Abstract
Purpose of the review
TB incidence has declined ~1.5% annually since 2000, but continued to affect 10.4 million individuals in 2015, with 1/3 remaining undiagnosed or under reported. The diagnosis of TB among those co-infected with HIV is challenging as TB remains the leading cause of death in such individuals. Accurate and rapid diagnosis of active TB will avert mortality in both adults and children, reduce transmission, and assist in timeous decisions for ART initiation. This review describes advances in diagnosing TB, especially among HIV co-infected individuals, highlights national program’s uptake, and impact on patient care.
Recent Findings
The TB diagnostic landscape has been transformed over the last 5 years. Molecular diagnostics such as Xpert MTB/RIF, which simultaneously detects M. tuberculosis resistance to rifampicin, has revolutionised TB control programs. WHO endorsed the use of Xpert MTB/RIF in 2010 for use in HIV/TB co-infected patients, and later in 2013 for use as the initial diagnostic test for all adults and children with signs and symptoms of pulmonary TB. Line probe assays (LPAs) are recommended for the detection of rifampicin and isoniazid resistance in sputum smear-positive specimens and mycobacterial cultures. A second-line line probe assay has been recommended for the diagnosis of extensively drug-resistant (XDR)-TB Assays such as the urine lateral flow(LF)-lipoarabinomannan(LAM), can be used at the point of care (POC) and have a niche role to supplement the diagnosis of TB in seriously ill HIV infected, hospitalized patients with low CD4<100cells/μl. Polyvalent platforms such as the m2000 (Abbott Molecular, IL, USA) and GeneXpert (Cepheid, CA, USA) offer potential for integration of HIV and TB testing services. While the Research and Development (R&D) pipeline appears to be rich at first glance, there are actually few leads for true POC tests that would allow for earlier TB diagnosis or rapid, comprehensive drug susceptibility testing, especially when considering the very high attrition rates observed between biomarker discovery and product market entry.
Summary
In this review, we describe diagnostic strategies specifically for HIV and TB co-infected individuals. Molecular diagnostics in particular within the past 5 years have revolutionized and “disrupted” this field. They lend themselves to integration of services with platforms capable of polyvalent testing. Impact on patient care is, however, still debatable. What has been highlighted is the need for health system strengthening and for true POC testing that can be used in active case finding.
Keywords: Molecular TB diagnostics, HIV/TB care, Xpert MTB/RIF, drug resistance, implementation, TB control
Introduction
In 2010 it was true to report that TB curative drugs had not changed in 50 years, TB control programs were weak, treatment regimens were lengthy and medications toxic. There was limited attention to infection control, inadequate investment in R&D and with the HIV epidemic all served to make M. tuberculosis (MTB) disease an increasing global threat [1]. Five years on, we see the global incidence of TB slowly decreasing at around 1.5% per annum but still well below the 4–5% annual reduction in incidence needed to meet the first milestones (35% reduction in the number of TB deaths; 20% reduction in TB incidence) of the End TB strategy, set for 2020 [2]. WHO has recommended the use of a standardised shorter multi-drug resistant tuberculosis (MDR)-TB regimen of 9–12 months for the majority of patients (excluding pregnant women) with pulmonary MDR/rifampicin-resistant(RR)-TB that is not resistant to second-line drugs. WHO recommendations for the use of two new drugs, notably bedaquiline and delamanid, have helped to improve outcomes for patients with MDR/XDR-TB. Since 2010, WHO has endorsed the use of several new diagnostic technologies such as Xpert MTB/RIF (Cepheid, CA, USA), molecular line probe assays (Hain Lifesciences, Germany and Nipro Coorporation, Japan) for the diagnosis of MDR- and XDR-TB, TB-LAMP (Eiken, Japan) and LF-LAM (Alere Inc, MA, USA ).
In 2016, WHO reports the status of the TB epidemic with 10.4 million new incident cases [3]. TB remains the leading infectious disease causing mortality with an estimated 1.4 million TB deaths in 2015, and an additional 0.4 million deaths resulting from TB disease among people living with HIV. An estimated 11 % of TB patients are co-infected with HIV and high rates (3.9% of new TB cases and 21% of previously treated cases) of MDR-TB of which 9.5% have XDR-TB represent a major public health crisis [4]. Of note, 80% estimated incident TB cases are reported from 22 high burden countries, with those in sub-Saharan Africa bearing the brunt of dual HIV and TB epidemics.
TB (including drug-resistant (DR)-TB) is still the leading cause of death among HIV co-infected individuals. This is evident from autopsy studies, of which a systematic review and meta-analysis of 36 eligible studies [5] reported a pooled prevalence of 39.7% (confidence interval [CI] 32.4% – 47%) in adults. This varied by world region: 63% in South Asia, 43% in sub-Saharan and 27% in the Americas. A study in South Africa (SA) from the North West province [6] which has a high (13%) HIV prevalence, reported a quarter of home deaths had evidence of undiagnosed TB disease, emphasising the burden of TB in the community and underlining the fatal consequences of delayed TB diagnosis and treatment [7].
