | MCP-1 | TTCCCCTAGCTTTCCCCAGA | TCCCAGGGGTAGAACTGTGGT |
| nuclear factor kappa B subunit 1 | NFKB | TGC ATC TGG GGA TGA GGT TG | TGG TCA GAA GGA ATG CCA GG |
| heparin binding EGF like growth factor | HB-EGF | CAA GTC TCA GAA GAG GTT GGG C | CAC CAG AAG AAT GGC AGG AGT T |
| nerve growth factor | NGF | AAG CGG TCA TCA TCC CAT CC | CAC CTC CTT GCC CTT GAT GTC |
| nitric oxide synthase 1 | nNOS | ATC CAG TGC TCT TGA GCT GGG | TTG GGC CTT CTG GAA AAC CA |
| nitric oxide synthase 2 | iNOS | TCG GAG CCT CCT CTC TCA AAC T | GGT GCA CTC AGC AGC AAG TTC |
| nitric oxide synthase 3 | eNOS | TGG GTC CGC CTT AAT CTG G | TGT AAT CCA CAT GAG CTG GGG |
| cyclin dependent kinase inhibitor 2A | ARF | GAG GCT CTG AGA AAC CTC GGG | AAA ACT ACG AAA GCG GGG TGG |
| choline O-acetyltransferase | CHAT | ATG GCC ATT GAC AAC CAC CTG | GCA GCA GAA CAT CTC CGT GGT |
| solute carrier family 5 member 7 | CHT | TTG TAC CCA TCA TGT GCT CTG TT | TAT CCA CAG GTG TTG CCT TCC |
| jumping translocation breakpoint | PAR | TGG AAG TTC GAA GGG GCT G | CTC GAT TTG CTT CCG GAC CT |
| POU class 2 homeobox 1 | OCT_1 | AGC AGC TTG AAT GAG GCA GTG | TTT GGC TAA CAG GCA CTC TGG |
| nuclear receptor corepressor 2 | SMRT | TTA GCG CTC TGG ACA GAT GGA | TGG CCT GAC TTG GTT TCC AG |
| GLI transcription factor family | GLI-1 | CCA GCT GTG GTC ATC CTG AGG | AAG ATC AAG AGA GTC CAG GGG GTT |
| patched 1 | PTC-1 | AGT TAC CAT TGG CGA CCT AGC AT | TGA TGG CTC CAA CAC TAA CTG TCT C |
| Wnt family member 2B | WNT2B | CTC CCT GAT TTC CCG CTC TG | AGA AGT ATC GGG AAG CTG GTG C |
| Wnt family member 5A | WNT5A | AAT AGG CAC GAA GGC ACA GGT C | AAC ACG GCA TCT CTC TTT CAC CA |
| Wnt family member 4 | WNT4 | CAT CCT GCC CAA ACC ACT CTC | CGT CAC AAT GGC AAA GAG CC |
| Wnt family member 8A | WNT8A | GCA GAG GCG GAA CTG ATC TTT | GTT GTG GCT GTT CTG TAG GCA CT |
| Wnt family member 8B | WNT8B | GGG GTT GGT TCC TAG AGG CAG | TGT ATC TGG AGT CCC TCG GGT T |
| Wnt family member 11 | WNT11 | TCT TTG GGG TGG CAC TTC TC | TCT GCC GAG TTC ACT TGA CG |
| Wnt family member 10A | WNT10A | TTG GCT CTT GGG AAG AGG AGA | TGA GTG GTG GGG TTC AGA CAG |
| Wnt family member 10B | WNT10B | CCA GGC CCT TAG GGA AGT TG | CCA CCC TTC CTG CTG AAG AA |
Cell culture
HBlEpC, a primary epithelial cell line derived from the normal human bladder (Cell Applications, Inc, San Diego, CA) was maintained in Bladder Epithelial Cell Growth Medium (Cell Applications, Inc), according to the manufacturer’s instructions.
