Abstract
Upon tissue damage the plant secondary metabolites glucosinolates can generate various hydrolysis products, including isothiocyanates (ITCs). Their role in plant defence against insects and pest and their potential health benefits have been well documented, but our knowledge regarding the endogenous molecular mechanisms of their effect in plants is limited. Here we investigated the effect of allyl-isothiocyanate (AITC) on Arabidopsis thaliana mutants impaired in homeostasis of the low-molecular weight thiol glutathione. We show that glutathione is important for the AITC-induced physiological responses, since mutants deficient in glutathione biosynthesis displayed a lower biomass and higher root growth inhibition than WT seedlings. These mutants were also more susceptible than WT to another ITC, sulforaphane. Sulforaphane was however more potent in inhibiting root growth than AITC. Combining AITC with the glutathione biosynthesis inhibitor L-buthionine-sulfoximine (BSO) led to an even stronger phenotype than observed for the single treatments. Furthermore, transgenic plants expressing the redox-sensitive fluorescent biomarker roGFP2 indicated more oxidative conditions during AITC treatment. Taken together, we provide genetic evidence that glutathione plays an important role in AITC-induced growth inhibition, although further studies need to be conducted to reveal the underlying mechanisms.
Introduction
Glucosinolates (GSLs) are secondary metabolites produced by species belonging to the Brassicales order, hence are present in the model plant Arabidopsis thaliana. GSLs are hydrolysed by enzymes called myrosinases (β-thioglucosidases) when they come together upon tissue damage1,2. Due to this enzymatic reaction, a variety of glucosinolate hydrolysis products (GHPs) can be generated such as isothiocyanates (ITCs), nitriles, epithionitriles and thiocyanates depending on the GSL substrate and reaction conditions3. ITCs are the most commonly generated breakdown products and are considered as biologically active phytochemicals in plant-insect and plant-pathogen interactions4,5. Different ITCs have also been involved in the inhibition of carcinogenesis6–8.
Allyl-isothiocyanate (AITC) originates from the enzymatic cleavage of the aliphatic GSL sinigrin and has been shown to exhibit antibacterial, antifungal and antinematodal properties9–11. In addition, AITC affects the behaviour, growth and survival of insects12,13. A growing body of scientific evidence on mammalian systems indicates that AITC treatment arrests the cell cycle, causes apoptosis and inhibits the proliferation of cancer cells leading to reduced tumour formation in certain types of cancer14,15. Our understanding is however relatively poor about the biological effects of AITC and other isothiocyanates on plants. AITC was studied as a possible alternative to hazardous chemicals in weed control management since it can act as herbicide at high concentrations16,17. Exogenously applied AITC causes stomatal closure via the generation of reactive oxygen species (ROS) and enhances the heat tolerance in A. thaliana18–20. Our group has lately demonstrated that AITC causes a shift in the cell cycle distribution, leads to a disintegration of microtubules and inhibition of the actin-dependent intracellular transport in A. thaliana21–23.
ITCs are electrophilic compounds that have been shown to activate phase II detoxification enzymes, including glutathione S-transferases (GSTs), in several organisms24–26. Some GSTs are capable of mediating the conjugation of the available sulfhydryl group of reduced glutathione (GSH) to the electrophilic central carbon atom of the isothiocyanate group27–30. The conjugation of ITCs to glutathione can however also be non-enzymatic29,31,32. In mammals this chemical interaction results in the formation of mercapturic acid through enzymatic or non-enzymatic steps followed by excretion33,34. Compared to animal systems, there is limited knowledge about GST-mediated in vivo conjugation of AITC to glutathione for plants35,36. But some plant GSTs show activity on ITCs in vitro37–39, and exogenous application of AITC upregulates transcript levels of GSTs and depletes glutathione17,40,41.
Glutathione is synthesized in a two-step process, in which the first and rate-limiting enzyme γ-glutamylcysteine synthetase (γ–ECS or GSH1) converts glutamate and cysteine to γ–EC, then glutathione synthetase (GSH2) forms glutathione by the ligation of an additional glycine to the intermediate42,43 (Fig. 1). In plants glutathione plays a pivotal role in various physiological responses such as redox homeostasis and ROS scavenging, detoxification of xenobiotics and development44–46. Glutathione is also involved in the biosynthesis of phytochelatins and defence compounds such as camalexin and glucosinolates47–49.
Figure 1.
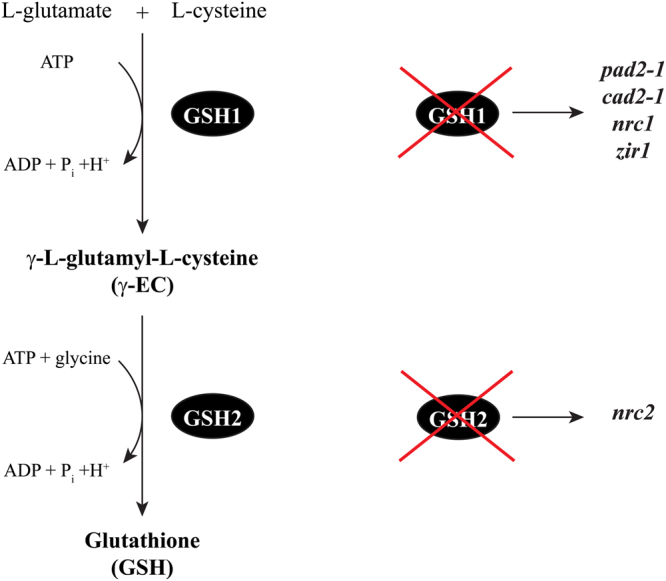
Glutathione biosynthesis and mutants. On the left, schematic representation of the glutathione biosynthetic pathway. On the right, simplified explanatory graph showing which gene of GSH biosynthesis is impaired in the particular mutants.
Nowadays, the importance of GHPs has gained significant attention, especially understanding their potential effects on plants. In the present study we investigated the role of glutathione in the response of A. thaliana to AITC in order to better understand the underlying molecular mechanisms of ITC-induced physiological responses. We show that A. thaliana GSH1 and GSH2 mutants, affected in the biosynthesis of glutathione, have reduced root growth and total biomass production compared to wild type (WT) seedlings when exposed to exogenous AITC. A series of mutants impaired in other genes putatively involved in glutathione homeostasis were however not more susceptible than WT. Our results show that the low-molecular weight thiol glutathione plays an essential role in ITC-triggered physiological responses.
Results
Glutathione-deficient mutants showed an increased susceptibility to AITC
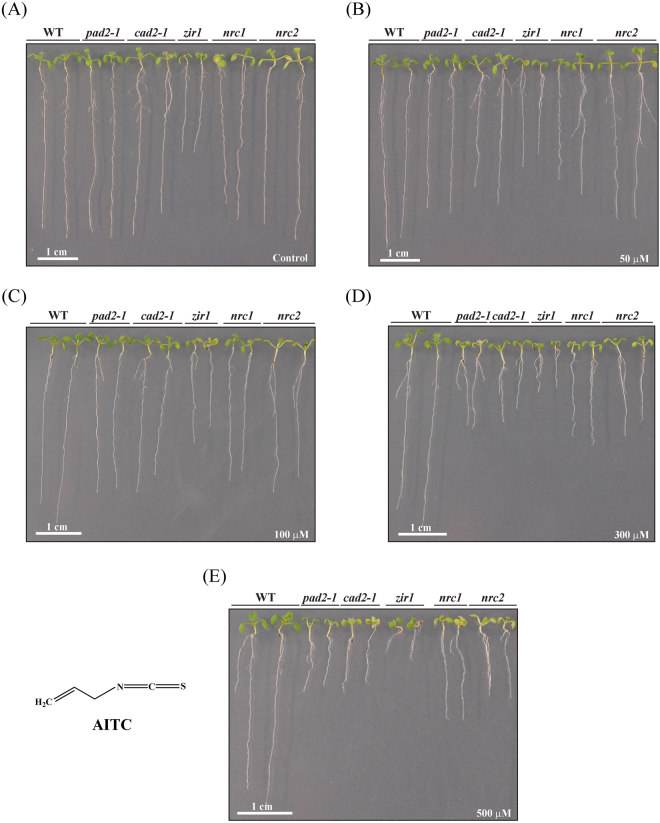
To investigate the importance of glutathione in the ITC-triggered response of A. thaliana the five mutants pad2-1, cad2-1, zir1, nrc1 and nrc2 that are affected in glutathione biosynthesis as they are either impaired in GSH1 or GSH2 (Fig. 1; Supplementary Table S1) and Col-0 WT seedlings were exposed to different concentrations of AITC. Under the control treatment all the mutants, especially zir1, nrc1 and nrc2, showed reduced growth compared to the WT (Fig. 2A), which is consistent with some earlier observations50,51. The AITC treatment led to a dose-dependent growth inhibition of Col-0 WT seedlings as shown in Fig. 2 and measured by primary root length on day 10 (Fig. 3).