Diagnosis of TB is particularly difficult among HIV co-infected individuals who may have atypical, non-specific clinical presentation, and more often (24–61%) smear-negative disease [8] with less cavitary lesions (due to impairment of granuloma formation, [9], along with higher rates of extra-pulmonary TB [10–12]. Sputum-based diagnosis is therefore less sensitive among HIV co-infected patients, resulting in more smear-negative TB disease leading to more empiric treatment among those at greatest risk of disease [13]. This requires caution after the REMEMBER Trial illustrated that empiric TB therapy did not reduce mortality at 24 weeks compared to isoniazid preventative therapy (IPT) in adult outpatients with advanced HIV disease initiating ART [14].
The emergence of MDR- and XDR-TB further highlights the need for sensitive and timely diagnosis. A meta-analysis performed by Mesfin et al [15] confirmed the association between MDR-TB and HIV, with the odds of having MDR-TB among HIV positive cases being 24% higher. However, recent studies show HIV co-infection not to be a direct driver for the emergence and transmission of resistant strains [16]. As mechanistic mathematical modelling approaches show [17] the vast majority (up to 80% in under-resourced settings) of MDR-TB is due to transmission and not acquisition, a change in dogma regarding DR acquisition versus strain transmission is being called for [18]. It is critical too to understand that MDR-TB strains are as equally transmissible as drug-susceptible [19]. As Van Rie and Warren [18] further highlight, transmission of MDR-TB drives the epidemic in high burden settings, and the TB epidemic can be contained by implementation of active case finding with rapid TB detection and drug resistance detection (at least for rifampicin) for all people with signs and symptoms of TB as the greatest number of MDR-TB cases will be among newly diagnosed TB cases [20].
TB remains one of the top ten leading causes of death in children (WHO reports 170 000 deaths in 2015) and of concern is ~57% children diagnosed with and treated for TB are HIV-infected in high burden countries [21]. In South Africa it is estimated children <14yrs account for 15–20% total TB burden. Diagnosing childhood TB is challenging since the most appropriate specimen to collect depends on age and clinical presentation. Specimens (especially sputum) are also paucibacillary [22], often of poor quality and quantity. A study of ART programmes showed sputum smear microscopy and chest X-ray (CXR) where available, were only used in 86% and 52% of TB diagnoses [23]. Although WHO recommends Xpert MTB/RIF testing for children, Xpert MTB/RIF (where available) was only used in 8% and culture in 17% cases [24]. Further, a study in Johannesburg showed 67% sputum collected from children <14yrs (median age 24months) was below the required volume for Xpert MTB/RIF testing [25]. Overall, this highlights the need for strengthening the capacity for diagnosis, proactive screening for TB and MDR-TB in in-patient settings and the community [26]. In addition, HIV co-infected individuals require antiretroviral therapy (ART) scale up, continuous monitoring and collaboration between HIV and TB control programs. This will require task shifting [27] and integration of services for adults and children [23]. Screening pregnant women for HIV and TB may also improve access to care [28], and health care providers are encouraged to increase competency in linkage to care and integration, which will also enhance prevention in young infants and children [23].
The poor sensitivity of smear microscopy (38–69%) in HIV infected individuals has been well described [29], where the presence of 5000–10,000 bacteria are required for visual detection[30], compared to liquid culture which remains the gold standard at the lowest limit of detection of MTB of ~10–100 cfu/ml [30, 31]. The contrast, however, is a poor (yet affordable and easy to perform in a standard laboratory) test that yields a result in <24hrs compared to a sensitive test that could take longer than 6 weeks (if DST is included) and becomes less clinically relevant, and requires a biosafety laboratory environment and skilled operation. The desired diagnostic needs to be fast, accurate, affordable, and capable of being performed in the household or community, and on a range of specimen types (sputum, urine, stool), simultaneously with HIV diagnosis and monitoring. This is becoming a reality through molecular technology specifically for HIV co-infected individuals [11]. WHO recommends that the Xpert MTB/RIF assay is used as the initial diagnostic for TB for all adults and children with signs and symptoms of TB and especially where the burden of HIV is high and high rates of DR-TB are suspected. Another desirable component becoming available on several of the molecular instruments is the ability to perform >1 type of test on the same platform. Xpert HIV-1 Qualitative test for early infant diagnosis is now pre-qualified for use by WHO and provides an opportunity for integration of TB and HIV diagnosis, and extend testing services closer to POC. This would also be the case for the Xpert HIV-1 Quantitative test for HIV viral load monitoring (Gous NM, unpublished data), once approved, to improve patient access and impact on the 90/90/90 goals.
