Abstract
Plasma prorenin is commonly elevated in diabetic patients and appears to predict the development of diabetic nephropathy. However, the pathological role of prorenin is unclear. In the present study, a transgenic, inducible, hepatic prorenin-overexpressing rat model was generated and the effect of prorenin in organ injury was examined. Four groups of rats (cyp1a1 prorenin transgenic male and female rats and non-transgenic littermates) were assigned to receive a diet containing 0.3% of the transgene inducer indole-3-carbinol (I3C) for 4 weeks. Plasma prorenin concentration was increased and mean arterial pressure (MAP) increased from 80 ± 18 to 138 ± 17 (mmHg), whereas renal prorenin/renin protein expression was unchanged, in transgenic rats fed with I3C diet. The intact prorenin, not renin, in plasma and urine samples was further observed by Western blot analysis. Importantly, transgenic rats with high levels of prorenin developed albuminuria, glomerular and tubulointerstitial fibrosis associated with increased expression of transforming growth factor β (TGFβ) 1 (TGFβ1), plasminogen activator inhibitor-1 (PAI-1), collagen, and fibronectin (FN). These rats also exhibited cardiac hypertrophy determined by echocardiography, with elevated ratio of heart weight to body weight (HW/BW). Cardiac collagen in interstitial and perivascular regions was prominent, accompanied by the increase in mRNA contents of atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), β-myosin heavy chain (β-MHC), TGFβ1, PAI-1, and collagen in the heart tissue. Furthermore, renal protein levels of p-NF-κB-p65 and monocyte chemoattractant protein-1 (MCP-1), NAPDH oxidases, malondialdehyde (MDA) and 8-isoprostane (8-IP), p-ERK, p-β-catenin, and p-Akt were dramatically increased in prorenin overexpressing rats. These results indicate that prorenin, without being converted into renin, causes hypertension, renal and cardiac fibrosis via the induction of inflammation, oxidative stress and the ERK, β-catenin, and Akt-mediated signals.
Keywords: cardiac disease, hypertension, Prorenin, renin, renal disease
Introduction
Elevated plasma prorenin levels are commonly found in diabetic patients and it has been known for many years that plasma prorenin levels correlate with microvascular diseases of diabetes, including nephropathy [1–5]. The mechanism that is responsible for the increase in plasma prorenin in diabetes is not apparent. Importantly, the potential role of prorenin in the pathogenesis of kidney disease is unclear.
Although there is no report of the relationship between prorenin and cardiac events or mortality, the major cause of morbidity and mortality in persons with diabetes is cardiovascular disease (CVD) [6]. Furthermore, myocardial hypertrophy, interstitial fibrosis, capillary endothelial changes, and capillary basal laminate thickening, observed in the heart associated with diabetes mirror what occurs in the kidneys of diabetics [7]. Although mechanisms responsible for these alterations occurring both in the heart and in the kidney might be similar, details remain incompletely understood.
A prorenin/renin receptor, (P)RR, has been identified. (P)RR is present not only on human mesangial cells (MCs) [8,9] but also in the subendothelium of arteries of the heart and kidney and other places. Prorenin binding to this receptor causes non-proteolytic activation of the pro-enzyme and generation of Ang I [9]. In addition, accumulated data in renal and cardiac cells indicated that receptor-bound prorenin further leads to the activation of intracellular signaling proteins, such as mitogen-activated protein kinases (MAPKs), and their downstream events, such as Hsp-27, transforming growth factor-β (TGFβ), and plasminogen activator inhibitor-1 (PAI-1) that are mediators of fibrosis, without angiotensin generation and was not altered by angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB) or direct renin inhibitors (DRI), such as aliskiren (it binds to renin and prorenin and prevents the conversion of AGT into Ang I) [10]. The capability of the prorenin-(P)RR binding for activating the pro-enzyme and intracellular signals involved in tissue damage has raised the expectation that prorenin may be directly responsible for renal and cardiac injury in disease including diabetes, without being converted into renin.
To examine the pathogenic role of elevated prorenin in vivo independent of hyperglycemia, early animal studies have shown that when plasma prorenin levels were markedly increased in transgenic rats engineered to secrete prorenin constitutively from the liver, glomerulosclerosis and cardiomyocyte hypertrophy occurred, even in the absence of elevated plasma renin levels [11]. However, results from the conditional cyp1a1 mouse ren-2 transgenic rats with increased plasma concentrations of mouse prorenin have been inconsistent, with some studies failing to observe histological signs of glomerulosclerosis or cardiac fibrosis [12–14]. The reasons for these inconsistencies are unclear but may be related to differences in species, i.e. prorenin does not work similarly in mice and rats.
In the present study, we thus generated inbred transgenic rats with conditional expression of rat prorenin within the same species primarily in the liver using the cytochrome P450 promoter, cyp1a1, to drive and direct expression of the rat prorenin gene to the liver. The transgene is induced by xenobiotics, such as indole-3-carbinol (I3C) [15], which act via the aryl hydrocarbon receptor. I3C is a naturally occurring agent found in cruciferous vegetables that acts as a benign inducer with a short biological half-life. Using this transgenic rat model, we elucidated the potential role of prorenin in the development of renal and cardiac pathology associated with human diabetes, where plasma prorenin levels are elevated.
Materials and methods
Unless specified, all reagents were purchased from Sigma Chemical Co. (St. Louis, Missouri, U.S.A.).
Animals
The breeding, maintenance, and all study procedures of animals described herein were performed according the guidelines of the Public Health Service Policy on Use of Laboratory Animals and were approved by the Animal Care Committee of the University of Utah.
Generation of transgenic rats
The rat cyp1a1 promoter was isolated by NotI-SalI double digestion of pAhIR1-lacZ (kindly provided by Dr John J. Mullins, University of Edinburgh) to yield an 11.5-kb fragment containing 5′-flanking regions exon 1 and part of exon 2 and cloned upstream of rat prorenin cDNA and an SV40 poly(A) signal in pBluescript SK2(+) (described in Supplementary Figure S1A). The injection fragment was excised by NotI-BssHII digestion (Supplementary Figure S1B), purified and introduced into WKY rat single-cell embryos by microinjection, which was performed in the University of Michigan Transgenic Animal Model Core (Ann Arbor, MI, U.S.A.). Founders were identified by PCR with the rat prorenin specific probes covering the cyp1a1 promoter and rat prorenin (sense, 5′GAGAGAGCAGCAGCACACA3′; antisense, 5′TGAACCCGTGTCAAAGAT-GA3′, the size of the PCR production was 511 bp) (Supplementary Figure S1C). Rat β-globin was amplified as the endogenous control (sense, 5′GCTTTCCAGCAGGCACTAAC3′; antisense, 5′TCAACAATCCACAGGGCA-TA3′, the size of the PCR production was 410 bp). Founders with normal blood pressure measured by tail-cuff method (MC4000 multi-channel blood pressure analysis system, Hatteras Instrument, Inc., Cary, NC, U.S.A.), normal urine volume, urine albumin/creatinine (A/C) value, and urine albumin excretion measured by the DC2000+ microalbumin reagent kit (Bayer Healthcare) were bred with wild-type WKY rats. Qualified transgenic F1 rats were then used to continually breed with wild-type WKY rats to establish the transgenic line. The transgenic animals were fertile and developed normally. Transgenic rats after F3 and qualified to be normotensive and absent for albuminuria were used for experiments described below. Transgenic rats, which exhibited varying degrees of hypertension and urinary albuminuria in the absence of inducer, were not studied further.
Induction of transgene expression by I3C
Cyp1a1 is not constitutively expressed but is highly induced when a basic helix–loop–helix transcription factor binds to specific DNA elements in the cyp1a1 promoter [16,17]. Effective induction of a cyp1a1 promoter-driven transgene by I3C (0.3% w/w) in cyp1a1 ren-2 transgenic rats has been previously demonstrated [14]. I3C (0.3% w/w) was then mixed with the rat standard chow by Harlan Laboratories (Harlan™, Madison, WI, U.S.A.) and given to transgenic rats and non-transgenic littermates.
Four groups of eight rats each (transgenic male and female rats and non-transgenic male and female littermates (10–12 weeks of age)) were assigned to 0.3% I3C diet for 4 weeks. At the same time, four groups of eight rats each (transgenic male and female rats, and non-transgenic male and female rats (10–12 weeks of age)) receiving standard food without I3C for 4 weeks served as controls. All animals were allowed free access to water. Twenty-four-hour urine was obtained from each rat after placement in metabolic cages at the end of each experiment. Urine A/C ratio was measured using the DC2000+ microalbumin reagent kit (Bayer Healthcare).