Figure 2.
Effect of AITC on seedling growth. Ten day old WT and glutathione mutants grown in a vertical position on solid in vitro medium supplemented with AITC at the desired concentrations are shown: (A) control, (B) 50 μM, (C) 100 μM, (D) 300 μM and (E) 500 μM. The structure of AITC is also shown. For illustration purposes two plants of each line from plates at the same concentration were transferred together to a separate plate on day 10.
Figure 3.
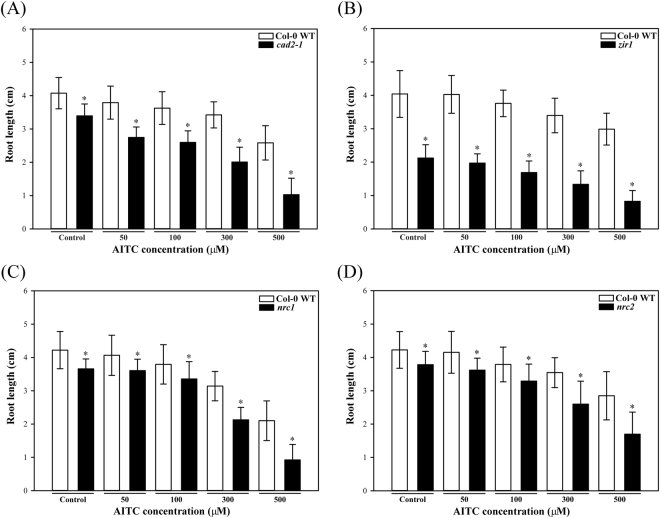
Root growth inhibition by AITC. Mean root length (n = 60) of 10 day old seedlings of the WT and the glutathione mutants (A) cad2-1, (B) zir1, (C) nrc1 and (D) nrc2 grown on solid in vitro medium supplemented with different concentrations of AITC. Asterisks indicate that the difference is significant between the WT and the particular mutant (P < 0.01, Student’s t-test) for that treatment. Error bars represent SDs.
Mutants that are impaired in glutathione biosynthesis exhibited a stronger growth inhibition than WT seedlings when exposed to AITC, independent of whether the mutation was present in the first or second enzyme of glutathione biosynthesis (Figs 2 and 3). The AITC-induced root growth reduction was greater for the glutathione mutants than the WT, especially at higher doses, with the largest AITC inhibition effect observed for nrc1 and cad2-1. To account for the fact that glutathione mutants had shorter roots on the control plates, ratios between the average root length on each AITC concentration and on control medium are shown in Supplementary Figure S1. Although zir1 had much shorter roots on control medium and exhibited the strongest phenotype on AITC (Fig. 2), the ratio to the control treatment showed that the root growth of zir1 was affected similarly to that of the other glutathione mutants (Supplementary Figure S1).
We supplemented the growth medium with glutathione monoethyl ester in addition to AITC but this did not mitigate the AITC-induced root growth inhibition of WT or mutant seedlings (data not shown).
Dynamics of the AITC-induced effect on root elongation in glutathione mutants
To assess the dynamics of the differential AITC effect on root growth between glutathione mutants and WT seedlings the root elongation on 300 µM AITC was monitored at different time points. The results clearly indicated that the five glutathione mutants had shorter roots on control plates than the WT already after 4 days (Supplementary Figure S2). At this time point the reduction of root growth by AITC was substantially smaller for zir1 than for the other lines. Between day 4 and day 7 the root elongation of all glutathione mutants was remarkably different from that of WT. While AITC affected the root length increment of WT by 20% over this period it had a much stronger effect on the glutathione mutants. From day 7 to day 10 the differences between control and AITC-treated seedlings were smaller than those observed during the second period, indicating that the effect of AITC on root growth persisted until day 10 but decreased over time (Supplementary Figure S2).
Glutathione mutants produced lower biomass upon AITC treatment
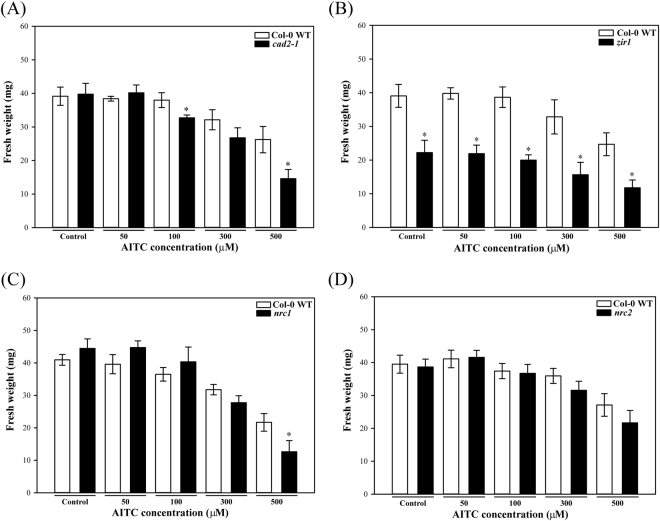
The total biomass was also investigated following AITC treatment and showed that in contrast to root length, fewer significant differences in fresh weight values were observed between WT and the glutathione mutants (Fig. 4). On control plates the biomass of glutathione mutants was not different from the WT, except for zir1 (Fig. 4B). The biomass of glutathione mutants was consistently more inhibited by 300 μM and 500 μM AITC treatments than that of the WT, which is similar to the root length results described above. Despite the slightly higher fresh weight of nrc1 at low AITC concentrations the mutant coped less well at the two highest doses relative to WT (Fig. 4C and Supplementary Figure S3).
Figure 4.
Effect of AITC on seedling biomass. The mean fresh weight of WT and glutathione mutants: (A) cad2-1, (B) zir1, (C) nrc1, (D) nrc2 grown on medium supplemented with different concentrations of AITC was measured on day 10 (n = 4, each replicate consists of a pool of 15 seedlings). Statistically significant differences between the WT and the particular mutant for a given treatment are marked by asterisks (P < 0.01, Student’s t-test). Error bars represent SDs.
Glutathione mutants showed also a higher susceptibility to SFN treatment
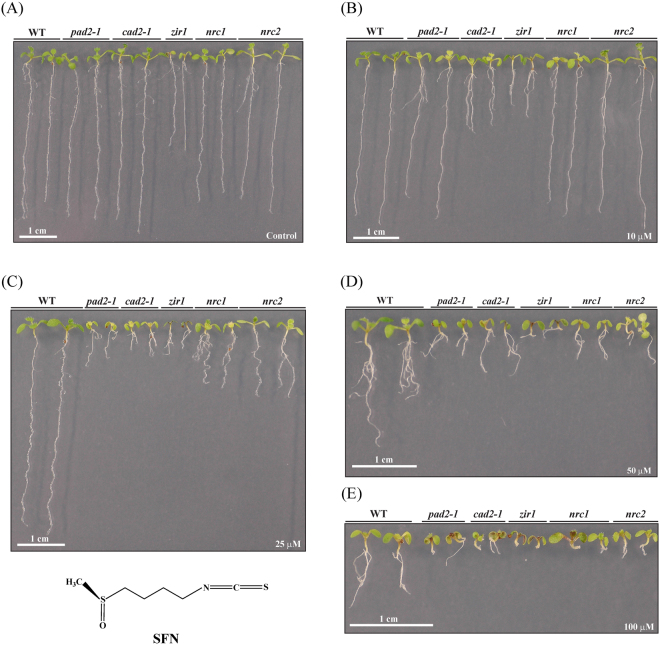
To address the question if the more susceptible phenotype of glutathione mutants towards AITC was unique for this GHP, sulforaphane (SFN), an aliphatic ITC possessing a longer side chain than AITC, was tested. Similar to the exposure to high AITC concentrations the five glutathione mutants were more severely affected by SFN than WT. Mutants such as cad2-1 and zir1 exhibited shorter roots already at the lowest SFN dose, and clear differences to the WT were observed for all glutathione mutants on 25 μM and 50 μM SFN (Fig. 5). Furthermore the highest SFN concentration tested (100 µM) led to extreme root growth inhibition (Fig. 5), revealing a much higher potency for SFN than for AITC.