WHO endorsed diagnostic technologies
Xpert MTB/RIF (Cepheid)
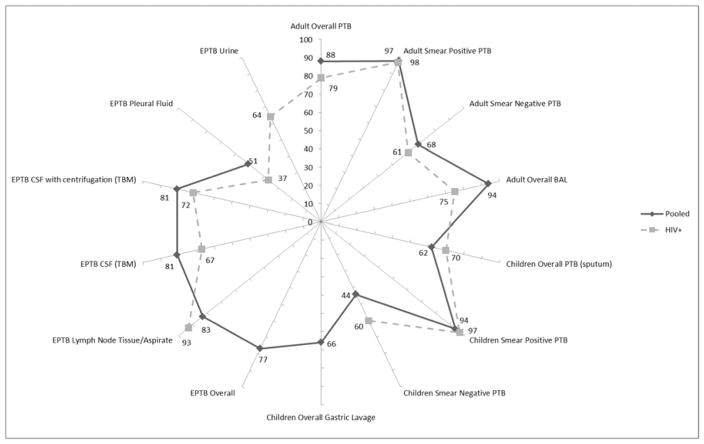
The Xpert MTB/RIF (Cepheid, CA, USA) is a cartridge based molecular test that is fully automated only requiring addition of reagent buffer to liquefy and inactivate any TB bacilli present in a clinical specimen [32–34]. Results are reported within 2 hours, and include the detection of rifampicin resistance conferring mutations (RIF). The limit of detection of MTB complex (MTBC) with Xpert MTB/RIF on clinical specimens is 150cfu/ml [33]. Figure 1 simplistically outlines the overall performance of Xpert MTB/RIF with specific reference to performance among HIV infected.
Figure 1.
A radar plot of the performance (represented as sensitivity values 0–100%) of Xpert MTB/RIF compared to liquid culture for various specimen types. PTB-pulmonary tuberculosis, BAL-bronchoalveolar lavage, EPTB, CSF-cerebrospinal fluid and TBM-tuberculosis meningitis. The solid radial arms illustrate sensitivity (%) of overall pooled data, and the dashed radial arms report studies among HIV/TB co-infected individuals.
Adults Overall: n=27 studies (7 by HIV status), Smear Positive PTB: n=5 studies, Smear Negative PTB: n=5 studies [35]; BAL: n=1 study, centrifuged BAL [36]. Children Overall PTB (sputum): n=12 studies, Smear Positive PTB: n=6 studies, Smear Negative PTB: n=7 studies, Overall Gastic Lavage: n=7 studies [37]. EPTB Overall EPTB: n=36 studies [38]; Lymph Node Tissue/Aspirate: n=18 studies [39], n= 1 HIV positive cohort [40]; CFS(TBM): n=18 studies [39], n=1 HIV positive cohort, mixed CSF samples [41], n=1 HIV positive cohort, centrifuged CSF [42]; Pleural fluid: n=24 studies [43], n=1 high TB-HIV prevalence cohort [44]; Urine: n=1 hospitalised, HIV positive cohort [45].
In summary, Xpert MTB/RIF performs well on adult respiratory specimens compared to culture reference, but less well in sputum smear-negative specimens. This is similar among childhood specimens, with somewhat increased performance of Xpert MTB/RIF in HIV infected children. Performance of Xpert MTB/RIF is good on lymph node and other tissue specimens and CSF, with greater sensitivity in HIV infected individuals’ lymph node tissue. Xpert MTB/RIF is known to have poorer performance in pleural fluid irrespective of smear status.
The impact of Xpert MTB/RIF on patient care has, to date, been described in 33 studies from 22 countries, including 10 sub-Saharan African countries. Of these, 20 studies (refer to Table 1) discuss the impact of the Xpert MTB/RIF in HIV infected populations: Xpert MTB/RIF increases detection of TB and dramatically reduces treatment initiation times for DR-TB. The placement of GeneXpert instruments at treatment facilities and at POC facilities results in shortened treatment initiation times than centralised testing. Changes in empiric treatment practice, however varies, and overall impact on mortality has not been shown, and in fact studies undertaken to measure it were underpowered [64].
Table 1.