By the end of 4-week induction of transgene expression, rats were killed under isoflurane anesthesia. Three rats in each group were used for collection of blood samples from the tail vein and then received bilateral nephrectomy (BNX). After BNX for 24 h, 5–10 ml blood/each rat was drawn from the lower abdominal aorta to verify the excess of circulating prorenin of hepatic origin by Western blot assay compared with before BNX. The remaining five rats in each group were used for blood pressure measurement and collection of samples. Mean arterial pressure (MAP) was recorded by the Digi-Med Blood Pressure Analyzer™ (BPA-400, Micro-Med, Inc., Louisville, KY, U.S.A.) via lower abdominal aorta catheterization with heparin-filled pressure transducers (25 IU/ml). Five to ten milliliters blood was then drawn from the lower abdominal aorta and kidneys and other organs were perfused with 60 ml ice-cold PBS. One piece of renal cortical tissue and one piece of heart tissue (left ventricle) were fixed in 10% neutral-buffered formalin for histological examination. One piece of renal cortical tissue was snap-frozen for frozen sectioning. Other pieces of renal cortex, heart tissue, and liver tissue were harvested by dissection and stored in liquid nitrogen for Western blot or treated with Tri Reagent for isolation of RNA or treated with 100 mM NaCl and 20 mM HEPES to be sonicated for 30 s, three times on ice and centrifuged at 12000 rpm for 15 min at 4°C. The supernatant was then collected and stored at −80°C for transforming growth factor β1 (TGFβ1) ELISA (R&D Systems Inc., Minneapolis, MN, U.S.A.) and protein measurement by BCA protein assay kit (Pierce, Rockford, IL, U.S.A.).
Verifying prorenin expression in transgenic rats
Total RNA was isolated from renal cortex and liver tissue of transgenic rats and their wild-type littermates receiving I3C diet or standard diet to determine rat prorenin gene expression by RT-PCR as described below. The prorenin/renin protein in the plasma and renal tissue was observed by Western blot using the polyclonal rabbit anti-rat prorenin/renin IgG, which was generated against the whole rat prorenin protein described previously [18–20] and purified by using the protein A IgG purification kit (Pierce) and peptide affinity chromatography. The specificity of the antibody for detecting rat prorenin and renin was confirmed by Western blot assay.
To further verify if elevated plasma prorenin in induced transgenic rats originated from the liver, not the kidney, and if elevated circulating prorenin was able to be converted into renin, plasma at the same amount from each rat in a group before and after BNX was pooled to represent the individual group. The plasma and urine levels of prorenin and renin were observed by Western blot assay using the polyclonal anti-rat prorenin/renin antibody. Briefly, plasma samples (2 µl each) or individual urine samples (one-thousandth of the total 24 h urine volume in each rat) were subjected to SDS/PAGE in 4–12% gradient gel (Invitrogen, Carlsbad, CA, U.S.A.) and immunoblotting on immobilon-P transfer membranes (Millipore Corporation, Bedford, MA, U.S.A.). Proteins of prorenin/renin were assessed on the Western blots and the immunostaining band was visualized and quantitated as described below.
Determination of plasma renin/prorenin catalytic activity and plasma Ang I, Ang II, and aldosterone levels
The catalytic activity of plasma renin/prorenin (PRA) was determined by an Ang I generation assay [18]. Ang I was quantitated using a commercially available RIA kit (Phoenix Pharmaceuticals, Inc., Burlingame, CA, U.S.A.). Plasma Ang II and aldosterone levels were measured using the commercially available ELISA kits (Enzo Life Sciences, Inc., Farmingdale, NY, U.S.A. and Alpha Diagnostic Intl. Inc., San Antonio, TX, U.S.A.).
Determination of renal function
Plasma BUN and creatinine (Cr) concentrations were measured by using the QuantiChrom™ urea assay kit and Cr assay kit (BioAssay System, Hayward, CA, U.S.A.).
Histological analysis
Formalin-fixed renal cortex and heart tissues were subsequently embedded in paraffin. Three-micrometer sections were cut from these tissue blocks and stained with periodic acid-Schiff (PAS) and Masson’s trichrome (TRI) for kidney tissue, and Hematoxylin and Eosin (H&E) and TRI for heart tissue to evaluate gross morphology and fibrosis. To quantitate individual myocyte size and cardiac collagen volume, left ventricular (LV) heart tissue sections were stained with FITC-conjugated wheat germ agglutinin (WGA) (Invitrogen, Carlsbad, CA, U.S.A.) and Picrosirius Red (PSR). The PAS-positive glomerular extracellular matrix (ECM) was quantitated in a blinded fashion by a computer-assisted color image analysis system as previously described [21]. At least 20 glomeruli from each individual rat were assessed under high magnification (400×). The PAS-positive material area in the mesangium was normalized by that of the total glomerular tuft where the percentage of mesangial matrix occupying each glomerulus area was rated. The average glomerular sclerosis was obtained by averaging scores from all glomeruli on one section. TRI stained kidney tissue sections were viewed with bright field illumination at 200× magnification. Five non-overlapping fields of renal interstitial area were scored using ImageJ software by quantitating the stained collagen content (in percentage) relative to that of entire chosen tissue area. The mean cardiomyocyte area was evaluated by measurement of 100 cells per heart (five hearts per experimental group) or five non-overlapping fields of cardiac interstitial area or perivascular area in PSR stained tissue sections with ImageJ. All examinations were performed independently in a blinded manner by two individuals.
Immunofluorescent staining for fibronectin (FN) and type I collagen (Col I) was performed on frozen renal sections and evaluated in 20 glomeruli from each rat as described [21]. Intraglomerular positive staining of FN and Col I was quantitated separately in a blinded fashion by a computer-assisted method as described previously [21].
Western blot analysis
Renal cortex tissue (15 mg) from each rat was homogenized in lysis buffer (Cell Signaling Technology, Inc., Beverly, MA, U.S.A.) with 1% NP40, 1 mM PMSF, and 1 tablet/5 ml protease inhibitor mix (Complete, Mini; Roche Diagnostics Corporation, Indianapolis, IN). Protein sample at the same amount from each rat in a group was pooled to represent the individual group for further examination. For Western blot analysis, protein samples (20 µg each) were subjected to SDS/PAGE in 4–12% gradient gel (Invitrogen, Carlsbad, CA, U.S.A.) and immunoblotting on immobilon-P transfer membranes (Millipore Corporation, Bedford, MA, U.S.A.). Proteins of prorenin/renin, PAI-1, FN, Col I, sirtuin 1 (SirT1), p-nuclear factor κ-B p65 subunit (p-NF-κB-p65), NADPH oxidase gp91phox (Nox2), p47phox, Nox4, p-β-catenin, β-catenin, GSK-3β, p-GSK-3β, phosphoinositide 3-kinase (PI3K)-p85, pAkt, phosphorylated and total ERK1/2 were assessed on the Western blots and the immunostaining band was visualized and quantitated as described previously [22–24].
RNA preparation and real-time RT-PCR assay
Total RNA was extracted from renal cortex tissue or heart tissue using Tri reagent according to the manufacturer’s instructions. Two micrograms of total RNA were reverse-transcribed using the Superscript III First-strand Synthesis System for RT-PCR kit (Invitrogen). Real-time RT-PCR was performed using the SYBR green dye I (Applied Biosystems, Foster City, CA, U.S.A.) and the ABI 7900 Sequence Detection System (Applied Biosystems) as described previously [25]. Samples were run as triplicates in separate tubes to permit quantitation of the target gene normalized to GAPDH. Sequences of primers used for fibrotic markers such as TGFβ1, PAI-1, FN and Col, (pro)renin, (P)RR, β-actin, and GAPDH were described previously [24,26]. Sequences of new primers used as the molecular markers of pathologic cardiac hypertrophy such as atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), α-myosin heavy chain (α-MHC), β-myosin heavy chain (β-MHC), type III collagen, and markers of oxidative stress and inflammation such as Nox2, Nox4, endothelin-1 (ET-1), NF-κB-p65, interleukin-6 (IL-6), and intercellular adhesion molecule-1 (ICAM-1) are listed in the Supplementary Table S1. The specificity of the PCR products was confirmed on a 1.5% agarose gel by showing a specific single band with the expected size.
Measurement of urinary monocyte chemoattractant protein-1, thiobarbituric acid reactive substances, and 8-isoprostane levels
Twenty-four-hour urine collected from individual rats at the end of the experimental protocol was analyzed for the levels of monocyte chemoattractant protein-1 (MCP-1), malondialdehyde (MDA), and 8-isoprostane (8-IP) by using a commercially available ELISA kit (R&D Systems or Cayman Chemical Company, Ann Arbor MI, U.S.A.). MDA was estimated by determining thiobarbituric acid (TBA)-reactive substances (TBARS) that are derived from lipid hydroperoxides produced by oxidative stress that reacts with TBA, using a colorimetric assay (Cayman Chemical Company). TBARS concentration was obtained from a standard curve produced by serially diluting MDA. Urinary MCP-1 and TBARS excretion were corrected by urinary Cr levels. Urinary 8-IP concentration was expressed as the total amount excreted in 24 h.
Statistical analysis
Data are expressed as mean ± S.D. with n representing the number of animals. Groups were analyzed by one-way ANOVA and subsequent Student–Newman–Keuls or Dunnett’s testing for multiple comparisons. P<0.05 was considered statistically significant.