Figure 5.
Effect of SFN on seedling growth. Representative 10 day old WT and glutathione mutant seedlings grown on plates supplemented with SFN at the indicated concentrations are shown: (A) control, (B) 10 μM, (C) 25 μM, (D) 50 μM and (E) 100 μM. The structure of SFN is also shown.
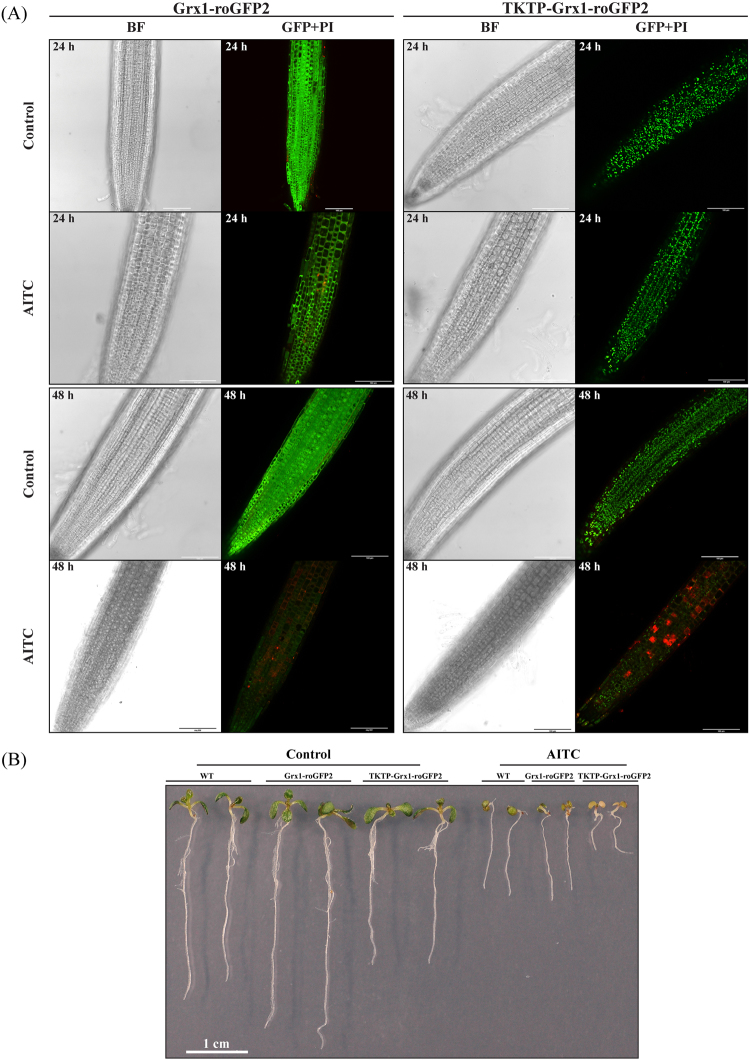
Transgenic lines expressing roGFP2 indicated a change in the glutathione redox potential upon AITC treatment
The application of AITC led to stronger responses for the glutathione mutants than for WT, which may suggest an essential role for this tripeptide in the AITC-induced physiological responses. In order to elucidate whether the glutathione redox potential was changed or not during the treatment we used two transgenic Arabidopsis lines expressing the versatile redox-sensitive fluorescent protein roGFP2 that is a biosensor for the glutathione redox potential52. The cytosolic Grx1-roGFP2 and to a lesser extent the plastidic TKTP-Grx1-roGFP2 lines exhibited lower fluorescence intensity when excited at 488 nm after 24 h or 48 h of AITC treatment compared to their respective control, indicating oxidative conditions and/or lower levels of glutathione (Fig. 6A). In roots of seedlings that were exposed to AITC for 72 h roGFP2 fluorescence was almost completely absent (data not shown). In addition to the intensive propidium iodide fluorescence of root cell nuclei indicating cell death in AITC-treated roots (Fig. 6A), seedlings also exhibited growth inhibition especially following the 72 h treatment (Fig. 6B).
Figure 6.
Fluorescence of roGFP2 seedlings at different time points of a 1 mM AITC treatment. (A) Representative confocal images (BF: brightfield, GFP + PI: overlay image of GFP excited at 488 nm and propidium iodide fluorescent signals) of roots exposed to AITC for 24 h and 48 h. Intensive red staining in AITC-treated roots indicates cell death. Scale bars = 100 µm. (B) Representative pictures of WT and roGFP2 plantlets after 72 h of AITC treatment as under (A) demonstrate the AITC-triggered growth phenotype.
The combination of L-buthionine-sulfoximine and AITC exacerbated the growth inhibition
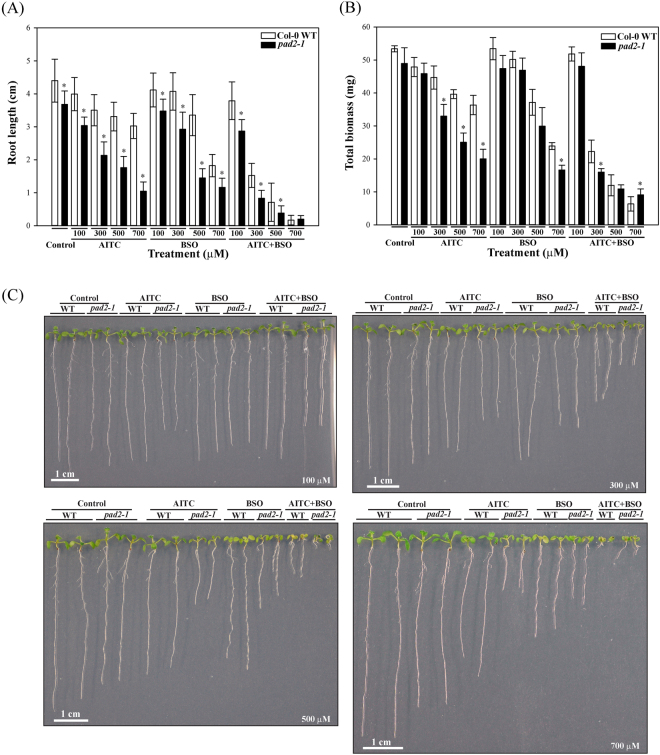
L-buthionine-sulfoximine (BSO) is a specific inhibitor of GSH1. Its application to plants leads to depletion of the cellular glutathione pool53, the inhibition of root growth54 and a growth reduction similar to that observed for the severely glutathione deficient rml1 (root meristemless1)55. As AITC treatment led to a similar biological outcome we compared the growth inhibition effects caused by AITC, BSO and a combination of the two chemicals on WT and pad2-1 seedlings.
AITC led to reduced root growth, with a more severe reduction for pad2-1 than for WT (Fig. 7A and Supplementary Figure S4). Col-0 WT seedlings exposed to BSO showed similar root inhibition as when exposed to equivalent amounts of AITC, except at the highest concentration. BSO had similar effects on Col-0 WT and pad2-1 seedlings at 100 µM whereas it inhibited the root growth of pad2-1 more than of WT at the three highest BSO concentrations. The growth inhibition effect of BSO on pad2-1 seemed however to reach a plateau at the highest dose, in contrast to Col-0. The combination of AITC and BSO resulted in more severe root growth inhibition compared to the single treatments (Fig. 7A and C). The effect of the combined treatment was also stronger on pad2-1 than on WT, except for a concentration of 700 µM each (Fig. 7C and Supplementary Figure S4).
Figure 7.