Impact of Xpert MTB/RIF specifically among HIV co-infected individuals
| Main study investigations | Main study findings | |
|---|---|---|
| Detection of TB | Passive TB case detection and treatment outcomes [46] | Increased detection, earlier treatment, decreased empiric treatment and more patients completed treatment with fewer LTFU |
| Single Xpert MTB/RIF in intensified case findings among prisoners [47] | ||
| Xpert MTB/RIF among smear-negative or sputum-scarce using BAL [36] | ||
| TB management and outcome in hospitalized patients [48] | ||
| Drug resistant (DR)-TB | DR Screening in a referral hospital [49, 50] | Presumptive screening enabled rapid diagnosis (2days with Xpert MTB/RIF, 8 days to confirm with LPA) and reduced treatment time (8days vs 40days) with potential to decrease transmission |
| Decentralised care on Rif Resistant treatment initiation [51] | ||
| Placement (services) | Xpert MTB/RIF impact depends on service coordination [52] | Shortened time to treatment only in TB treatment facilities, |
| Drug resistant screening in referral hospital [49] | Added little to clinical decision | |
| POC sites [53–55] and intensified case finding [56] | Increased number patients evaluated for DR-TB, time to treat reduced 0 – 1 days POC vs 5–11 days vs centralised Xpert testing | |
| Empiric treatment | POC [54] | Empiric treatment resulted in no difference in treatment numbers |
| at PHC [57] | Proportion of patients treated without confirmation was halved | |
| Replaces routine [58] | Health-care workers more confident to withhold TB treatment when Xpert MTB/RIF negative and no HIV. | |
| High rates of empiric undermine Xpert MTB/RIF potential [59] | No change in morbidity, | |
| ART associated TB | RCT [60] | No difference in treatment initiation times, many patients still treated empirically, mortality not reduced |
| Mortality | TB management and outcome in hospitalized patients [48] and Intensified case finding [56] | No difference in time to treatment or 2month mortality |
| Cluster randomized trial at early country implementation [61] | No difference in mortality at 6 months | |
| Stepped-wedge RCT [58] | 35% reduction in TB related mortality but no increase in treatment success or decrease in LTFU | |
| Single POC Xpert MTB/RIF in PHC [62] | No impact on treatment initiation or mortality among HIV positive cohort | |
| Dynamic simulation and economic evaluation [63] | Xpert MTB/RIF could reduce morbidity and mortality, but modestly reduce TB incidence and requires better TB case finding, but at substantial program cost |
FIND and collaborating partners is currently undertaking a multi-centre study in 8 countries of the much anticipated Xpert Ultra (Cepheid, CA, USA) test, which promises to reduce the limit of detection of MTB in sputum to the realm of liquid culture (10–100cfu/ml) [65]. Much has been learnt from the implementation of Xpert MTB/RIF as summarised in Table 2 and includes [66, 67] cost and forecast models [68, 69], program interfacing [70], socio-economic trends [71], and quality assessment [72]. Additional innovations, being reported for the first time for possible TB control (currently, developed for GeneXpert instruments), is remote connectivity [73, 74]. Cepheid’s C360 is a web-based software that remotely connects all instruments and centrally collects result run information. This, together with a laboratory information system (LIS) allows for central program and laboratory monitoring on test and module performance (potentially in real time), with its further application in TB control [75].
Table 2.
Considerations for implementation of new diagnostics (experience drawn from Xpert MTB/RIF implementation [66, 67])
| Build a good team | Trainers (technical, clinical), program managers, data analysts, TB and HIV specialists, R&D scientists |
| Develop models and trend analyses | Cost, forecast [68, 69], program interface [70], socioeconomic trends [71] |
| Maintain networks | WHO, new developers, NDOH – stakeholders, funders, clinical lab interface |
| Sustain quality management system | * EQA [72], Connectivity [73–75], manage assay change over, SOPs, infrastructure, HR, stock control, algorithms |
| Monitoring and evaluation | Investment case update, epidemiology and surveillance |
| Align Parallel programs | Correctional facilities, children, EPTB, rural/remote communities through mobile/POC |
| Integration of services | Platform optimization and service refinement |
| Linkage to care | Maximize laboratory LIS for faster data streams (SMS printers, m-health) |
some of the 12 components of the quality assurance model mentioned
Line probe assays (LPA)
GenoType® MTBDRplus, (Hain Lifescience, Germany) was the first commercial LPA recommended for use by WHO in 2008 [76]. It remains the most widely studied LPA. Further data has since been published on the use of LPAs and newer versions of LPA technology have since been developed: i) Hain Genotype MTBDRplus version 2 [77–80]; and ii) the Nipro NTM+MDRTB detection kit developed by the Nipro Corporation (Japan) DR-TB [81]. These newer LPAs aim to improve sensitivity for MTBC detection and to simultaneously detect resistance to rifampicin and isoniazid. These LPAs are recommended for use in regional or centralised high through-put laboratories for the rapid detection of rifampicin and isoniazid resistance in sputum-smear positive specimens and from mycobacterial cultures. The tests are not recommended for use on sputum smear-negative specimens [82]. LPA require laboratory trained personnel skilled in PCR, to perform the assay in well managed laboratories. LPA identify drug resistance through manual extraction of MTBC DNA and PCR amplification of the resistance hotspot regions in the rpoB, inhA and katG genes. The impact of expanded testing using the LPA in South Africa resulted in a substantial increase in the proportion of new cases identified as MDR-TB, and although the time to treatment was reduced, it still took 2 months [83].