Gene knockdown
For RNA interference mediated gene silencing, shRNA constructs were designed to target 19 base-pair gene-specific regions of WNT2B, WNT5A, WNT10A, or HB-EGF and then then cloned into a pLenti6/BLOCK-iT™-DEST lentiviral vector using the Gateway Technology reaction as previously described28,30. The lentivirus was generated using a four-plasmid transfection system (Invitrogen, Waltham, MA). Two days after transfection into the 293 FT packaging cell line, supernatants containing recombinant pseudo-lentiviral particles were collected and concentrated by precipitation using Lenti-X Concentrator kit (Clontech, Mountain View, CA) according to the manufacturer’s instructions (Invitrogen). HBlEpC cells were infected with the concentrated virus using 6 μg/ml polybrene (Invitrogen). The effect of gene knockdown was examined using RT-PCR 5 days after infection. The sequences for the top and bottom oligonucleotides for each shRNA are listed in Table 3.
Table 3.
Sequences of the top and bottom oligonucleotides for each shRNA used in this study.
| WNT2B_shRNA_ Top | CAC CGC ACG AGT GAT CTG TGA CAA TCG AAA TTG TCA CAG ATC ACT CGT GC |
| WNT2B_shRNA_ Bottom | AAA AGC ACG AGT GAT CTG TGA CAA TTT CGA TTG TCA CAG ATC ACT CGT GC |
| WNT5A_ shRNA_Top | CAC CGA AGT GCA ATG TCT TCC AAG TCG AAA CTT GGA AGA CAT TGC ACT TC |
| WNT5A_ shRNA_ Bottom | AAA AGA AGT GCA ATG TCT TCC AAG TTT CGA CTT GGA AGA CAT TGC ACT TC |
| WNT5A_ shRNA_Top | CAC CGC AAG TTG GTA CAG GTC AAC ACG AAT GTT GAC CTG TAC CAA CTT GC |
| WNT5A_ shRNA_Top | AAA AGC AAG TTG GTA CAG GTC AAC ATT CGT GTT GAC CTG TAC CAA CTT GC |
| WNT10A _shRNA_ Top | CAC CGA GAC ATC CAC GCG AGA ATG ACG AAT CAT TCT CGC GTG GAT GTC TC |
| WNT10A_ shRNA_ Bottom | AAA AGA GAC ATC CAC GCG AGA ATG ATT CGT CAT TCT CGC GTG GAT GTC TC |
| WNT11_shRNA_ Top | CAC CGC GTG TGC TAT GGC ATC AAG TCG AAA CTT GAT GCC ATA GCA CAC GC |
| WNT11_ shRNA_ Bottom | AAA AGC GTG TGC TAT GGC ATC AAG TTT CGA CTT GAT GCC ATA GCA CAC GC |
| HB-EGF_shRNA_ Top | CAC CGA AAG TCC GTG ACT TGC AAG ACG AAT CTT GCA AGT CAC GGA CTT TC |
| HB-EGF_ shRNA_ Bottom | AAA AGA AAG TCC GTG ACT TGC AAG ATT CGT CTT GCA AGT CAC GGA CTT TC |
| HB-EGF_shRNA_ Top | CAC CGG GAC CCA TGT CTT CGG AAA TCG AAA TTT CCG AAG ACA TGG GTC CC |
| HB-EGF_ shRNA_ Bottom | AAA AGG GAC CCA TGT CTT CGG AAA TTT CGA TTT CCG AAG ACA TGG GTC CC |
Immunocytochemistry and western blot analysis
HBlEpC cells cultured on glass coverslips were fixed with 4% paraformaldehyde, and permeabilised with 0.1% Triton X-100. Cells were blocked with 1% bovine serum albumin in phosphate buffer solution for 30 min at room temperature and subsequently stained with antibodies against Phospho-Smad2 (#3101; Cell Signaling Technology, Danvers, MA), vimentin (sc-6260; Santa Cruz Biotechnology, Santa Cruz, CA), and fibronectin (sc-8422; Santa Cruz Biotechnology). Immunostaining was visualized using Alexa 488 (A11001) or Alexa 488 (A11008)-conjugated anti-mouse or rabbit antibodies (Molecular Probes, Grand Island, NY). Nuclei were counterstained with 4,6-diamidino-2-phenylindole (D9542; DAPI, Sigma-Aldrich). Images were obtained using a fluorescence microscope (EVOS® FL Imaging System, Life Technologies Microscopy, Carlsbad, CA).