Growth inhibition effects of AITC, BSO and their combination on WT and pad2-1 seedlings. (A) Mean root lengths of 10 day old seedlings (n = 60) grown on solid in vitro medium supplemented with the chemicals at the given concentration levels. (B) Mean biomass of 10 day old seedlings (n = 4 and each of them consists of a pool of 15 seedlings) grown on plates supplemented with different concentrations of the compounds. (C) Ten day old WT and pad2-1 plants exposed to the chemicals at the indicated concentrations (bottom right corners). In (A) and (B) error bars represent SDs and asterisks mark a significant difference (P < 0.01, Student’s t-test) between pad2-1 and WT for a given treatment.
The total biomass was also affected by these treatments, although fewer significant differences were detected (Fig. 7B). The biomass of pad2-1 was more affected than that of WT by both AITC and BSO. The combined application of AITC and BSO caused more severe effects on fresh weight than the single treatments, consistent with results of the combined treatment on root length. Moreover, pad2-1 exhibited significantly higher biomass than WT at 700 μM (Fig. 7B and Supplementary Figure S4).
Mutants affected in the transport and degradation of glutathione and glutathione S-conjugates did not exhibit differences in their susceptibility to AITC
While glutathione is synthesized in the chloroplast and the cytosol it is also present in the mitochondrion, nucleus and the apoplast, indicating the necessity of transport56. Oxidized glutathione and glutathione S-conjugates that the low-molecular weight thiol forms with endogenous and xenobiotic electrophilic compounds can also be transported between subcellular compartments and across the plasma membrane. As these transport mechanisms take part in glutathione homeostasis we tested the susceptibility of different transporter mutants (Supplementary Table S1) to AITC. As none of these showed any difference with WT in their susceptibility to AITC the data is not presented in detail. The triple mutant clt1clt2clt3 is deficient in the transport of γ–EC and glutathione from the plastid to the cytosol and shows lower cytosolic and higher plastidic glutathione levels57, but did not exhibit any difference in root length compared to WT when exposed to AITC. Multidrug Resistance-associated Protein 1 (MRP1) and MRP2, of the ABC (ATP-Binding Cassette)-type subfamily C transporters, transport oxidized glutathione and glutathione S-conjugates when expressed in yeast58,59 and might be involved in their transport from the cytosol into the vacuole in plants. MRP3 has also been reported to transport glutathione S-conjugates60. The root growth of mutants affected in these three MRPs was not distinguishable from that of WT upon AITC treatment. Members of the oligopeptide transporters (OPTs) might be responsible for transport of glutathione and its derivates across the plasma membrane, however the opt1-3 and opt6 mutants tested in our study did not exhibit any phenotype difference to WT when exposed to AITC.
Breakdown of glutathione, oxidized glutathione and glutathione S-conjugates happens upon uptake into the vacuole but can also take place in the cytosol and apoplast56. As γ-glutamyl transpeptidases (GGTs) and γ-glutamyl peptidases (GGPs) have been implicated in these catabolic processes, we investigated mutants affected in GGT1 to GGT4 and GGP1, but none showed a higher susceptibility to AITC than WT seedlings when root growth was assessed. The double mutant pcs1pcs2 impaired in phytochelatin synthesis and the catabolism of glutathione conjugates61,62 showed also a WT-like phenotype when exposed to AITC.
Glutathione is an important storage and transport form of reduced sulfur and exerts a negative feedback on sulfate uptake63. Glutathione is also involved in the synthesis of GSLs which upon hydrolysis to ITCs release sulfate3,47. We tested therefore also mutants impaired in the three genes SULTR1;2, SULTR4;1 and SULTR4;2 encoding sulfate transporters64–66 but did not observe an altered susceptibility to AITC. SULTR1;2 is the major high-affinity sulfate transporter responsible for uptake in Arabidopsis roots while SULTR4;1 and SULTR4;2 mediate the efflux of sulfate from vacuoles to the cytoplasm64,67.
Discussion
In the present study we used a genetic approach to better understand the role of glutathione in the growth inhibition effect of AITC on A. thaliana that was indicated by recent studies17,40,41.
Glutathione is synthesized in a two-step process through the action of γ-glutamylcysteine synthetase (γ–ECS or GSH1) and glutathione synthetase (GSH2)42,43. Several mutants that are impaired in glutathione biosynthesis and accumulate lower levels of glutathione than WT plants under normal growth conditions have been reported. The levels of glutathione depend on the mutation in question, and nrc1, cad2-1, pad2-1 and zir1, which are affected in GSH1 show glutathione levels that range from 45 to 15% of normal levels50,51,54,68. The essential role of glutathione in plant development has been demonstrated by the mutant rml1 which is impaired in GSH1 and produces approximately 3% of wild type glutathione levels, leading to extremely short roots55. Moreover a null mutant in GSH1 is embryo lethal69. GSH2 null mutants are lethal at the early seedling stage, but weaker alleles with reduced levels of glutathione, such as nrc251, have been described too. All the mutants in GSH1 that we tested, i.e. pad2-1, cad2-1, nrc1 and zir1, showed reduced root growth under control conditions and a more severe AITC-triggered root inhibition than the WT. Although less pronounced, a similar effect was observed for seedling biomass production. Hence, all these lines impaired in glutathione biosynthesis indicate its important role in AITC-induced physiological responses. The fact that nrc2, a mutant affected in GSH2, also exhibited greater sensitivity in its response to AITC confirmed that the root length phenotype was due to the impairment in the glutathione biosynthetic pathway, irrespective of whether GSH1 or GSH2 carried the mutation.
It was recently reported that a short-term exposure of A. thaliana to AITC (0.01 M) vapours led to a rapid depletion of the cellular glutathione pool and that removing the stressor allowed effective recovery of glutathione levels40. Taking this into consideration, we postulate that in our treatment AITC also led to a decrease of glutathione levels. As these were already lower in the mutants compared to the WT this led to stronger phenotypes regarding root length and total biomass production. In addition, as these mutants are affected in glutathione biosynthesis they would respond less efficiently than the WT to an increased demand for glutathione as a consequence of the AITC treatment.
In plants, GSH has been shown to be involved in maintaining the redox homeostasis and mediating signals from the ever-changing environment to intracellular target proteins70. That AITC exposure triggered an oxidation and/or depletion of GSH was shown by analysing transgenic lines expressing roGFP2. Both the cytosolic Grx1-roGFP2 and the plastidic TKTP-Grx1-roGFP2 lines exhibited a noticeable decrease in fluorescence intensity when excited at 488 nm following 24 h and 48 h AITC treatments. As we did not observe a clear increase in fluorescence intensity at 405 nm excitation our results indicated that a decrease in glutathione levels was responsible for the lower roGFP2 fluorescence intensity. It has been shown that roGFP2 is almost fully reduced in intact tissues and that a treatment with the GSH1 inhibitor BSO or the electrophilic xenobiotic 1-chloro-2,4-dinitrobenzene led to fully oxidized state of the biosensor accompanied by decreased fluorescence intensity52. We cannot exclude the possibility that loss of fluorescence during AITC treatment was due to its interference with the biomarker, but it has been reported that exogenously supplied chemicals responsible for GSH depletion are not likely to interfere with roGFP252.
Our results thus indicated that AITC treatment led to glutathione depletion which contributed to the higher susceptibility of glutathione mutants, but the molecular mechanism behind this physiological response is likely more complicated. We aimed at further depleting the intracellular glutathione levels by applying BSO and AITC simultaneously. The combined treatment resulted in a more severe reduction than the individual treatments for both WT and pad2-1 in terms of root length and total biomass, indicating an additive effect. However, the two individual treatments had clearly different effects on root growth as e.g. the AITC treatment increased even more the difference for pad2-1 through the concentration range than for the WT, whereas the reduction between the two highest doses of BSO was less pronounced on the mutant compared to WT. This finding supports the idea that the mutation in pad2-1 close to the glutamate conjugating residues of GSH1 might influence the affinity of the enzyme for BSO, as it was proposed for cad2-154,71. Supplementing the medium with glutathione monoethyl ester in addition to AITC did not diminish the effect of AITC on root growth. This might be due to the oxidation and/or degradation of glutathione over time72 or again indicate that the effect triggered by AITC is more complex than a change in glutathione homeostasis.