A second-line line probe assay (SL-LPA) for the detection of resistance to second line anti-TB drugs - MTBDRsl assay (Hain LifeScience, Germany), incorporates probes to detect mutations within genes (gyrA and rrs) for version 1.0 [84] and, in addition, gyrB and the eis promoter for version 2.0 [85–87]), which are associated with resistance to the class of fluoroquinolones or the second-line injectable agents. The presence of mutations in these regions does not necessarily imply resistance to all the drugs within that class. Although specific mutations within these regions may be associated with different levels of resistance (i.e. different minimum inhibitory concentrations) to each drug within these classes, the extent of cross resistance is not completely understood. WHO recommends the use of SL-LPA as an initial test to detect resistance directly on sputum from patients diagnosed with resistance to RIF or MDR-TB [87].
The loop mediated isothermal amplification assay (TB-LAMP)
Loop-mediated isothermal amplification (LAMP) is a unique, temperature-independent technique for amplifying DNA that is simple to use, providing a visual display that is easy to read. TB-LAMP does not require sophisticated instrumentation and can be used at a peripheral health center level, given biosafety requirements similar to microscopy. A meta-analysis of 10 studies reported a sensitivity of 80% (78–83) and specificity of 96% (95–97) to diagnose pulmonary TB [88]. One of the data sets included in the meta-analysis stood out in terms of its findings: the study conducted in Malawi among individuals with cough (44% HIV positivity) reported a sensitivity of 65% (48–79), specificity of 100% (98–100), similar performance (p=0.132) to Xpert, but lower performance compared to concentrated fluorescent smear microscopy with duplicate reading (p=0.02) [89]. TB-LAMP, however, provides better results than sputum smear microscopy, detecting 15% more patients with pulmonary TB, if performed in all persons presenting with signs and symptoms. If used as an add-on test after microscopy has been performed, >40% increase in TB cases were detected among those with smear-negative results compared to other rapid tests that have been recommended by WHO in recent years.
TB-LAMP only detects TB (therefore only suitable for testing of patients at low risk of multidrug-resistant TB (MDR-TB)), and therefore should not replace Xpert MTB/RIF, which simultaneously detects TB and rifampicin resistance. TB-LAMP may be a plausible alternative in settings with low prevalence of HIV and low prevalence of drug resistance, especially where environmental conditions (unstable electricity, temperature, humidity, excessive dust [90]) and possible cost limit access to implementation of Xpert MTB/RIF. The test does not detect drug resistance. It can be performed outside of conventional laboratories but requires training of health care staff, similar to the training needed for performing sputum smear microscopy [91].
Lipoarabinomannan assay
LAM is a lateral flow assay requiring 60μl urine and visual reading of band intensity compared to the manufacturer’s supplied reference line on a card to report a result within 25 minutes, making it applicable to identify active TB at POC, but will require a good quality framework to ensure accuracy. WHO only recommends its use in HIV positive hospitalised individuals, whose CD4 count <100cells/μl [92], and for seriously ill persons irrespective of their CD4 count [93]. A meta-analysis in this population reports the LAM used to diagnose TB with a pooled sensitivity of 56% (41–70) and pooled specificity 90% (81–95). Combining Xpert MTB/RIF testing of urine with urine LF-LAM improved overall TB diagnostic sensitivity 75% (61–87) and specificity of 93% (81–97) with the added advantage of Xpert MTB/RIF simultaneously detecting susceptibility to RIF [94]. Peter et al showed that POC LAM reduced mortality at 8 weeks in hospitalised patients [95].
Technologies under evaluation
Abbott RealTime MTB and MTB-RIF/INH assays (Abbott Molecular, IL, USA)
The m2000 platform is widely used for centralized HIV viral load testing. The platforms’ flexible, automated extraction and closed real time PCR systems (testing 93 specimens/8 hour day), lend itself to other molecular assays such as the Abbott RealTime MTB (amplification and detection of IS6110 and protein antigen B) and MTB-RIF/INH (similar region detection to those reported by MTBDRplus) assays for qualitative detection of MTBC [96, 97]. Similar performance to Xpert MTB/RIF in high TB and HIV settings has been noted (Scott LE, unpublished data), and with the added advantage of reporting RIF and INH susceptibility simultaneously [98]. The m2000 platform has full connectivity functionality, and training and quality management systems are in place in many HIV and TB high burden countries. Placement for TB testing would be similar to HIV viral load testing, therefore lending itself to integration of HIV and TB laboratory services. This principle of platform integration is not new to molecular testing services and is now also being investigated by Cepheid to provide HIV viral load testing (Xpert HIV-1) on their GeneXpert platform. Therefore integration of HIV and TB services is not only patient centric now but platform and testing service centric too.