For western blot analysis, cell extracts (30 μg) were prepared in RIPA lysis buffer (Santa Cruz Biotechnology) and separated on 12% SDS-PAGE gels. The expression level of the indicated proteins was assessed by probing with same antibodies used in immunocytochemistry assay. For loading control, β-actin (A5441; Sigma-Aldrich) was used. Uncropped western blot images were found in Supplementary Fig. 3.
Statistics
Data were analysed using GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA) and are expressed as mean ± standard error of the mean (SEM). The differences and significance were verified using one-way ANOVA followed by Bonferroni post hoc tests. A p-value < 0.05 was considered statistically significant.
Electronic supplementary material
Acknowledgements
This research was supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant no. HI14C3365) and by grants (2017-098, 2017-528, 2018-609, and 2018-098) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
Author Contributions
D.C., J.-Y.H.; Collection and/or assembly of data, data analysis and interpretation. J.-H.S., C.-M.R. and H.Y.Y.; Collection and/or assembly of data. A.K., S.L. and J.L.; Data analysis and interpretation. D.-M.S. and M.-S.C.: Conception and design, Financial support, Data analysis and interpretation, manuscript writing, final approval of manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Daeheon Choi and Ju-Young Han contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28093-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dong-Myung Shin, Email: d0shin03@amc.seoul.kr.
Myung-Soo Choo, Email: mschoo@amc.seoul.kr.
References
- 1.Kim A, Shin DM, Choo MS. Stem Cell Therapy for Interstitial Cystitis/Bladder Pain Syndrome. Curr Urol Rep. 2016;17:1. doi: 10.1007/s11934-015-0563-1. [DOI] [PubMed] [Google Scholar]
- 2.Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis) J Urol. 1991;145:732–735. doi: 10.1016/S0022-5347(17)38437-9. [DOI] [PubMed] [Google Scholar]
- 3.Lokeshwar VB, et al. Urinary uronate and sulfated glycosaminoglycan levels: markers for interstitial cystitis severity. J Urol. 2005;174:344–349. doi: 10.1097/01.ju.0000161599.69942.2e. [DOI] [PubMed] [Google Scholar]
- 4.Sant GR, Theoharides TC. The role of the mast cell in interstitial cystitis. The Urologic clinics of North America. 1994;21:41–53. [PubMed] [Google Scholar]
- 5.Clemens JQ, Brown SO, Calhoun EA. Mental health diagnoses in patients with interstitial cystitis/painful bladder syndrome and chronic prostatitis/chronic pelvic pain syndrome: a case/control study. J Urol. 2008;180:1378–1382. doi: 10.1016/j.juro.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Merwe JP. Interstitial cystitis and systemic autoimmune diseases. Nature clinical practice. Urology. 2007;4:484–491. doi: 10.1038/ncpuro0874. [DOI] [PubMed] [Google Scholar]
- 7.Fowler JE, Jr., et al. Interstitial cystitis is associated with intraurothelial Tamm-Horsfall protein. J Urol. 1988;140:1385–1389. doi: 10.1016/S0022-5347(17)42051-9. [DOI] [PubMed] [Google Scholar]
- 8.Hunner GL. A rare type of bladder ulcer: Further notes, with a report of eighteen cases. Journal of the American Medical Association. 1918;70:203–212. doi: 10.1001/jama.1918.02600040001001. [DOI] [Google Scholar]
- 9.Ryu J, et al. Elimination of Hunner’s Ulcers by Fulguration in Patients With Interstitial Cystitis: Is It Effective and Long Lasting? Korean journal of urology. 2013;54:767–771. doi: 10.4111/kju.2013.54.11.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messing EM, Stamey TA. Interstitial cystitis: early diagnosis, pathology, and treatment. Urology. 1978;12:381–392. doi: 10.1016/0090-4295(78)90286-8. [DOI] [PubMed] [Google Scholar]
- 11.Kim A, et al. Histopathological characteristics of interstitial cystitis/bladder pain syndrome without Hunner lesion. Histopathology. 2017;71:415–424. doi: 10.1111/his.13235. [DOI] [PubMed] [Google Scholar]