Results
Continuous induction of prorenin
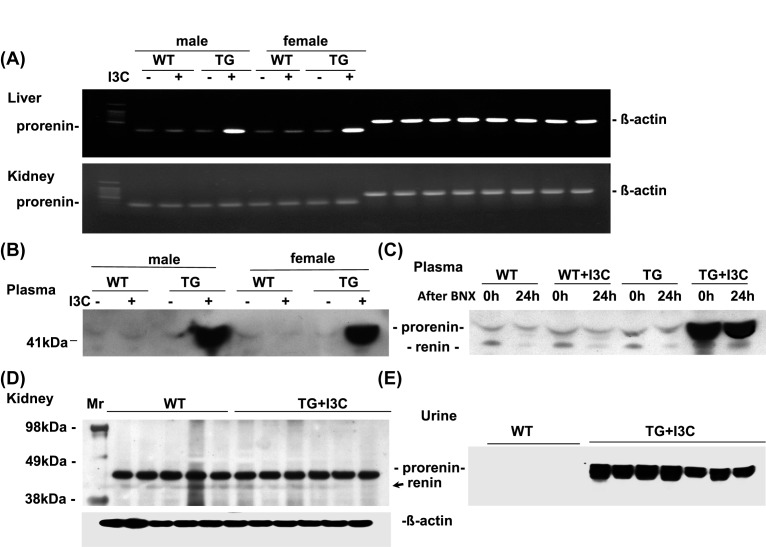
Transgenic and non-transgenic male and female rats were induced with 0.3% dietary I3C for 4 weeks. The induction of prorenin mRNA expression by I3C was observed in the liver tissue, but not in the kidney tissue, of the transgenic animals (Figure 1A). The induction of transgene expression by I3C was associated with a significant increase in plasma prorenin levels detected by Western blot assay (Figure 1B). Moreover, at 24 h after BNX, the induced plasma prorenin by I3C in transgenic rats did not fall (Figure 1C). Plasma renin levels were not increased in these transgenic rats fed by I3C with or without BNX, compared with non-transgenic controls. These increases in plasma prorenin levels were not observed in transgenic rats receiving the normal diet without I3C induction or in any wild-type rats. There was no increase either in renal prorenin/renin production in these transgenic rats fed by I3C (Figure 1D). Interestingly, the intact prorenin but no renin was observed in the urine samples in transgenic rats fed with I3C compared with non-transgenic controls (Figure 1E). These results indicate that the engineered overexpression of prorenin from the liver of transgenic rat after I3C induction results in exclusive release of unprocessed prorenin into circulation. The increased prorenin might be filtered by kidney and was excreted in urine. These prorenin molecules were not converted into renin in circulation since the necessary cleavage enzyme is absent from the circulation [27].
Figure 1. Expression of transgene prorenin.
Prorenin/renin was measured in the liver and kidney tissues from wild-type (WT) and transgenic rats (TG) fed with I3C (+) or without I3C (−) by RT-PCR (n=5/each group) (A), in plasma (n=5/each group) (B), renal cortex tissue (n=5/each group) (D), and urine samples (n=5/each group) (E) by Western blot assay. Further, 24 h after BNX, plasma prorenin/renin levels were determined by Western blot assay (C) (n=3/each group). β-actin served as control.
Systemic effect of elevated prorenin
Adding I3C diet did not alter MAP levels measured by the Digi-Med Blood Pressure Analyzer™ via lower abdominal aorta catheterization in non-transgenic control littermates. MAP in non-induced cyp1a1-prorenin male and female rats remained within the normotensive range. In contrast, I3C induction resulted in a rise in MAP from 80.6 to 137.8 mmHg (P<0.01) in male cyp1a1-prorenin rats and a rise in MAP from 103.8 to 159.6 mmHg (P<0.01) in female cyp1a1-prorenin rats (Table 1). These data demonstrate that high levels of prorenin in the circulation result in an increase in blood pressure.
Table 1. Parameters of the experimental groups of rats.
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| WT rats | WT rats | TG rats | TG rats | WT rats | WT rats | TG rats | TG rats | |
| With 13C diet | With 13C diet | With 13C diet | With 13C diet | |||||
| MAP (mmHg) | 77.0 ± 5.8 | 86.2 ± 8.2 | 80.6 ± 18.8 | 137.8 ± 17.1* | 89.6 ± 6.7 | 84.7 ± 17.2 | 103.8 ± 18.1 | 159.6 ± 27.0† |
| Plasma BUN (mg/dl) | 47.0 ± 17.2 | 42.5 ± 11.1 | 49.1 ± 13.6 | 69.3 ± 7.5* | 38.0 ± 10.6 | 32.3 ± 24.7 | 39.1 ± 5.8 | 108.7 ± 37.6† |
| Plasma Cr (mg/dl 100g BW) | 0.307 ± 0.1 | 0.314 ± 0.1 | 0.340 ± 0.0 | 0.694 ± 0.3* | 0.346 ± 0.1 | 0.276 ± 0.06 | 0.252 ± 0.2 | 0.812 ± 0.2† |
| Urine A/C (mg/g) | 28.0 ± 7.8 | 53.8 ± 35.2 | 34.2 ± 21.0 | 1282.4 ± 436.2* | 45.5 ± 35.8 | 76.6 ± 10.5 | 59.4 ± 46.1 | 805.4 ± 229.8† |
| KW/BW (mg/g) | 3.8 ± 0.5 | 3.9 ± 0.3 | 3.8 ± 0.5 | 7.3 ± 0.6* | 3.9 ± 0.2 | 4.0 ± 0.4 | 3.9 ± 0.5 | 7.0 ± 0.9† |
| HW/BW (mg/g) | 3.3 ± 0.5 | 3.5 ± 0.3 | 3.6 ± 0.7 | 6.6 ± 1.2* | 3.5 ± 0.1 | 3.6 ± 0.2 | 3.5 ± 0.1 | 6.6 ± 0.3† |
Wild-type (WT) and transgenic (TG) rats were treated with either normal diet or normal diet with 0.3% I3C (n=5 for each group) for 4 weeks. Plasma Cr levels were corrected by BW.
Abbreviations: BW, body weight; HW/BW, ratio of heart weight to body weight; KW/BW, the ratio of kidney weight to body weight.
*P<0.05 compared with male WT rats; †P<0.05 compared with female WT rats.
Plasma BUN and Cr concentrations of non-induced cyp1a1-prorenin rats including male and female rats were identical to those of non-transgenic control littermates. However, plasma BUN and Cr levels in I3C-induced cyp1a1-prorenin rats were significantly higher than those of control littermates, consistent with impaired kidney function (Table 1). I3C-induced female cyp1a1-prorenin rats may have had more severe kidney injury since their plasma BUN and Cr levels were increased more than those of the male rats.
Of note, at the end of 4-week induction of I3C, a marked elevation of urine A/C value by more than ten-fold compared with non-induced transgenic rats was observed in I3C-induced cyp1a1-prorenin rats. The urine A/C value in non-transgenic controls was not increased with I3C diet and remained similar to the values seen in those rats with normal diet. These data indicate that high levels of prorenin in the circulation result in albuminuria.
Organ effect of elevated prorenin
To determine if high levels of circulating prorenin can contribute to tissue remodeling or injury, we compared the kidneys and hearts from the I3C-induced cyp1a1-prorenin transgenic rats with those of non-transgenic littermates.
After 4-week induction of prorenin, the ratio of kidney weight to body weight (KW/BW) was increased in both male and female cyp1a1-prorenin transgenic rats (Table 1). Representative glomeruli stained with PAS are shown in Figure 2A. The glomeruli of I3C induced both male and female transgenic rats demonstrated increased glomerulosclerosis with evident accumulation of PAS-positive ECM proteins (Figure 2A) including FN (Figure 2B) and collagen I (Figure 2C) in the mesangium compared with glomeruli of non-transgenic controls or non-induced transgenic controls. Furthermore, I3C-induced cyp1a1-prorenin transgenic rats also had increased deposition of collagen in renal tubulointerstitial area compared with non-transgenic controls or non-induced transgenic controls, as determined by TRI staining where collagen was stained blue and quantitated by computer-assisted color image analysis (Figure 3A,B).
Figure 2. Effect of elevated prorenin on renal glomerulosclerosis.
Representative photomicrographs of glomeruli stained with PAS staining (A) and glomerular immunofluorescent staining for FN (B) and Col I (C) from male and female wild-type (WT) and transgenic (TG) rats fed with I3C or without I3C. Magnification 400×. Graphic quantitative evaluations of glomerular matrix score, FN staining score, and Col I staining score are shown on the right. *P<0.05 compared with male WT rats fed without I3C. #P<0.05 compared with female WT rats fed without I3C. n=5/each group.
Figure 3. Effect of elevated prorenin on renal tubulointerstitial fibrosis.