Future
The development pipeline for diagnosing active (including drug resistant) tuberculosis appears rich from a molecular diagnostics perspective [99], including a large focus on whole genome sequencing and next generation sequencing [100, 101]. The aim is to improve sensitivity, speed, ease of use, ability to discriminate TB from other inflammatory or autoimmune diseases and identify subclinical TB in HIV infection [102]. However, very few candidate assays are in the R&D pipeline for true point-of-care tests in RDT format, with disappointing results from biomarker research [103]. There are molecular platforms in the pipeline that will get us closer to patients, but it remains unclear whether test implementation would be cost-effective [104, 105]. Xpert Omni (Cepheid, CA, USA) may address the criticism of GeneXpert, which requires a laboratory infrastructure (e.g. because of the need for continuous and stable electrical supply) and has limited utility for community testing. The anticipated launch of Xpert Omni in 2017 does not leave much time to address issues for implementers of regulatory assurance, quality control and maintenance, staff resources, logistic support and cost [106]. Mobile phone and thus platform connectivity may be a particularly challenging field for countries to address. Future evaluation studies will also require broader design to assess impact of TB diagnostics and more attention paid to analyses in methodology studies [107]. This too will apply to evaluation of high throughput centralised testing platforms (e.g. m2000) that will require flexibility around the informed consent process required for trials to match the platforms daily testing throughput.
Conclusion
The last few years have seen improvements in the integration of TB and HIV diagnosis and care. New polyvalent platforms should ease integration from a diagnostic standpoint. The major gaps today are true POCT for early and active case detection and universal rapid DST. While new tests have transformed TB control and acted as catalyst for change, impact is lower than anticipated. The linkage to care must be optimized to fully capitalize on the potential of new TB diagnostics [108]. Innovation and support is needed not only in the form of new tests, but more importantly for the strengthening of health care and delivery services to improve the cascade of care. Only with a comprehensive approach will we be able to achieve the sustainable development goals.
Key points.
TB remains the leading infectious disease causing mortality especially among HIV co-infected individuals.
For several years, rapid and reliable tests such as Xpert MTB/RIF have been available but implementation has been slow in many LMICs and the evidence of its impact on reducing the burden of TB is limited.
Greater investment in diagnostic innovations that can allow for early TB diagnosis and universal access to drug susceptibility testing for patients at their first encounter with the health system will be essential to end the TB epidemic.
Active case finding strategies have been shown to be cost-effective and need to be coupled with the right diagnostics.
Further improvements in assay sensitivity are needed especially for HIV co-infected, as current molecular tests perform suboptimally.
Footnotes
Conflicts of interest: none
Financial support and sponsorship: none
Contributor Information
Lesley Scott, Department of Molecular Medicine and Haematology, Faculty of Health Sciences, University of the Witwatersrand, 7 York Road, Parktown, Johannesburg, Gauteng, South Africa.
Pedro da Silva, National Priority Programs, National Health Laboratory Service, South Africa.
Catharina C. Boehme, Foundation for Innovative New Diagnostics (FIND), Geneva, Switzerland
Wendy Stevens, Department of Molecular Medicine & Haematology, University of the Witwatersrand & National Priority Programs, National Health Laboratory Service, South Africa.
Chris Gilpin, Global TB Program, WHO, Geneva, Switzerland.
References
- 1.Mayer KH, Dukes Hamilton C. Synergistic pandemics: confronting the global HIV and tuberculosis epidemics. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(Suppl 3):S67–70. doi: 10.1086/651475. Epub 2010/04/20. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organisation. Implementing the End TB Strategy: The Essentials. Geneva, Switzerland: World Health Organisation; 2015. Available from: http://www.who.int/tb/publications/2015/end_tb_essential.pdf. [Google Scholar]
- 3.World Health Organisation. Global Tuberculosis Report 2016. Geneva, Switzerland: World Health Organisation; 2016. Available from: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf. [Google Scholar]
- 4.World Health Organisation. Global Tuberculosis Report. Geneva, Switzerland: World Health Organisation; 2015. [Google Scholar]