In order to assess whether the stronger AITC-triggered phenotype of glutathione mutants was specific to this particular GHP or not, mutants were subjected to SFN treatment, which is another isothiocyanate produced by A. thaliana accessions73. Our results indicated indeed also a higher susceptibility of glutathione mutants than WT towards SFN, which demonstrates that the phenotype induced by AITC is not unique but can be triggered by other ITCs. The assay revealed however also a higher potency for SFN than for AITC, which might be due to differences in physicochemical properties such as their ability to cross the cell membrane, compound stability or differences in reactivity. This is also consistent with our recent work showing that different ITCs lead to various degree of growth inhibition depending on their side chain structure74. That SFN injected directly into Arabidopsis leaves has been reported to lead to a rapid decrease of the cellular glutathione pool75 further strengthens the involvement of glutathione in ITC-triggered growth inhibition.
In animal cells the fate of ITCs is well characterized: conjugation to glutathione by GSTs resulting in its intracellular depletion and subsequent degradation of dithiocarbamates through enzymatic events catalysed by γ-glutamyltranspeptidase, cysteinylglycinase and N-acetyltransferase via the mercapturic acid pathway76.
Plants have evolved specific strategies too in order to neutralize potentially harmful compounds such as xenobiotics and heavy metals. As an electrophilic chemical, ITCs might react covalently with the thiol groups of (proteins and) glutathione in plants, similar to what has been demonstrated in different in vitro systems38,77,78 as well as in insects feeding on GSL or ITC diets79,80. This might be one of the possible mechanisms by which AITC leads to lower GSH levels in plants. Conjugation of indolic ITCs with glutathione in the biosynthesis of indole-sulfur phytoalexins and in PEN2-mediated indole GSL hydrolysis has been reported recently35,81. Conjugation of aliphatic ITCs with glutathione, the further processing of these conjugates and the molecular mechanism of ITC-triggered reduction of glutathione levels in planta still requires further investigation. A conjugation of AITC to glutathione in planta might be non-enzymatic or catalysed by GSTs, as indicated by the AITC-induced upregulation of several GST-encoding genes17,40,41 and similar to what was recently shown for indol-3-ylmethyl-ITC35. Glutathione S-conjugates are usually sequestered by distinct ABC transporters from the cytosol into the vacuole for degradation, although breakdown of glutathione, oxidized glutathione and glutathione S-conjugates also happens outside the vacuole56. We aimed at investigating the possible intracellular fate of glutathione-ITC conjugates by analysing different mutants that might be involved in the processing and/or transport of these compounds. However, the chosen mutants impaired in ABC-type MRP transporters, oligopeptide transporters (OPTs) and γ-glutamyl transpeptidases (GGTs) did not exhibit a difference in root growth inhibition during AITC treatment compared to the WT. This suggests that these proteins do not play a major role in the transport and breakdown of putative glutathione-AITC conjugates. Alternatively functional redundancy might have prevented us from observing any phenotype for single mutants. Hence, further studies are needed to elucidate the fate of AITC in plant tissue.
GSLs, the precursors of ITCs, constitute in addition to their role in defence responses a storage for reduced sulfur as well as a good example on how the partitioning of this macronutrient is controlled between primary and secondary metabolism82. Glutathione is also an important storage and transport form of reduced sulfur owing to the thiol-containing Cys residue incorporated into the tripeptide and its biosynthesis is strongly dependent on the sulfur assimilatory pathway including necessary feedback inhibition mechanisms63. A mutant impaired in the major high-affinity sulfate transporter SULTR1;2 responsible for uptake in Arabidopsis roots was reported earlier to contain decreased amounts of glutathione83 but our data indicate that sultr1;2 seedlings exposed to AITC have a WT-like response. The sultr4;1 and sultr4;2 mutants impaired in mediating the efflux of sulfate from vacuoles to the cytoplasm67 displayed also WT-like growth under AITC treatment. Taking into consideration that mutants affected in glutathione biosynthesis exhibited a susceptible phenotype while, under our conditions, none of the tested sulfate transporter mutants did so, the latter did not seem to affect glutathione homeostasis sufficiently to result in increased susceptibility to AITC.
In the present study we provided genetic evidence about the important role of GSH in AITC-induced growth inhibition. Our analysis of a series of mutants has revealed that those directly impaired in glutathione biosynthesis exhibited a higher degree of growth reduction than WT. Our results indicate glutathione depletion upon treatment with AITC, corroborating earlier studies17,40,75. The underlying molecular mechanism of AITC-induced physiological responses -especially the consequences of GSH depletion- seems to be complex. Glutathione plays an essential role in the control of root development via cell cycle regulation as it was comprehensively demonstrated in the rml1 mutant55. Interestingly, previous transcriptional analysis uncovered that AITC treatment affected the expression profile of certain genes involved in the regulation of cell cycle23, indicating the phytochemical-induced growth inhibition might be an indirect consequence of cell cycle misregulation due to GSH depletion. According to our current knowledge the most likely scenario is that AITC treatment causes ROS production19,20 and induces the expression of several GSTs17,40,41 as part of the plant’s detoxification system to conjugate AITC to glutathione leading to its intracellular depletion. Recruitment of glutathione from the cytosol into the nucleus is essential at the beginning of cell proliferation to provide proper redox environment84,85. However, the import of glutathione into the nucleus under normal physiological conditions occurs at the expense of the cytosolic pool85 which together with AITC generates an extreme demand for glutathione. Plants have to adjust their metabolism in order to supply enough glutathione for AITC neutralization and in the same time to provide enough redox buffer for the nucleus to maintain proper cell proliferation. However, based on our results mutants abolished in glutathione biosynthesis failed to accomplish this mission. The appropriate glutathione supply within different subcellular compartments is necessary to maintain the physiological redox balance within the cell86. Although in our study roGFP2 expressed in the cytosol or in the plastid exhibited reduced fluorescence intensity upon AITC treatment, it is currently not known if AITC can hijack glutathione only in the cytosol and therefore interrupt the nuclear import, or is able to deplete different pools at the same time. Is AITC able to deplete directly the nuclear glutathione pool? The nuclear glutathione pool is more resistant to depletion by BSO than the cytosolic as it was demonstrated in 3T3 fibroblasts and other human cell lines87,88, hence we cannot exclude the possibility that AITC -which presumably acts differently from BSO- is able to deplete efficiently the nuclear pool. Depletion of glutathione affects root development also by interfering with auxin transport84,89,90. Although transcriptional analysis of AITC-treated seedlings revealed down-regulation of many auxin-inducible genes, auxin marker lines exposed to AITC did not indicate a change in auxin response and auxin-resistant mutants did not reveal an altered resistance to AITC74. Whether alternative pathways such as thioredoxins90 or other members of the redox hub such as ascorbate44 might be involved in the process also remains to be elucidated. Moreover, the possibility that AITC in planta can interact with oxidized glutathione -as it was shown in vitro77- in addition to the reduced form also needs to be investigated. The identification of additional members involved in the AITC-induced responses is a key step to fully understand the complexity of this physiological process.
Methods
Chemicals
Allyl-isothiocyanate (AITC, CAS 57-06-7; 377430; 95%), sulforaphane (SFN, CAS 142825-10-3; S6317; 95%), dimethyl sulfoxide (DMSO, CAS 67-68-5; D8418) and L-buthionine-sulfoximine (BSO, CAS 83730-53-4; B2515) were purchased from Sigma-Aldrich. Propidium iodide (PI, CAS 25535-16-4; Molecular Probes P3566) was purchased from Thermo Fisher Scientific.
Plant material and growth conditions
Seeds of A. thaliana mutant lines used in our study were ordered from the European Arabidopsis Stock Centre (NASC) or kindly donated by authors (see Supplementary Table S1 for further details). Seeds were surface sterilised using chlorine gas for 4 h, and then stratified for at least 3 days at 4 °C in water. Seeds were sown on square plates (Greiner Bio One; 120 × 120 × 17 mm) containing 75 ml solid ½ strength Murashige-Skoog medium (pH 5.7) (Sigma-Aldrich, M5524 supplemented with 2% sucrose and 1% agar). Plates were sealed and placed in a vertical position into a controlled growth chamber (VB1514 Vötsch Industrietechnik) under a 16 h photoperiod (75 µmol m−2 s−1) at 22 °C/18 °C for 10 days.