(A) Representative photomicrographs of renal tubulointerstitial fibrosis stained with TRI (200× magnification) from male and female wild-type (WT) and transgenic (TG) rats fed with I3C or without I3C. (B) Graphic representation of renal tubulointerstitial collagen staining score of each group. *P<0.05 compared with male WT rats fed without I3C. #P<0.05 compared with female WT rats fed without I3C. n=5/each group.
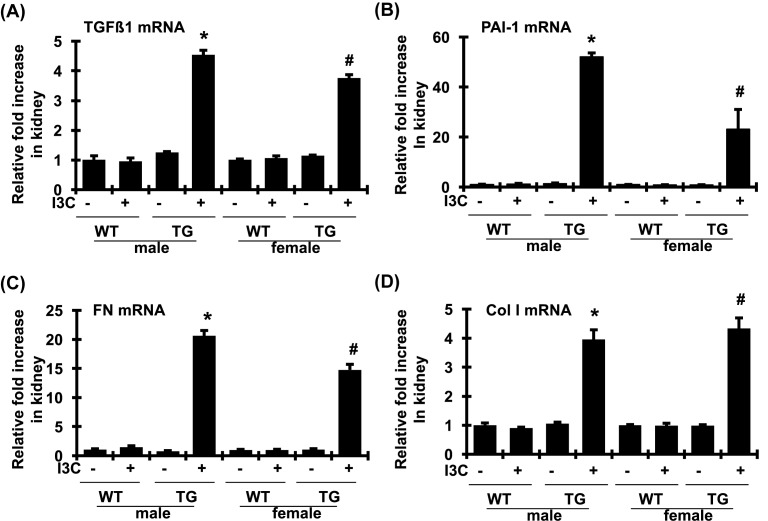
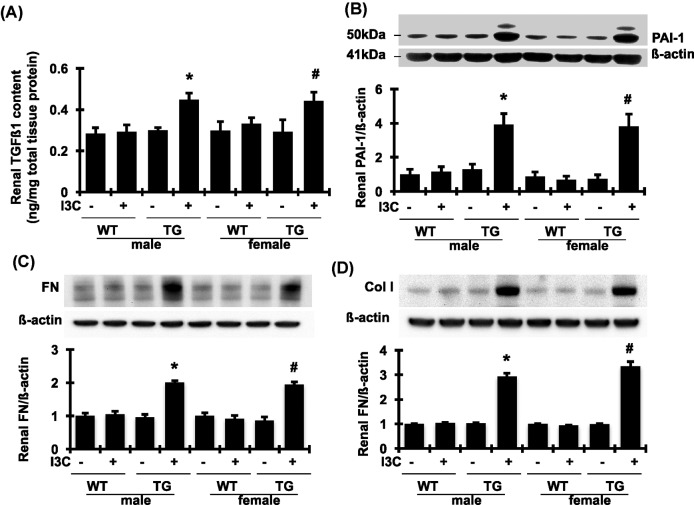
As two key modulators of matrix accumulation, both TGFβ1 and PAI-1 play an important role in fibrosis in both kidney and heart tissues. As shown in Figure 4A,B, renal mRNA expression of TGFβ1 and PAI-1 was increased by 4.4- and 49.6-fold, respectively, in I3C-induced cyp1a1-prorenin transgenic male rats, compared with those of male control littermates. A similar elevation of renal mRNA expression of TGFβ1 and PAI-1 was also observed in I3C-induced female transgenic rats but not in female control rats. In addition, the mRNA levels of FN and Col I in renal cortex tissue, the major ECM proteins that contribute to renal fibrosis, were also significantly increased in I3C-induced cyp1a1-prorenin transgenic male and female rats, compared with those of control littermates (Figure 4C,D). In agreement with the levels of mRNA expression, the levels of TGFβ1, PAI-1, FN, and Col I in renal cortex were markedly increased in I3C-induced cyp1a1-prorenin transgenic male and female rats, compared with non-transgenic control rats (Figure 5A–D). Thus, I3C-induced cyp1a1-prorenin transgenic rats developed renal hypertrophy, glomerulosclerosis, and tubulointerstitial fibrosis in the kidney associated with significantly increased mRNA expression and protein production of TGFβ1, PAI-1, FN, and Col I following exposure to the high circulating levels of prorenin.
Figure 4. Changes of profibrotic molecules in mRNA expression in renal cortex tissue.
mRNA expression of TGFβ1 (A), PAI-1 (B), FN (C), and Col I (D) was determined by real time RT-PCR. mRNA values were normalized by the levels of GAPDH mRNA and then expressed relative to the mean value for the wild-type (WT) control group. *P<0.05, compared with male WT rats fed without I3C. #P<0.05, compared with female WT rats fed without I3C. n=5/each group.
Figure 5. Changes of profibrotic molecules in protein production in renal cortex tissue.
Renal content of total TGFβ1(A) determined by ELISA and PAI-1 (B), FN (C), and Col I (D) determined by Western blot assay was significantly increased in both male and female transgenic (TG) rats fed with I3C for 4 weeks. Protein values of PAI-1, FN, and Col I are expressed relative to the wild-type (WT) control fed without I3C, which was set at unity. *P<0.05, compare with male WT rats fed without I3C. #P<0.05, compared with female WT rats fed without I3C. n=5/each group.
Similarly, at the end of the experiment, I3C-induced cyp1a1-prorenin transgenic male and female rats also both developed a greater than 80% increase in the ratio of heart weight to body weight (HW/BW) relative to the non-induced transgenic controls or non-transgenic controls (Table 1). Importantly, the pattern of LV remodeling in response to 4 weeks of high levels of prorenin correlated with the increased HW/BW in induced transgenic rats. Functional assessment using load-sensitive parameters obtained by non-invasive echocardiography (Figure 6A,B) demonstrated maintained cardiac function in all rats, as evidenced by preserved fractional shortening (FS; 34.51 ± 5.65% compared with 38.93 ± 4.79%, P>0.05). However, the interventricular septum (IVS) thickness, an indicator of LV hypertrophy, was significantly greater in I3C-induced transgenic rats than in non-transgenic rats. Consistently, the LV end-diastolic dimension (LVDd) was greater but the LV end-diastolic and end-systolic internal dimensions (LVID-D and LVID-S) were smaller in I3C-induced transgenic rats than in non-transgenic rats. I3C-induced transgenic rats also showed a small but significant increase in LV posterior wall (LVPW) thickness. Taken together, these parameters revealed that I3C-induced cyp1a1-prorenin transgenic rats after exposure to the high circulating levels of prorenin for 4 weeks developed concentric cardiac hypertrophy, without ventricular dilation or overt heart failure.
Figure 6. Echocardiography at 4 weeks after induction of prorenin overexpression.
(A) Representative M-mode findings and B-mode findings in the wild-type (WT + I3C) and prorenin transgenic (TG) rats fed with 0.3% I3C (TG + I3C). (B) The detailed echocardiography changes (n=5/each group). *P<0.05 compared with WT + I3C rats.
Histological examination of the hearts of the I3C-induced transgenic rats confirms the cardiac hypertrophy (Figure 7), as evidenced by an increase in cardiomyocyte cross-sectional area determined by H&E staining and WGA staining, compared with non-transgenic controls. We then evaluated the cardiac fibrosis in response to chronic pressure overload. Heart sections were stained with TRI and PSR to assess the extent of fibrosis, which was quantitated as the LV collagen volume. I3C-induced transgenic rats exhibited a significant increased extent of cardiac interstitial and perivascular fibrosis staining compared with non-transgenic controls 4 weeks after prorenin induction.
Figure 7. Histological changes in the heart.
(A) Representative images of heart sections subjected to H&E, WGA, TRI, and PSR staining (200× magnification) from the left ventricles of wild-type (WT + I3C) and prorenin transgenic (TG) rats fed with 0.3% I3C (TG + I3C). (B) The statistical results of cardiomyocyte cross-sectional areas, LV collagen volume and perivascular collagen volume in each group (n=5/each group). *P<0.05 compared with WT + I3C rats.