- 5.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. Aids. 2015;29(15):1987–2002. doi: 10.1097/QAD.0000000000000802. Epub 2015/08/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Omar T, Variava E, Moroe E, et al. Undiagnosed TB in adults dying at home from natural causes in a high TB burden setting: a post-mortem study. Int J Tuberc Lung Dis. 2015;19(11):1320–5. doi: 10.5588/ijtld.15.0222. Epub 2015/10/16 TB-related mortality is under-reported, with serious implications for TB control in high TB burden settings. [DOI] [PubMed] [Google Scholar]
- 7.Bates M, Mudenda V, Shibemba A, et al. Burden of tuberculosis at post mortem in inpatients at a tertiary referral centre in sub-Saharan Africa: a prospective descriptive autopsy study. The Lancet Infectious diseases. 2015;15(5):544–51. doi: 10.1016/S1473-3099(15)70058-7. Epub 2015/03/15. [DOI] [PubMed] [Google Scholar]
- 8.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369(9578):2042–9. doi: 10.1016/S0140-6736(07)60284-0. Epub 2007/06/19. [DOI] [PubMed] [Google Scholar]
- 9.Palmieri F, Girardi E, Pellicelli AM, et al. Pulmonary tuberculosis in HIV-infected patients presenting with normal chest radiograph and negative sputum smear. Infection. 2002;30(2):68–74. doi: 10.1007/s15010-002-2062-9. Epub 2002/05/23. [DOI] [PubMed] [Google Scholar]
- 10.Black A. A new algorithm for the diagnosis of all forms of tuberculosis is required for South Africa. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2013;103(6):355–6. doi: 10.7196/samj.6896. Epub 2013/06/04. [DOI] [PubMed] [Google Scholar]
- *11.Vittor AY, Garland JM, Gilman RH. Molecular Diagnosis of TB in the HIV Positive Population. Annals of global health. 2014;80(6):476–85. doi: 10.1016/j.aogh.2015.01.001. Epub 2015/05/12 A comprehensive review of molecular diagnosis of TB in the HIV positive population. [DOI] [PubMed] [Google Scholar]
- 12.Aaron L, Saadoun D, Calatroni I, et al. Tuberculosis in HIV-infected patients: a comprehensive review. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2004;10(5):388–98. doi: 10.1111/j.1469-0691.2004.00758.x. Epub 2004/04/29. [DOI] [PubMed] [Google Scholar]
- 13.Lawn SD, Ayles H, Egwaga S, et al. Potential utility of empirical tuberculosis treatment for HIV-infected patients with advanced immunodeficiency in high TB-HIV burden settings. Int J Tuberc Lung Dis. 2011;15(3):287–95. Epub 2011/02/22. [PubMed] [Google Scholar]
- **14.Hosseinipour MC, Bisson GP, Miyahara S, et al. Empirical tuberculosis therapy versus isoniazid in adult outpatients with advanced HIV initiating antiretroviral therapy (REMEMBER): a multicountry open-label randomised controlled trial. Lancet. 2016;387(10024):1198–209. doi: 10.1016/S0140-6736(16)00546-8. Epub 2016/03/31 Multicentre REMEMBER trial comparing empirical versus preventative TB therapy in HIV positive populations. Notably, empirical treatment did not decrease mortality at 6 months and preventative therapy is recommended. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesfin YM, Hailemariam D, Biadgilign S, Kibret KT. Association between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysis. PloS one. 2014;9(1):e82235. doi: 10.1371/journal.pone.0082235. Epub 2014/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.Eldholm V, Rieux A, Monteserin J, et al. Impact of HIV co-infection on the evolution and transmission of multidrug-resistant tuberculosis. eLife. 2016;5 doi: 10.7554/eLife.16644. Epub 2016/08/10. HIV does not increase emergence of TB resistance, but can drive the epidemic by increasing the number of susceptible hosts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Kendall EA, Fofana MO, Dowdy DW. Burden of transmitted multidrug resistance in epidemics of tuberculosis: a transmission modelling analysis. The Lancet Respiratory medicine. 2015;3(12):963–72. doi: 10.1016/S2213-2600(15)00458-0. Epub 2015/11/26. MDR-TB in high burden settings is transmitted, rather than acquired. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Rie A, Warren RM. MDR tuberculosis control: time to change the dogma? The Lancet Respiratory medicine. 2015;3(12):907–9. doi: 10.1016/S2213-2600(15)00477-4. Epub 2015/11/26. [DOI] [PubMed] [Google Scholar]
- 19.Salvatore PP, Becerra MC, Abel zur Wiesch P, et al. Fitness Costs of Drug Resistance Mutations in Multidrug-Resistant Mycobacterium tuberculosis: A Household-Based Case-Control Study. The Journal of infectious diseases. 2016;213(1):149–55. doi: 10.1093/infdis/jiv347. Epub 2015/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *20.Martin LJ, Roper MH, Grandjean L, et al. Rationing tests for drug-resistant tuberculosis - who are we prepared to miss? BMC medicine. 2016;14:30. doi: 10.1186/s12916-016-0576-8. Epub 2016/03/24 DST based only on risk factors misses unacceptable numbers of patients, fuelling the MDR-TB epidemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Hesseling AC, Rabie H. Tuberculosis and HIV remain major causes of death in African children. Int J Tuberc Lung Dis. 2016;20(8):996–7. Epub 2016/07/10 Important study detailingongoing causes of childhood mortality in Africa. [Google Scholar]