Validation of mutant lines
All mutants were either homozygous for the mutation when obtained or propagated to homozygosity. The homozygosity of T-DNA insertion mutants was verified by PCR using T-DNA specific and target specific primers (Supplementary Table S2). In case of ethyl methanesulfonate mutants, the region surrounding the expected point mutation was amplified by PCR and the single point mutation was verified by sequencing. Genotyping results of the mutants are presented in Supplementary Figure S5.
Treatment with ITCs and BSO
A. thaliana seedlings were exposed to ITC for 10 days while growing vertically on square plates containing solid in vitro medium as described above. Seeds were sown in parallel on medium supplemented with ITC, which was added just before pouring the plates, and on control plates (medium without ITC). The AITC concentrations used were 50 µM, 100 µM, 300 µM and 500 µM, based on our recent study on the effect of AITC on root growth inhibition74. Selected mutants were also exposed to SFN (30 mM stock solution prepared in DMSO) at the concentrations of 10 µM, 25 µM, 50 µM and 100 µM. In this case control plates consisted of DMSO-supplemented medium. Unless stated otherwise four replicate plates were prepared for each treatment and mutant seedlings were grown together with WT seedlings on each plate.
BSO treatment was performed similarly as for ITCs by adding it (100 mM stock solution prepared in water) to the medium (either on its own or in combination with ITC) to obtain BSO concentrations ranging from 50 µM to 700 µM.
Measurement of growth parameters
Primary root length and total seedling biomass were measured in order to evaluate the ITC-triggered growth inhibition of mutants compared to WT. Pictures of vertically grown seedlings were taken at three different time points (4, 7 and 10 days) after sowing out, and primary root length was measured using the ImageJ software91. Total biomass of the seedlings was measured at day 10. Seeds that did not germinate or develop were not taken into consideration when calculating the average root length or biomass. Statistical significance testing was done by Student’s t-test using SigmaPlot (Systat Software; ver. 13.0).
Confocal laser scanning microscopy (CLSM) analysis
Seeds of two transgenic lines expressing redox-sensitive GFP2 (roGFP2) either in the cytosol (Grx1-roGFP2) or the plastids (TKTP-Grx1-roGFP2)92 and Col-0 WT seedlings were sown onto 6-well cell culture plates (Sigma-Aldrich, CLS3516) containing 3 ml of liquid ½ MS medium (pH 5.7) supplemented with 2% sucrose. Plates were sealed and placed into a controlled growth chamber for 7 days under the same conditions as mentioned above. On day 7 seedlings were transferred to fresh growth medium, and then some of the plates were supplemented with AITC to a final concentration of 1 mM whereas others remained non-treated (control). Plates were sealed and placed back into the growth cabinet.
Samples from two replicate wells were investigated after 24 h, 48 h and 72 h of AITC treatment. Prior to imaging, seedlings were rinsed in sterile water and stained with 500 nM propidium iodide (PI, 50 µM stock solution in H2O) for 1 minute, then rinsed again with water to get rid of the excess staining. PI was used as a marker to monitor the cell viability during live cell imaging. Samples were mounted onto glass slides using water as a medium and investigated under a Leica (DMI 6000 CS AFC Bino inverted microscope) TCS SP8 instrument. The following settings were applied in our analysis: HCX IRAPO L 25x/0.95 NA (FWD = 2.4 mm) water immersion objective; 1 airy unit pinhole size; sequential scanning between frames; UV (405 nm) and white light (WLL 470–670 nm) lasers. GFP was excited at 405/488 nm and detected at 498–530 nm, whereas PI was excited at 561 nm and detected between 571 and 715 nm. Leica HyD detectors were used in standard mode for recording the signal of fluorophores. Images were processed by Leica Application Suite X (LAS X 2.0), Adobe Photoshop CC and Adobe Illustrator CC softwares.
Electronic supplementary material
Acknowledgements
The present study was supported by the Research Council of Norway [grant number: 214329]. The authors would like to thank Prof. Dr. Andreas Meyer (University of Bonn) for kindly providing the roGFP2 lines. In addition, we are grateful to the following people for donating seeds: cad2-1 and clt1clt2clt3 (Christopher S. Cobbett, University of Melbourne), ggt4 and pcs1pcs2 (Erwin Grill, Technische Universität München), ggt1 (Dongtao Ren, China Agricultural University), nrc1 and nrc2 (Julian Schroeder, University of California San Diego), zir1 (Kuo-Chen Yeh, Academia Sinica).
Author Contributions
J.U., A.M.B. and R.K. conceived and designed the research. J.U. conducted experiments. J.U. and R.K. analyzed data and wrote the manuscript. All authors read and approved the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28099-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bones AM, Rossiter JT. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol. Plant. 1996;97:194–208. doi: 10.1111/j.1399-3054.1996.tb00497.x. [DOI] [Google Scholar]
- 2.Kissen R, Rossiter JT, Bones AM. The ‘mustard oil bomb’: not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem. Rev. 2009;8:69–86. doi: 10.1007/s11101-008-9109-1. [DOI] [Google Scholar]
- 3.Bones AM, Rossiter JT. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry. 2006;67:1053–1067. doi: 10.1016/j.phytochem.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Wittstock, U., Kliebenstein, D. J., Lambrix, V., Reichelt, M. & Gershenson, J. In Integrative Phytochemistry: from Ethnobotany to Molecular Ecology Vol. 37 Recent Advances in Phytochemistry (ed. J. T. Romeo) 101–125 (Elsevier, 2003).
- 5.Hopkins RJ, van Dam NM, van Loon JJA. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009;54:57–83. doi: 10.1146/annurev.ento.54.110807.090623. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Talalay P. Anticarcinogenic activities of organic isothiocyanates: chemistry and mechanisms. Cancer Res. 1994;54:1976s–1981s. [PubMed] [Google Scholar]
- 7.Traka M, Mithen R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009;8:269–282. doi: 10.1007/s11101-008-9103-7. [DOI] [Google Scholar]
- 8.Verkerk R, et al. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009;53:S219. doi: 10.1002/mnfr.200800065. [DOI] [PubMed] [Google Scholar]
- 9.Calmes B, et al. Glucosinolate-derived isothiocyanates impact mitochondrial function in fungal cells and elicit an oxidative stress response necessary for growth recovery. Front. Plant Sci. 2015;6:414. doi: 10.3389/fpls.2015.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dias C, Aires A, Saavedra M. Antimicrobial activity of isothiocyanates from cruciferous plants against methicillin-resistant Staphylococcus aureus (MRSA) Int. J. Mol. Sci. 2014;15:19552–19561. doi: 10.3390/ijms151119552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder NE, MacGuidwin AE. Mortality and behavior in Heterodera glycines juveniles following exposure to isothiocyanate compounds. J. Nematol. 2010;42:194–200. [PMC free article] [PubMed] [Google Scholar]
- 12.Agrawal AA, Kurashige NS. A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J. Chem. Ecol. 2003;29:1403–1415. doi: 10.1023/A:1024265420375. [DOI] [PubMed] [Google Scholar]
- 13.Pope TW, et al. Comparative innate responses of the aphid parasitoid Diaeretiella rapae to alkenyl glucosinolate derived isothiocyanates, nitriles, and epithionitriles. J. Chem. Ecol. 2008;34:1302–1310. doi: 10.1007/s10886-008-9531-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res. 2010;54:127–135. doi: 10.1002/mnfr.200900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharya A, et al. Inhibition of bladder cancer development by allyl isothiocyanate. Carcinogenesis. 2010;31:281–286. doi: 10.1093/carcin/bgp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bangarwa SK, Norsworthy JK, Gbur EE. Allyl isothiocyanate as a methyl bromide alternative for weed management in polyethylene-mulched tomato. Weed Technol. 2012;26:449–454. doi: 10.1614/WT-D-11-00152.1. [DOI] [Google Scholar]