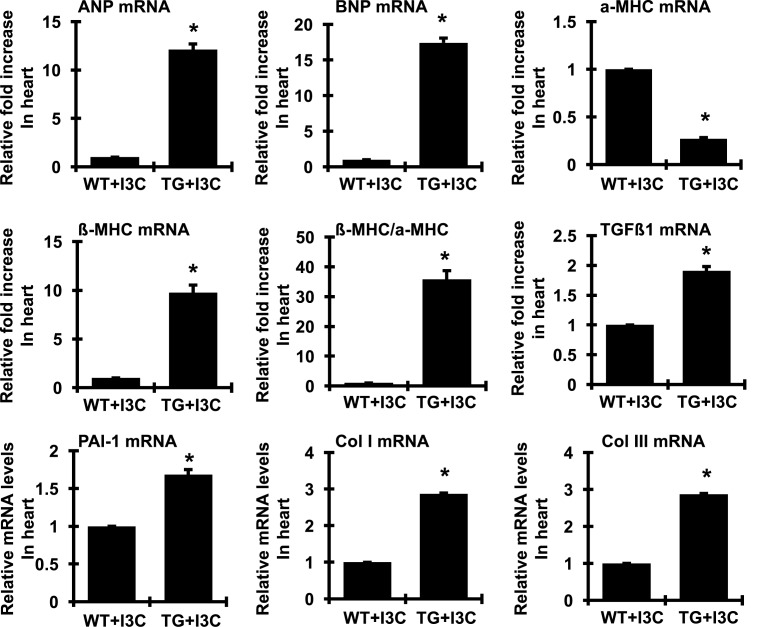
To determine the expression of molecular genes previously shown to be involved in the progression of cardiac hypertrophy and fibrosis, we conducted real time RT-PCR of total cardiac RNA. As expected and shown in Figure 8, cardiac ANP, BNP, and the fetal type MHC, β-MHC, mRNA expressions were markedly induced in I3C-induced transgenic rats. These changes were accompanied by a concomitant reduction in the adult type α-MHC, mRNA levels with a significant switch toward the fetal pattern of gene expression, manifested by a significant increase in the β-MHC:α-MHC ratio. TGFβ1 is a multifunctional growth factor that becomes elevated in pathological fibrosis as seen in kidney tissue and recent studies have shown that TGFβ1 is also an important mediator of cardiac hypertrophy [28]. As shown in Figure 8, TGFβ1 expression in the cardiac tissue of the I3C-induced transgenic rats was increased, to almost two-times that of non-transgenic controls. In addition, cardiac PAI-1, and Col I and type III collagen (Col III) mRNA levels, molecular markers of fibrosis, were increased in I3C-induced cyp1a1-prorenin transgenic rats, compared with non-transgenic controls (Figure 8). Thus, I3C-induced cyp1a1-prorenin rats also developed cardiac hypertrophy, which was accompanied by prominent cardiac interstitial and perivascular fibrosis.
Figure 8. Expression of hypertrophic and profibrotic genes in the hearts of wild-type (WT) and prorenin transgenic rats fed with I3C (TG) for 4 weeks.
The amount of mRNA for the indicated proteins in the left ventricle was determined by real time RT-PCR. mRNA values were normalized by the levels of GAPDH mRNA and then expressed relative to the mean value for the WT control group (n=5/each group). The switch toward the fetal-like state was indicated by a change in the β-MHC:α-MHC ratio. *P<0.05 comapared with WT control group.
Together, these results indicate that high levels of circulating prorenin cause arterial hypertension, renal and cardiac fibrosis in both male and female I3C-fed transgenic rats.
Effects of elevated prorenin on plasma renin/prorenin catalytic activity, Ang I, Ang II, and aldosterone levels
As shown in Figure 9, plasma catalytic activities of PRA and plasma Ang I, Ang II, and aldosterone levels were dramatically increased in in I3C-fed transgenic rats, compared with I3C-fed wild-type rats.
Figure 9. Plasma renin/prorenin catalytic activities and content of Ang I, Ang II, and aldosterone in wild-type (WT + I3C) and prorenin transgenic (TG) rats fed with I3C (TG + I3C) for 4 weeks (n=10/each group).
*P<0.05 compared with WT control group.
Signal transduction involved in elevated prorenin-induced organ injury
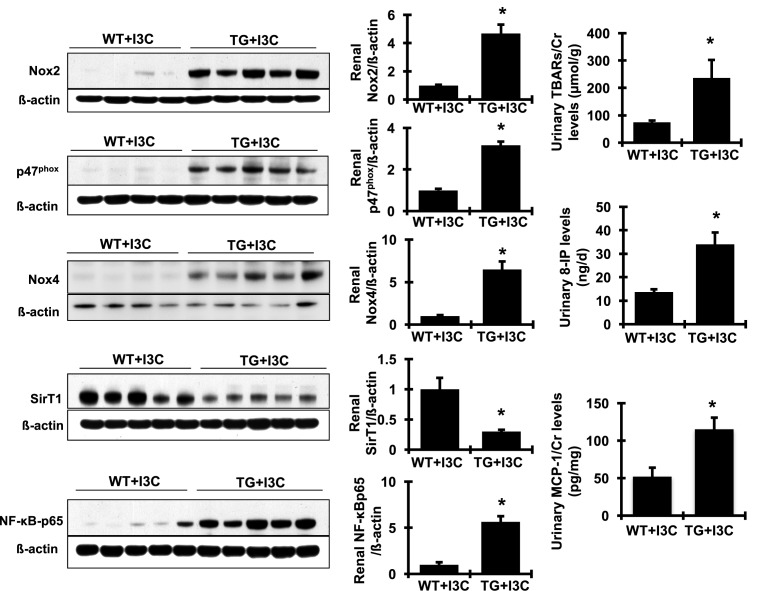
The involvement of oxidative stress and inflammation in hypertension and in renal disease and CVD has been intensively studied for almost three decades. Increasing observations have revealed a close relationship between oxidative stress and RAS overactivation. We thus investigated whether either oxidative stress or inflammation pathway were involved in elevated prorenin-promoted hypertension and renal and cardiac fibrosis in cyp1a1-prorenin transgenic rats. As shown in Figure 10, protein production of Nox2 and its regulatory subunit, p47phox, and Nox4, the family members of NAPDH oxidase in renal cortex tissue, were much greater in I3C-induced cyp1a1-prorenin transgenic rats, compared with non-transgenic counterparts, indicating significant activation of Nox2 and Nox4 in kidney tissue. Lipid peroxidation, a marker of oxidative stress, was measured by TBARS levels. In parallel with renal activation of NAPDH oxidase, urine levels of TBARS were consistently and dramatically increased in I3C-induced cyp1a1-prorenin transgenic rats compared with non-transgenic controls. Furthermore, urine levels of 8-IP, which is produced in vivo through free radical-catalyzed peroxidation of arachidonic acid and reflects oxidative stress, were also elevated in transgenic rats overexpressing prorenin compared with non-transgenic controls. Accordingly, renal production of p-NF-κB-p65 and urine levels of MCP-1 (Figure 10) were increased markedly in I3C-induced cyp1a1-prorenin transgenic rats, compared with non-transgenic controls. In contrast, SirT1 is an NAD+-dependent protein deacetylase, which has been shown to suppress NF-κB signaling through deacetylation of the p65 subunit of NF-κB [29]. As expected, I3C-induced prorenin transgenic rats had significant reduction in renal production of SirT1 compared with wild-type controls (Figure 10). It is likely that the decreased SirT1 may contribute to NF-κB-p65 activation and subsequent NF-κB-mediated inflammation. Similarly, mRNA expression of the common markers of oxidative stress and inflammation in the heart, such as Nox2, Nox4, ET-1, NF-κB-p65, IL-6, and ICAM-1, was also significantly increased in I3C-induced cyp1a1-prorenin transgenic rats compared with non-transgenic controls (Figure 11). Together, these results indicate that increased prorenin markedly enhances oxidative stress and inflammation both in the kidney and in the heart in I3C-induced cyp1a1-prorenin transgenic rats.
Figure 10. Production of related signaling molecules involved in tissue oxidative stress and inflammation in the kidney.
Representative Western blots illustrated protein expression of Nox2, p47phox, Nox4, SirT1, NF-κB-p65, and β-actin in the renal cortex tissue. Quantitation of the band density is shown on the right of Western blot (n=4 or 5). Protein values are expressed relative to the wild-type (WT + I3C) control, which was set at unity. Urine levels of TBARS were determined by a colorimetric assay and urine levels of 8-IP and MCP-1 were determined by ELISA (n=10/each group). *P<0.05 compared with WT + I3C rats.
Figure 11. mRNA expression of related signaling molecules involved in tissue oxidative stress and inflammation in the heart was determined by real time RT-PCR.
mRNA values were normalized by the levels of GAPDH mRNA and then expressed relative to the mean value for the WT control group (n=5/each group). *P<0.05 compared with WT + I3C rats.
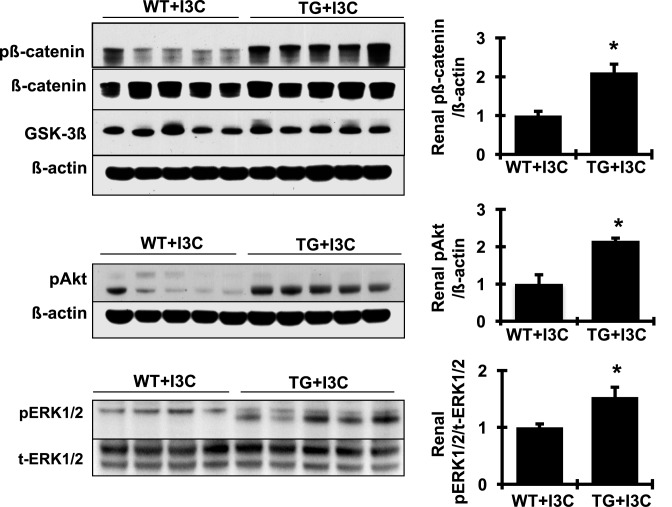
In addition, many specific cellular/molecular pathways including the mitochondrial pathways, PI3K/Akt, mammalian target of rapamycin (mTOR), and the canonical Wnt/β-catenin signaling pathway have also been shown to be involved in the progression of both renal and cardiac hypertrophy and fibrosis [30–33]. We asked which of these molecular pathways might be implicated in elevated prorenin-promoted organ injury in cyp1a1-prorenin transgenic rats. As shown in Figure 12, protein levels of p-β-catenin, pAkt, and p-ERK1/2, not the total β-catenin protein or the total ERK1/2, were dramatically increased in the kidney in I3C-induced cyp1a1-prorenin transgenic rats, compared with non-transgenic controls. These results indicate that increased prorenin not only enhances oxidative stress and inflammation, but also activates cellular MAPK/ERK, β-catenin, and Akt related signal pathways per se, which may contribute to prorenin-induced hypertension and organ injury in these transgenic rats.