- 12.Kim A, et al. Improved efficacy and in vivo cellular properties of human embryonic stem cell derivative in a preclinical model of bladder pain syndrome. Scientific Reports. 2017;7:8872. doi: 10.1038/s41598-017-09330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song M, et al. Mesenchymal Stem Cell Therapy Alleviates Interstitial Cystitis by Activating Wnt Signaling Pathway. Stem Cells and Development. 2015;24:1648–1657. doi: 10.1089/scd.2014.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastakoty D, Young PP. Wnt/β-catenin pathway in tissue injury: roles in pathology and therapeutic opportunities for regeneration. The FASEB Journal. 2016;30:3271–3284. doi: 10.1096/fj.201600502R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edeling M, et al. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol. 2016;12:426–439. doi: 10.1038/nrneph.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrisey EE. Wnt signaling and pulmonary fibrosis. The American journal of pathology. 2003;162:1393–1397. doi: 10.1016/S0002-9440(10)64271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao H, Yang JJ, Shi KH, Li J. Wnt signaling pathway in cardiac fibrosis: New insights and directions. Metabolism: clinical and experimental. 2016;65:30–40. doi: 10.1016/j.metabol.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Miao CG, et al. Wnt signaling in liver fibrosis: progress, challenges and potential directions. Biochimie. 2013;95:2326–2335. doi: 10.1016/j.biochi.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Li M, et al. Epithelial-mesenchymal transition: An emerging target in tissue fibrosis. Exp Biol Med (Maywood) 2016;241:1–13. doi: 10.1177/1535370215597194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nusse R, Clevers H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Farahmand L, Darvishi B, Majidzadeh-A K, Madjid Ansari A. Naturally occurring compounds acting as potent anti-metastatic agents and their suppressing effects on Hedgehog and WNT/β-catenin signalling pathways. Cell Proliferation. 2017;50:e12299–n/a. doi: 10.1111/cpr.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chai TC, et al. Bladder stretch alters urinary heparin-binding epidermal growth factor and antiproliferative factor in patients with interstitial cystitis. J Urol. 2000;163:1440–1444. doi: 10.1016/S0022-5347(05)67638-0. [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ. Update on the Pathology and Diagnosis of Interstitial Cystitis/Bladder Pain Syndrome: A Review. Int Neurourol J. 2016;20:13–17. doi: 10.5213/inj.1632522.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo HC. Potential urine and serum biomarkers for patients with bladder pain syndrome/interstitial cystitis. Int J Urol. 2014;21(Suppl 1):34–41. doi: 10.1111/iju.12311. [DOI] [PubMed] [Google Scholar]
- 25.Niimi, A. et al. Diagnostic value of urinary CXCL10 as a biomarker for predicting Hunner type interstitial cystitis. Neurourol Urodyn (2017). [DOI] [PubMed]
- 26.Chancellor, M. B., Bartolone, S. N., Veerecke, A. & Lamb, L. E. Crowdsourcing Disease Biomarker Discovery Research; The IP4IC Study. J Urol (2017). [DOI] [PubMed]
- 27.Galicia-Moreno M, Favari L, Muriel P. Antifibrotic and antioxidant effects of N-acetylcysteine in an experimental cholestatic model. European journal of gastroenterology & hepatology. 2012;24:179–185. doi: 10.1097/MEG.0b013e32834f3123. [DOI] [PubMed] [Google Scholar]
- 28.Heo J, et al. Sirt1 Regulates DNA Methylation and Differentiation Potential of Embryonic Stem Cells by Antagonizing Dnmt3l. Cell reports. 2017;18:1930–1945. doi: 10.1016/j.celrep.2017.01.074. [DOI] [PubMed] [Google Scholar]
- 29.Jeong, E. M. et al. Real-Time Monitoring of Glutathione in Living Cells Reveals that High Glutathione Levels Are Required to Maintain Stem Cell Function. Stem Cell Reports (2018). [DOI] [PMC free article] [PubMed]
- 30.Kim, Y. et al. Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.