- 17.Hara M, Yatsuzuka Y, Tabata K, Kuboi T. Exogenously applied isothiocyanates enhance glutathione S-transferase expression in Arabidopsis but act as herbicides at higher concentrations. J. Plant Physiol. 2010;167:643–649. doi: 10.1016/j.jplph.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Hara M, Harazaki A, Tabata K. Administration of isothiocyanates enhances heat tolerance in Arabidopsis thaliana. Plant Growth Regul. 2013;69:71–77. doi: 10.1007/s10725-012-9748-5. [DOI] [Google Scholar]
- 19.Khokon MAR, et al. Allyl isothiocyanate (AITC) induces stomatal closure in Arabidopsis. Plant Cell Environ. 2011;34:1900–1906. doi: 10.1111/j.1365-3040.2011.02385.x. [DOI] [PubMed] [Google Scholar]
- 20.Hossain MS, et al. Glucosinolate degradation products, isothiocyanates, nitriles, and thiocyanates, induce stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2013;77:977–983. doi: 10.1271/bbb.120928. [DOI] [PubMed] [Google Scholar]
- 21.Sporsheim B, Øverby A, Bones A. Allyl isothiocyanate inhibits actin-dependent intracellular transport in Arabidopsis thaliana. Int. J. Mol. Sci. 2015;16:29134–29147. doi: 10.3390/ijms161226154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Øverby A, Silihagen Bævre M, Bones A. Disintegration of microtubules in Arabidopsis thaliana and bladder cancer cells by isothiocyanates. Front. Plant Sci. 2015;6:6. doi: 10.3389/fpls.2015.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Åsberg SE, Bones AM, Øverby A. Allyl isothiocyanate affects the cell cycle of Arabidopsis thaliana. Front. Plant Sci. 2015;6:364. doi: 10.3389/fpls.2015.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munday R, Munday CM. Induction of Phase II detoxification enzymes in rats by plant-derived isothiocyanates: comparison of allyl isothiocyanate with sulforaphane and related compounds. J. Agric. Food Chem. 2004;52:1867–1871. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa K, Miwa S, Tsutsumiuchi K, Miwa J. Allyl isothiocyanate that induces GST and UGT expression confers oxidative stress resistance on C. elegans, as demonstrated by nematode biosensor. PLoS ONE. 2010;5:e9267. doi: 10.1371/journal.pone.0009267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis F, Vanhaelen N, Haubruge E. Glutathione S-transferases in the adaptation to plant secondary metabolites in the Myzus persicae aphid. Arch. Insect Biochem. Physiol. 2005;58:166–174. doi: 10.1002/arch.20049. [DOI] [PubMed] [Google Scholar]
- 27.Kolm RH, Danielson UH, Zhang Y, Talalay P, Mannervik B. Isothiocyanates as substrates for human glutathione transferases: structure-activity studies. Biochem J. 1995;311:453–459. doi: 10.1042/bj3110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer DJ, Crease DJ, Ketterer B. Forward and reverse catalysis and product sequestration by human glutathione S-transferases in the reaction of GSH with dietary aralkyl isothiocyanates. Biochem. J. 1995;306:565. doi: 10.1042/bj3060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YS, Kolm RH, Mannervik B, Talalay P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem. Biophys. Res. Commun. 1995;206:748–755. doi: 10.1006/bbrc.1995.1106. [DOI] [PubMed] [Google Scholar]
- 30.Wiktelius E, Stenberg G. Novel class of glutathione transferases from cyanobacteria exhibit high catalytic activities towards naturally occurring isothiocyanates. Biochem. J. 2007;406:115–123. doi: 10.1042/BJ20070328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brüsewitz G, et al. The metabolism of benzyl isothiocyanate and its cysteine conjugate. Biochem. J. 1977;162:99–107. doi: 10.1042/bj1620099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartling D, Radzio R, Steiner U, Weiler EW. A glutathione S-transferase with glutathione-peroxidase activity from Arabidopsis thaliana. Molecular cloning and functional characterization. Eur. J. Biochem. 1993;216:579–586. doi: 10.1111/j.1432-1033.1993.tb18177.x. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol. Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 34.Vermeulen M, van den Berg R, Freidig AP, van Bladeren PJ, Vaes WHJ. Association between consumption of cruciferous vegetables and condiments and excretion in urine of isothiocyanate mercapturic acids. J. Agric. Food Chem. 2006;54:5350–5358. doi: 10.1021/jf060723n. [DOI] [PubMed] [Google Scholar]
- 35.Pislewska-Bednarek M, et al. Glutathione transferase U13 functions in pathogen-triggered glucosinolate metabolism. Plant Physiol. 2018;176:538–551. doi: 10.1104/pp.17.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanschen FS, et al. Characterization of products from the reaction of glucosinolate-derived isothiocyanates with cysteine and lysine derivatives formed in either model systems or broccoli sprouts. J. Agric. Food Chem. 2012;60:7735–7745. doi: 10.1021/jf301718g. [DOI] [PubMed] [Google Scholar]
- 37.Dixon DP, Cole DJ, Edwards R. Purification, regulation and cloning of a glutathione transferase (GST) from maize resembling the auxin-inducible type-III GSTs. Plant Mol. Biol. 1998;36:75–87. doi: 10.1023/A:1005958711207. [DOI] [PubMed] [Google Scholar]
- 38.Dixon DP, Hawkins T, Hussey PJ, Edwards R. Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase superfamily. J. Exp. Bot. 2009;60:1207–1218. doi: 10.1093/jxb/ern365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner U, Edwards R, Dixon DP, Mauch F. Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol. Biol. 2002;49:515–532. doi: 10.1023/A:1015557300450. [DOI] [PubMed] [Google Scholar]
- 40.Øverby A, Stokland RA, Åsberg SE, Sporsheim B, Bones AM. Allyl isothiocyanate depletes glutathione and upregulates expression of glutathione S-transferases in Arabidopsis thaliana. Front. Plant Sci. 2015;6:277. doi: 10.3389/fpls.2015.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kissen R, Overby A, Winge P, Bones AM. Allyl-isothiocyanate treatment induces a complex transcriptional reprogramming including heat stress, oxidative stress and plant defence responses in Arabidopsis thaliana. BMC Genomics. 2016;17:740. doi: 10.1186/s12864-016-3039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.May MJ, Leaver CJ. Arabidopsis thaliana gamma-glutamylcysteine synthetase is structurally unrelated to mammalian, yeast, and Escherichia coli homologs. Proc. Natl. Acad. Sci. USA. 1994;91:10059–10063. doi: 10.1073/pnas.91.21.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ullmann P, Gondet L, Potier S, Bach TJ. Cloning of Arabidopsis thaliana glutathione synthetase (GSH2) by functional complementation of a yeast gsh2 mutant. Eur. J. Biochem. 1996;236:662–669. doi: 10.1111/j.1432-1033.1996.00662.x. [DOI] [PubMed] [Google Scholar]
- 44.Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Considine MJ, Foyer CH. Redox regulation of plant development. Antioxid. Redox Signal. 2014;21:1305–1326. doi: 10.1089/ars.2013.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cummins I, Dixon DP, Freitag-Pohl S, Skipsey M, Edwards R. Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Metab. Rev. 2011;43:266–280. doi: 10.3109/03602532.2011.552910. [DOI] [PubMed] [Google Scholar]
- 47.Geu-Flores F, et al. Cytosolic γ-glutamyl peptidases process glutathione conjugates in the biosynthesis of glucosinolates and camalexin in Arabidopsis. Plant Cell. 2011;23:2456–2469. doi: 10.1105/tpc.111.083998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlaeppi K, Bodenhausen N, Buchala A, Mauch F, Reymond P. The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates and is more susceptible to the insect herbivore Spodoptera littoralis. Plant J. 2008;55:774–786. doi: 10.1111/j.1365-313X.2008.03545.x. [DOI] [PubMed] [Google Scholar]
- 49.Cobbett C, Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- 50.Shanmugam V, Tsednee M, Yeh K-C. Zinc tolerance induced by iron 1 reveals the importance of glutathione in the cross-homeostasis between zinc and iron in Arabidopsis thaliana. Plant J. 2012;69:1006–1017. doi: 10.1111/j.1365-313X.2011.04850.x. [DOI] [PubMed] [Google Scholar]
- 51.Jobe TO, et al. Feedback inhibition by thiols outranks glutathione depletion: a luciferase-based screen reveals glutathione-deficient γ-ECS and glutathione synthetase mutants impaired in cadmium-induced sulfate assimilation. Plant J. 2012;70:783–795. doi: 10.1111/j.1365-313X.2012.04924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer AJ, et al. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J. 2007;52:973–986. doi: 10.1111/j.1365-313X.2007.03280.x. [DOI] [PubMed] [Google Scholar]
- 53.Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine) J. Biol. Chem. 1979;254:7558–7560. [PubMed] [Google Scholar]