Figure 12. Representative Western blots illustrated protein expression of p-β-catenin, total β-catenin, GSK-3β, pAkt and β-actin and pERK1/2, and total ERK1/2 in the renal cortex tissue.
Quantitation of the band density is shown on the right of blots (n=4 or 5). Protein values are expressed relative to the wild-type (WT + I3C) control, which was set at unity. *P<0.05 compared with WT + I3C rats.
Discussion
The present study reveals that an excess of circulating rat prorenin of hepatic origin within the same species can be induced by oral administration of I3C to cyp1a1 prorenin transgenic rats. These prorenin molecules are enzymatically active against their substrate in the circulation and result in elevated plasma Ang I, Ang II, and aldosterone contents. Moreover, the activation of prorenin is not due to proteolytic cleavage of prorenin since the intact prorenin was still present in the circulation, filtered by kidney and secreted in urine and no renin conversion was found in the circulation and kidney by the end of the experiment. Importantly, these transgenic rats with induced high levels of circulating prorenin have hypertension, albuminuria, and renal and cardiac hypertrophy, and fibrosis accompanied by locally activated oxidative stress, inflammation, and cellular MAPK/ERK, β-catenin, and Akt related signals. Our studies demonstrate a direct role of prorenin in the development of hypertension and renal and cardiac pathology that may be independent of renin.
Previously, TGR(mRen2)27 transgenic rats that were generated by random integration of the mouse Ren2 renin gene and its flanking sequences into the rat genome have been shown to be severely hypertensive with target organ injury [34]. In these transgenic rats, the transgene expression was found and varied in different organs including kidneys [34]. Therefore, an enhanced production of active renin as the cause for the hypertensive phenotype and organ damage in the TGR(mRen2)27 transgenic rat model cannot be excluded. In order to understand the contribution of elevated prorenin, not renin, in organ damage, Mullins et al. designed and generated the TGR(hAT-rpR) rat model in which the rat prorenin gene expression was directed to the liver constitutively under the control of the human a1-antitrypsin (hAT) promoter [11,35]. Since renal juxtaglomerular cells are the only known sites of production of renin and removal of the kidneys cause active renin to disappear from the circulation [36], it is believed that no prorenin-renin conversion occurs outside the kidney. The vasculotoxic effect of the overexpressed prorenin of hepatic origin observed in the TGR(hAT-rpR) rat model, including hypertension and/or cardiac and renal hypertrophy and fibrosis, was believed to be independent of renin. Instead of constitutively overexpressing rat prorenin in rats, an inducible liver promoter that regulates the release of mouse Ren-2 prorenin into the circulation of rats was generated recently [12–15]. Although cardiac or kidney fibrosis was not observed in the cyp1a1 Ren-2 transgenic rats that received the low concentration I3C (0.125%) diet, this contrasted with rats that received the higher concentration I3C (0.3%) diet. These animals with increased circulating prorenin, with no change in renin, still had a severe hypertension that was attributed to the increased generation of Ang II [12,13]. Taken together, the elevated catalytic activity of overexpressed prorenin no matter whether from overexpressed rat prorenin or the mouse Ren-2 gene was observed consistently. These prior reports are in agreement with those from our inducible prorenin transgenic rat model, and may possibly be independent of renin.
Activation of prorenin can be proteolytic or non-proteolytic. Proteolytic activation of prorenin involves removal of the propeptide (conversion of prorenin into renin). Non-proteolytic activation of prorenin may occur when the inhibitory propeptide presumably becomes diverted from the enzymatic active sites, resulting in an open conformation that is capable of converting AGT into Ang I [37]. Since no renin conversion was detected in either the current cyp1a1 prorenin transgenic rat model or previous TGR(hAT-rpR) rat model or cyp1a1 Ren-2 transgenic rat model, prorenin in these transgenic rats could possibly be activated in a non-proteolytic manner. It has been shown that non-proteolytic action of prorenin can be induced by exposure to low pH or cold temperature. Consequently, under regular measurement <2% of prorenin is activated by temperature- and pH-mediated non-proteolytic activation [37]. Based on angiotensin generation, the catalytic activity of plasma prorenin/renin in I3C-induced cyp1a1 prorenin transgenic rats is approximately 30-fold that of non-transgenic controls, similar to other transgenic rats, much higher than the expected 2% activity of total soluble prorenin. In addition, a number of recent observations in vitro have revealed that prorenin activation involves binding the (p)RR, which may induce unfolding of the propeptide of the prorenin exposing the enzymatic cleft and thereby allowing prorenin to cleave AGT locally [38–42]. Consistent with these in vitro results are data also from studies in double-transgenic mice overexpressing human native prorenin and human AGT [43]. When transgene expression was targetted specifically to the somatotrophs of the pituitary gland by putting the human prorenin and AGT cDNAs under the transcriptional control of the rat growth hormone gene promoter, both human prorenin and AGT mRNAs were detectable only within the pituitary gland of the transgenic mouse. These double-transgenic mice had elevated pituitary Ang I content, which suggests that human prorenin is enzymatically active against its substrate within the mouse pituitary gland [43]. Notably, when mice expressing human AGT were crossed with a line expressing a human mutant prorenin that was non-cleavable, the mice still had elevated Ang I content in the pituitary gland suggesting that prorenin activation within the pituitary gland is not due to proteolytic cleavage of prorenin [43]. Based on the fact that (P)RR is widely distributed in the body including the pituitary gland, heart, and kidneys [44], it is likely that (P)RR-mediated non-proteolytic activation of prorenin may be involved. Furthermore, a truncated extracellular N-terminal part of (P)RR called the soluble (P)RR was found in rat and human plasma and was able to bind prorenin [45]. Whether the circulating soluble (P)RR contributes to prorenin binding and activation in these prorenin transgenic rats remains to be determined. Nonetheless, it appears that the activation of Ang II-forming pathways by prorenin promoted hypertension in the constitutive or inducible prorenin transgenic rats including our I3C-induced cyp1a1 prorenin transgenic rat model. Moreover, it has been well established that Ang II is a pleiotropic peptide that plays a critical role in the pathogenesis of organ damage through stimulating the generation of reactive oxygen species, promoting inflammation and activating multicellular signaling [46,47]. It is perhaps therefore not surprising that increased Ang II generation induced by prorenin overexpression is actively involved in local activation of oxidative stress, inflammation, and cellular MAPK/ERK, β-catenin, and Akt related signal pathways and organ damage such as renal and cardiac hypertrophy and fibrosis that occurred in our I3C-induced cyp1a1 prorenin transgenic rat model. The reasons for the inconsistency in organ damage observed either on the same transgenic rat line (the TGR(hAT-rpR) rat model) in different experiments [35] or from different prorenin transgenic rat models (the cyp1a1 Ren-2 transgenic rat model) [12,13] are unclear but may be related to species differences, i.e. prorenin does not work similarly in mice and rats or to differences in circulating prorenin concentrations.
In addition, it has been shown that plasma levels of prorenin in diabetes are elevated compared with normal subjects. There will be great value in utilizing an animal model in which prorenin levels are in a disease relevant range. The inducer, I3C-controlled expression of the cyp1a1-rat prorenin transgene makes this goal achievable by adjusting the given concentration of I3C. Therefore, the current transgenic rats with conditional expression of rat prorenin within the same species should be valuable tools for further examining the pathophysiological role of prorenin especially in diabetes.
However, it is unanswered yet whether prorenin bound to its receptor activates intracellular signaling, independent of Ang II’s generation and action, observed in vitro also occurs in vivo, in these inducible prorenin overexpressing transgenic rats, particularly in the heart and the kidneys. Interestingly, mRNA expression of (P)RR either in the kidney (2.257 ± 0.088 compared with 1.0 ± 0.029, P<0.05) or in the heart tissue (2.867 ± 0.025 compared with 1.0 ± 0.029, P<0.05) was up-regulated in I3C-induced cyp1a1-prorenin transgenic rats compared with non-transgenic controls. Further study is needed to define the role of Ang II-independent that prorenin acts through its receptor in fibrotic or diabetic disease by using prorenin mutant transgenic rats that lack enzymatic activity.
In summary, our data demonstrate that the inducible cyp1a1 prorenin transgenic rats are fertile and healthy and a better model for further studying the pathophysiological role of prorenin in disease. These transgenic rats with elevated plasma prorenin levels develop hypertension, albuminuria, and renal and cardiac fibrosis. Our data suggest that increasing prorenin is profibrotic in the development or progression of renal and cardiovascular diseases. The mechanism may involve prorenin binding to the (P)RR and activating multiply profibrotic cellular pathways, as summarized in the scheme in Figure 13. To further dissect the Ang II-dependent and Ang II-independent mechanisms of profibrotic action of prorenin in vivo by using transgenic rats with conditional expression of prorenin or mutant prorenin (i.e. non-cleavable mutant prorenin or mutant prorenin without enzymatic activity) may facilitate the identification of patients who may have limited long-term benefits from angiotensin-blockade therapy.
Figure 13. Schematic of how elevated prorenin may induce organ injury.
Prorenin, via (P)RR, activates both Ang II-dependent and Ang II-independent mechanisms and subsequent cellular inflammation, oxidative stress, and the MAPK/ERK, β-catenin, and Akt-mediated signaling pathways to promote renal and cardiac fibrosis.
Clinical perspectives
Plasma prorenin is commonly elevated in diabetic patients and appears to predict the development of diabetic nephropathy. However, the pathological role of prorenin is unclear.
In the present study, elevated circulating prorenin within the same species can be induced by oral administration of I3C to the inducible cyp1a1 prorenin transgenic rat model. The resulting high levels of prorenin per se cause hypertension, albuminuria, and renal and cardiac hypertrophy and fibrosis via induction of inflammation, oxidative stress, and the MAPK/ERK, Wnt/β-catenin and PI3K/Akt-mediated signaling pathways.
These findings clearly suggest that increases in plasma prorenin seen in diabetes may play an independent role in the development or progression of renal and cardiovascular diseases.
Acknowledgments
We thank Dr John J. Mullins, University of Edinburgh for his generous help to provide the inducible transgene vector for the present study. We also thank Dr Yan-Ting E. Shiu for her kind help with Picrosirius Red staining.
Abbreviations
- AGT
angiotensinogen
- Akt
protein kinase B
- ANP
atrial natriuretic peptide
- AngI
angiotensin I
- A/C
albumin/creatinine
- BNP
brain natriuretic peptide
- BNX
bilateral nephrectomy
- CVD
cardiovascular disease
- Col I
type I collagen
- Cr
creatinine
- ECM
extracellular matrix
- ERK
extracellular-signal-regulated kinase
- ET-1
endothelin-1
- FN
fibronectin
- Gsk-3beta
glycogen synthase kinase 3 beta
- hAT
human a1-antitrypsin
- HW/BW
ratio of heart weight to body weight
- H&E
hematoxylin and eosin
- ICAM-1
intercellular adhesion molecule-1
- IL-6
interleukin-6
- I3C
indole-3-carbinol
- LV
left ventricular
- MAP
mean arterial pressure
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein-1
- MDA
malondialdehyde
- Nox2
NADPH oxidase
- PAI-1
plasminogen activator inhibitor-1
- PAS
periodic acid-Schiff
- PI3K
phosphoinositide 3-kinase
- PRA
plasma renin/prorenin activity
- PSR
picrosirius red
- (P)RR
prorenin/renin receptor
- RT-PCR
reverse transcription polymerase chain reaction
- SirT1
sirtuin 1
- TBA
thiobarbituric acid
- TBARS
TBA-reactive substance
- TGFβ
transforming growth factor β
- TRI
Masson’s trichrome
- WGA
wheat germ agglutinin
- α-MHC
α-myosin heavy chain
- β-MHC
β-myosin heavy chain
- 8-IP
8-isoprostane
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Author contribution
J.W. constructed and verified the transgene, performed gene typing and gene sequencing and bred the transgenic animals for the present study. G.Z. and Y.H. performed the experiments and analyzed the data. C.G. performed the real time RT/PCR assay in heart tissue. B.W. performed the 8-isoprostane ELISA measurement and histology measurement in heart tissue. E.D.A. performed the cardiac functional measurements. Y.H., E.D.A. and A.C. designed the experiments and wrote and revised the manuscript.
Funding
This work was supported by the National Kidney Foundation of Utah and Idaho [grant number 51001724 (to Y.H.)]; the American Heart Association [grant number 16GRNT27610030 (to Y.H.)]; the American Diabetes Association [grant number 1-17-IBS-312 (to Y.H.)]; and the National Natural Science Foundation of China [grant number 81670665].
References
- 1.Luetscher J.A., Kraemer F.B., Wilson D.M., Schwartz H.C. and Bryer-Ash M. (1985) Increased plasma inactive renin in diabetes mellitus. A marker of microvascular complications. N. Engl. J. Med. 312, 1412–1417 10.1056/NEJM198505303122202 [DOI] [PubMed] [Google Scholar]
- 2.Yokota H., Mori F., Kai K., Nagaoka T., Izumi N., Takahashi A. et al. (2005) Serum prorenin levels and diabetic retinopathy in type 2 diabetes: new method to measure serum level of prorenin using antibody activating direct kinetic assay. Br. J. Ophthalmol. 89, 871–873 10.1136/bjo.2004.056580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson D.M. and Luetscher J.A. (1990) Plasma prorenin activity and complications in children with insulin-dependent diabetes mellitus. N. Engl. J. Med. 323, 1101–1106 10.1056/NEJM199010183231604 [DOI] [PubMed] [Google Scholar]
- 4.Deinum J., Tarnow L., van Gool J.M., de Bruin R.A., Derkx F.H., Schalekamp M.A. et al. (1999) Plasma renin and prorenin and renin gene variation in patients with insulin-dependent diabetes mellitus and nephropathy. Nephrol. Dial. Transplant. 14, 1904–1911 10.1093/ndt/14.8.1904 [DOI] [PubMed] [Google Scholar]
- 5.Chiarelli F., Pomilio M., De Luca F.A., Vecchiet J. and Verrotti A. (2001) Plasma prorenin levels may predict persistent microalbuminuria in children with diabetes. Pediatr. Nephrol. 16, 116–120 10.1007/s004670000514 [DOI] [PubMed] [Google Scholar]
- 6.Sowers J.R. and Stump C.S. (2004) Insights into the biology of diabetic vascular disease: what’s new? Am. J. Hypertens. 17 (S2), 2S–6S 10.1016/j.amjhyper.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 7.Asbun J. and Villarreal F.J. (2006) The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 47, 693–700 10.1016/j.jacc.2005.09.050 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen G., Delarue F., Berrou J., Rondeau E. and Sraer J.-D. (1996) Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigen. Kidney Int. 50, 1897–1903 10.1038/ki.1996.511 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen G., Delarue F., Burckle C., Bouzhir L., Giller T. and Sraer J.D. (2002) Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 109, 1417–1427 10.1172/JCI0214276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen G. (2006) Renin/prorenin receptors. Kidney Int. 69, 1503–1506 10.1038/sj.ki.5000265 [DOI] [PubMed] [Google Scholar]
- 11.Veniant M., Menard J., Bruneval P., Morley S., Gonzales M.F. and Mullins J. (1996) Vascular damage without hypertension in transgenic rats expressing prorenin exclusively in the liver. J. Clin. Invest. 98, 1996–1970 10.1172/JCI119000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters B., Grisk O., Becher B., Wanka H., Kuttler B., Ludemann J. et al. (2008) Dose-dependent titration of prorenin and blood pressure in Cyp1a1ren-2 transgenic rats: absence of prorenin-induced glomerulosclerosis. J. Hypertens. 26, 102–109 10.1097/HJH.0b013e3282f0ab66 [DOI] [PubMed] [Google Scholar]
- 13.Peters J., Schluter T., Riegel T., Peters B.S., Beineke A., Maschke U. et al. (2009) Lack of cardiac fibrosis in a new model of high prorenin hyperaldosteronism. Am. J. Physiol. Heart Circ. Physiol. 297, H1845–H1852 10.1152/ajpheart.01135.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graciano M.L., Mouton C.R., Patterson M.E., Seth D.M., Mullins J.J. and Mitchell K.D. (2007) Renal vascular and tubulointerstitial inflammation and proliferation in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am. J. Physiol. Renal Physiol. 292, F1858–F1866 10.1152/ajprenal.00469.2006 [DOI] [PubMed] [Google Scholar]