- 54.Cobbett CS, May MJ, Howden R, Rolls B. The glutathione-deficient, cadmium-sensitive mutant, cad2–1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. Plant J. 1998;16:73–78. doi: 10.1046/j.1365-313x.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 55.Vernoux T, et al. The root meristemless1/cadmium sensitive2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell. 2000;12:97–109. doi: 10.1105/tpc.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noctor G, Queval G, Mhamdi A, Chaouch S, Foyer CH. Glutathione. The Arabidopsis Book. 2011;9:1–32. doi: 10.1199/tab.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maughan SC, et al. Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proc. Natl. Acad. Sci. USA. 2010;107:2331–2336. doi: 10.1073/pnas.0913689107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu YP, Li ZS, Rea PA. AtMRP1 gene of Arabidopsis encodes a glutathione S-conjugate pump: isolation and functional definition of a plant ATP-binding cassette transporter gene. Proc. Natl. Acad. Sci. USA. 1997;94:8243–8248. doi: 10.1073/pnas.94.15.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu Y-P, et al. AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione S-conjugates and chlorophyll catabolites: functional comparisons with AtMRP1. Plant Cell. 1998;10:267–282. doi: 10.1105/tpc.10.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tommasini R, et al. An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J. 1998;13:773–780. doi: 10.1046/j.1365-313X.1998.00076.x. [DOI] [PubMed] [Google Scholar]
- 61.Blum R, et al. Function of phytochelatin synthase in catabolism of glutathione-conjugates. Plant J. 2007;49:740–749. doi: 10.1111/j.1365-313X.2006.02993.x. [DOI] [PubMed] [Google Scholar]
- 62.Blum R, Meyer KC, Wunschmann J, Lendzian KJ, Grill E. Cytosolic action of phytochelatin synthase. Plant Physiol. 2010;153:159–169. doi: 10.1104/pp.109.149922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takahashi H, Kopriva S, Giordano M, Saito K, Hell R. Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011;62:157–184. doi: 10.1146/annurev-arplant-042110-103921. [DOI] [PubMed] [Google Scholar]
- 64.Shibagaki N, et al. Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J. 2002;29:475–486. doi: 10.1046/j.0960-7412.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- 65.Zuber H, et al. Sultr4;1 mutant seeds of Arabidopsis have an enhanced sulphate content and modified proteome suggesting metabolic adaptations to altered sulphate compartmentalization. BMC Plant Biol. 2010;10:1–13. doi: 10.1186/1471-2229-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.El Kassis E, et al. Characterization of a selenate-resistant Arabidopsis mutant. Root growth as a potential target for selenate toxicity. Plant Physiol. 2007;143:1231–1241. doi: 10.1104/pp.106.091462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kataoka T, et al. Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis. Plant Cell. 2004;16:2693–2704. doi: 10.1105/tpc.104.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parisy V, et al. Identification of PAD2 as a γ-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J. 2007;49:159–172. doi: 10.1111/j.1365-313X.2006.02938.x. [DOI] [PubMed] [Google Scholar]
- 69.Cairns NG, Pasternak M, Wachter A, Cobbett CS, Meyer AJ. Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiol. 2006;141:446–455. doi: 10.1104/pp.106.077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.May MJ, Vernoux T, Leaver C, Montagu MV, Inzé D. Glutathione homeostasis in plants: implications for environmental sensing and plant development. J. Exp. Bot. 1998;49:649–667. [Google Scholar]
- 71.Hothorn M, et al. Structural basis for the redox control of plant glutamate cysteine ligase. J. Biol. Chem. 2006;281:27557–27565. doi: 10.1074/jbc.M602770200. [DOI] [PubMed] [Google Scholar]
- 72.Jamai A, Tommasini R, Martinoia E, Delrot S. Characterization of glutathione uptake in broad bean leaf protoplasts. Plant Physiol. 1996;111:1145–1152. doi: 10.1104/pp.111.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lambrix V, Reichelt M, Mitchell-Olds T, Kliebenstein DJ, Gershenzon J. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell. 2001;13:2793–2807. doi: 10.1105/tpc.13.12.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urbancsok J, Bones AM, Kissen R. Glucosinolate-derived isothiocyanates inhibit Arabidopsis growth and the potency depends on their side chain structure. Int. J. Mol. Sci. 2017;18:2372. doi: 10.3390/ijms18112372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andersson MX, et al. Involvement of the electrophilic isothiocyanate sulforaphane in Arabidopsis local defense responses. Plant Physiol. 2015;167:251–261. doi: 10.1104/pp.114.251892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y. The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis. 2012;33:2–9. doi: 10.1093/carcin/bgr255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kawakishi S, Kaneko T. Interaction of oxidized glutathione with allyl isothiocyanate. Phytochemistry. 1985;24:715–718. doi: 10.1016/S0031-9422(00)84882-7. [DOI] [Google Scholar]
- 78.Wadleigh RW, Yu SJ. Detoxification of isothiocyanate allelochemicals by glutathione transferase in three lepidopterous species. J. Chem. Ecol. 1988;14:1279–1288. doi: 10.1007/BF01019352. [DOI] [PubMed] [Google Scholar]
- 79.Schramm K, Vassao DG, Reichelt M, Gershenzon J, Wittstock U. Metabolism of glucosinolate-derived isothiocyanates to glutathione conjugates in generalist lepidopteran herbivores. Insect Biochem. Mol. Biol. 2012;42:174–182. doi: 10.1016/j.ibmb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 80.Jeschke V, Gershenzon J, Vassão DG. A mode of action of glucosinolate-derived isothiocyanates: Detoxification depletes glutathione and cysteine levels with ramifications on protein metabolism in Spodoptera littoralis. Insect Biochem. Mol. Biol. 2016;71:37–48. doi: 10.1016/j.ibmb.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Klein AP, Sattely ES. Biosynthesis of cabbage phytoalexins from indole glucosinolate. Proc. Natl. Acad. Sci. USA. 2017;114:1910–1915. doi: 10.1073/pnas.1615625114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mugford SG, Lee B-R, Koprivova A, Matthewman C, Kopriva S. Control of sulfur partitioning between primary and secondary metabolism. Plant J. 2011;65:96–105. doi: 10.1111/j.1365-313X.2010.04410.x. [DOI] [PubMed] [Google Scholar]
- 83.Maruyama-Nakashita A, Inoue E, Watanabe-Takahashi A, Yamaya T, Takahashi H. Transcriptome profiling of sulfur-responsive genes in Arabidopsis reveals global effects of sulfur nutrition on multiple metabolic pathways. Plant Physiol. 2003;132:597–605. doi: 10.1104/pp.102.019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schnaubelt D, et al. Low glutathione regulates gene expression and the redox potentials of the nucleus and cytosol in Arabidopsis thaliana. Plant Cell Environ. 2015;38:266–279. doi: 10.1111/pce.12252. [DOI] [PubMed] [Google Scholar]
- 85.Vivancos PD, et al. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J. 2010;64:825–838. doi: 10.1111/j.1365-313X.2010.04371.x. [DOI] [PubMed] [Google Scholar]
- 86.Noctor G, et al. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012;35:454–484. doi: 10.1111/j.1365-3040.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- 87.Markovic J, et al. The depletion of nuclear glutathione impairs cell proliferation in 3t3 fibroblasts. PLoS ONE. 2009;4:e6413. doi: 10.1371/journal.pone.0006413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomas M, Nicklee T, Hedley DW. Differential effects of depleting agents on cytoplasmic and nuclear non-protein sulphydryls: a fluorescence image cytometry study. Br. J. Cancer. 1995;72:45–50. doi: 10.1038/bjc.1995.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koprivova A, Mugford ST, Kopriva S. Arabidopsis root growth dependence on glutathione is linked to auxin transport. Plant Cell Reports. 2010;29:1157–1167. doi: 10.1007/s00299-010-0902-0. [DOI] [PubMed] [Google Scholar]
- 90.Bashandy T, et al. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell. 2010;22:376–391. doi: 10.1105/tpc.109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schwarzländer M, et al. Confocal imaging of glutathione redox potential in living plant cells. J. Microsc. 2008;231:299–316. doi: 10.1111/j.1365-2818.2008.02030.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.