- 15.Kantachuvesiri S., Fleming S., Peters J., Peters B., Brooker G., Lammie A.G. et al. (2001) Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J. Biol. Chem. 276, 36727–36733 10.1074/jbc.M103296200 [DOI] [PubMed] [Google Scholar]
- 16.Campbell S.J., Carlotti F., Hall P.A., Clark A.J. and Wolf C.R. (1996) Regulation of the CYP1A1 promoter in transgenic mice: an exquisitely sensitive on-off system for cell specific gene regulation. J. Cell Sci. 109, 2619–2625 [DOI] [PubMed] [Google Scholar]
- 17.Smith J.D., Wong E. and Ginsberg M. (1995) Cytochrome P450 1A1 promoter as a genetic switch for the regulatable and physiological expression of a plasma protein in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 92, 11926–11930 10.1073/pnas.92.25.11926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y., Wongamorntham S., Kasting J., McQuillan D., Owens R.T., Yu L. et al. (2006) Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 69, 105–113 10.1038/sj.ki.5000011 [DOI] [PubMed] [Google Scholar]
- 19.Zhang J., Noble N.A., Border W.A., Owens R.T. and Huang Y. (2008) Receptor-dependent prorenin activation and induction of PAI-1 expression in vascular smooth muscle cells. Am. J. Physiol. Endocrinol. Metab. 295, E810–E819 10.1152/ajpendo.90264.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J., Wu J., Gu C., Noble N.A., Border W.A. and Huang Y. (2012) Receptor-mediated nonproteolytic activation of prorenin and induction of TGF-beta1 and PAI-1 expression in renal mesangial cells. Am. J. Physiol. Renal Physiol. 303, F11–F20 10.1152/ajprenal.00050.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y., Haraguchi M., Lawrence D.A., Border W.A., Yu L. and Noble N.A. (2003) A mutant, noninhibitory plasminogen activator inhibitor type 1 decreases matrix accumulation in experimental glomerulonephritis. J. Clin. Invest. 112, 379–388 10.1172/JCI200318038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J., Gu C., Lawrence D.A., Cheung A.K. and Huang Y. (2014) A PAI-1 mutant retards diabetic nephropathy in db/db mice through protecting podocytes. Exp. Physiol. 99, 802–815 10.1113/expphysiol.2013.077610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou G., Cheung A.K., Liu X. and Huang Y. (2014) Valsartan slows the progression of diabetic nephropathy in db/db mice via a reduction in podocyte injury, and renal oxidative stress and inflammation. Clin. Sci. 126, 707–720 10.1042/CS20130223 [DOI] [PubMed] [Google Scholar]
- 24.Gu C., Zhou G., Noble N.A., Border W.A., Cheung A.K. and Huang Y. (2014) Targeting reduction of proteinuria in glomerulonephritis: maximizing the antifibrotic effect of valsartan by protecting podocytes. J. Renin Angiotensin Aldosterone Syst. 15, 177–189 10.1177/1470320312466127 [DOI] [PubMed] [Google Scholar]
- 25.Huang Y., Border W.A., Yu L., Zhang J., Lawrence D.A. and Noble N.A. (2008) A PAI-1 mutant, PAI-1R, slows progression of diabetic nephropathy. J. Am. Soc. Nephrol. 19, 329–338 10.1681/ASN.2007040510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J., Gu C., Noble N.A., Border W.A. and Huang Y. (2011) Combining angiotensin II blockade and renin receptor inhibition results in enhanced antifibrotic effect in experimental nephritis. Am. J. Physiol. Renal Physiol. 301, F723–F732 10.1152/ajprenal.00271.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batenburg W.W. and Danser A.H. (2012) (Pro)renin and its receptors: pathophysiological implications. Clin. Sci. 123, 121–133 10.1042/CS20120042 [DOI] [PubMed] [Google Scholar]
- 28.Williams B. (2001) Angiotensin II and the pathophysiology of cardiovascular remodeling. Am. J. Cardiol. 87, 10C–17C 10.1016/S0002-9149(01)01507-7 [DOI] [PubMed] [Google Scholar]
- 29.Chong Z.Z., Wang S., Shang Y.C. and Maiese K. (2012) Targeting cardiovascular disease with novel SIRT1 pathways. Future Cardiol. 8, 89–100 10.2217/fca.11.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wende A.R., O’Neill B.T., Bugger H., Riehle C., Tuinei J., Buchanan J. et al. (2015) Enhanced cardiac Akt/protein kinase B signaling contributes to pathological cardiac hypertrophy in part by impairing mitochondrial function via transcriptional repression of mitochondrion-targeted nuclear genes. Mol. Cell. Biol. 35, 831–846 10.1128/MCB.01109-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma S.K., Choi J.S., Joo S.Y., Kim H.Y., Kim C.S., Bae E.H. et al. (2012) Activation of the renal PI3K/Akt/mTOR signaling pathway in a DOCA-salt model of hypertension. Chonnam Med. J. 48, 150–154 10.4068/cmj.2012.48.3.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin J.C., Chang R.L., Chen Y.F., Yang J.J., Baskaran R., Chung L.C. et al. (2016) Beta-catenin overexpression causes an increase in inflammatory cytokines and NF-kappaB activation in cardiomyocytes. Cell. Mol. Biol. 63, 17–22 10.14715/cmb/2017.63.1.4 [DOI] [PubMed] [Google Scholar]
- 33.Tan R.J., Zhou D., Zhou L. and Liu Y. (2014) Wnt/beta-catenin signaling and kidney fibrosis. Kidney Int. Suppl. 4, 84–90 10.1038/kisup.2014.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullins J.J., Peters J. and Ganten D. (1990) Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature 344, 541–544 10.1038/344541a0 [DOI] [PubMed] [Google Scholar]
- 35.Campbell D.J., Karam H., Menard J., Bruneval P. and Mullins J.J. (2009) Prorenin contributes to angiotensin peptide formation in transgenic rats with rat prorenin expression targeted to the liver. Hypertension 54, 1248–1253 10.1161/HYPERTENSIONAHA.109.138495 [DOI] [PubMed] [Google Scholar]
- 36.Oates HF F.J. and Stokes G.S. (1974) Disappearance rate of circulating renin after bilateral nephrectomy in the rat. Clin. Exp. Pharmacol. Physiol. 1, 547–549 10.1111/j.1440-1681.1974.tb00576.x [DOI] [PubMed] [Google Scholar]
- 37.Danser A.H. and Deinum J. (2005) Renin, prorenin and the putative (pro)renin receptor. Hypertension 46, 1069–1076 10.1161/01.HYP.0000186329.92187.2e [DOI] [PubMed] [Google Scholar]
- 38.Batenburg W.W., Krop M., Garrelds I.M., de Vries R., de Bruin R.J., Burckle C.A. et al. (2007) Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J. Hypertens. 25, 2441–2453 10.1097/HJH.0b013e3282f05bae [DOI] [PubMed] [Google Scholar]
- 39.Sakoda M., Ichihara A., Kaneshiro Y., Takemitsu T., Nakazato Y., Nabi A.H. et al. (2007) (Pro)renin receptor-mediated activation of mitogen-activated protein kinases in human vascular smooth muscle cells. Hypertens. Res. 30, 1139–1146 10.1291/hypres.30.1139 [DOI] [PubMed] [Google Scholar]
- 40.Feldt S., Maschke U., Dechend R., Luft F.C. and Muller D.N. (2008) The putative (pro)renin receptor blocker HRP fails to prevent (pro)renin signaling. J. Am. Soc. Nephrol. 19, 743–748 10.1681/ASN.2007091030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakoda M., Ichihara A., Kurauchi-Mito A., Narita T., Kinouchi K., Murohashi-Bokuda K. et al. (2010) Aliskiren inhibits intracellular angiotensin II levels without affecting (pro)renin receptor signals in human podocytes. Am. J. Hypertens. 23, 575–580 10.1038/ajh.2009.273 [DOI] [PubMed] [Google Scholar]
- 42.Uraoka M., Ikeda K., Nakagawa Y., Koide M., Akakabe Y., Nakano-Kurimoto R. et al. (2009) Prorenin induces ERK activation in endothelial cells to enhance neovascularization independently of the renin-angiotensin system. Biochem. Biophys. Res. Commun. 390, 1202–1207 10.1016/j.bbrc.2009.10.121 [DOI] [PubMed] [Google Scholar]
- 43.Methot D., Silversides D.W. and Reudelhuber T.L. (1999) In vivo enzymatic assay reveals catalytic activity of the human renin precursor in tissues. Circ. Res. 84, 1067–1072 10.1161/01.RES.84.9.1067 [DOI] [PubMed] [Google Scholar]
- 44.Huang Y., Border W.A. and Noble N.A. (2007) Functional renin receptors in renal mesangial cells. Curr. Hypertens. Rep. 9, 133–139 10.1007/s11906-007-0024-4 [DOI] [PubMed] [Google Scholar]
- 45.Cousin C., Bracquart D., Contrepas A., Corvol P., Muller L. and Nguyen G. (2009) Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 53, 1077–1082 10.1161/HYPERTENSIONAHA.108.127258 [DOI] [PubMed] [Google Scholar]
- 46.Takimoto E. and Kass D.A. (2007) Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49, 241–248 10.1161/01.HYP.0000254415.31362.a7 [DOI] [PubMed] [Google Scholar]
- 47.Mezzano S.A., Ruiz-Ortega M. and Egido J. (2001) Angiotensin II and renal fibrosis. Hypertension 38, 635–638 10.1161/hy09t1.094234 [DOI] [PubMed] [Google Scholar]