- 22.Kunkel A, Abel Zur Wiesch P, Nathavitharana RR, et al. Smear positivity in paediatric and adult tuberculosis: systematic review and meta-analysis. BMC infectious diseases. 2016;16:282. doi: 10.1186/s12879-016-1617-9. Epub 2016/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Rabie H, Frigati L, Hesseling AC, Garcia-Prats AJ. Tuberculosis: opportunities and challenges for the 90-90-90 targets in HIV-infected children. Journal of the International AIDS Society. 2015;18(Suppl 6):20236. doi: 10.7448/IAS.18.7.20236. Epub 2015/12/08 Review detailing progress and needs in diagnosing and preventing childhood TB in HIV co-infected children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballif M, Nhandu V, Wood R, et al. Detection and management of drug-resistant tuberculosis in HIV-infected patients in lower-income countries. Int J Tuberc Lung Dis. 2014;18(11):1327–36. doi: 10.5588/ijtld.14.0106. Epub 2014/10/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gous N, Scott LE, Khan S, et al. Diagnosing childhood pulmonary tuberculosis using a single sputum specimen on Xpert MTB/RIF at point of care. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2015;105(12):1044–8. doi: 10.7196/SAMJ.2015.v105i12.8585. Epub 2016/01/23. [DOI] [PubMed] [Google Scholar]
- 26.Corbett EL, MacPherson P. Tuberculosis screening in high human immunodeficiency virus prevalence settings: turning promise into reality. Int J Tuberc Lung Dis. 2013;17(9):1125–38. doi: 10.5588/ijtld.13.0117. Epub 2013/08/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kredo T, Adeniyi FB, Bateganya M, Pienaar ED. Task shifting from doctors to non-doctors for initiation and maintenance of antiretroviral therapy. The Cochrane database of systematic reviews. 2014;7:CD007331. doi: 10.1002/14651858.CD007331.pub3. Epub 2014/07/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann CJ, Variava E, Rakgokong M, et al. High prevalence of pulmonary tuberculosis but low sensitivity of symptom screening among HIV-infected pregnant women in South Africa. PloS one. 2013;8(4):e62211. doi: 10.1371/journal.pone.0062211. Epub 2013/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott LE, McCarthy K, Gous N, et al. Comparison of Xpert MTB/RIF with other nucleic acid technologies for diagnosing pulmonary tuberculosis in a high HIV prevalence setting: a prospective study. PLoS medicine. 2011;8(7):e1001061. doi: 10.1371/journal.pmed.1001061. Epub 2011/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. American journal of respiratory and critical care medicine. 2000;161(4 Pt 1):1376–95. doi: 10.1164/ajrccm.161.4.16141. Epub 2000/04/14. [DOI] [PubMed] [Google Scholar]
- 31.Desikan P. Sputum smear microscopy in tuberculosis: is it still relevant? The Indian journal of medical research. 2013;137(3):442–4. Epub 2013/05/04. [PMC free article] [PubMed] [Google Scholar]
- 32.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. The New England journal of medicine. 2010;363(11):1005–15. doi: 10.1056/NEJMoa0907847. Epub 2010/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helb D, Jones M, Story E, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48(1):229–37. doi: 10.1128/JCM.01463-09. Epub 2009/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blakemore R, Story E, Helb D, et al. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol. 2010;48(7):2495–501. doi: 10.1128/JCM.00128-10. Epub 2010/05/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organisation. Policy Update. Geneva, Switzerland: 2014. Automated real-rime nucleic acid amplification technology for rapid and simultanteous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. [PubMed] [Google Scholar]
- 36.Theron G, Peter J, Meldau R, et al. Accuracy and impact of Xpert MTB/RIF for the diagnosis of smear-negative or sputum-scarce tuberculosis using bronchoalveolar lavage fluid. Thorax. 2013;68(11):1043–51. doi: 10.1136/thoraxjnl-2013-203485. Epub 2013/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Detjen AK, DiNardo AR, Leyden J, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. The Lancet Respiratory medicine. 2015;3(6):451–61. doi: 10.1016/S2213-2600(15)00095-8. Epub 2015/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penz E, Boffa J, Roberts DJ, et al. Diagnostic accuracy of the Xpert(R) MTB/RIF assay for extra-pulmonary tuberculosis: a meta-analysis. Int J Tuberc Lung Dis. 2015;19(3):278–84. i–iii. doi: 10.5588/ijtld.14.0262. Epub 2015/02/17. [DOI] [PubMed] [Google Scholar]
- 39.Denkinger CM, Schumacher SG, Boehme CC, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. The European respiratory journal. 2014;44(2):435–46. doi: 10.1183/09031936.00007814. Epub 2014/04/04. [DOI] [PubMed] [Google Scholar]
- 40.Van Rie A, Page-Shipp L, Mellet K, et al. Diagnostic accuracy and effectiveness of the Xpert MTB/RIF assay for the diagnosis of HIV-associated lymph node tuberculosis. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2013;32(11):1409–15. doi: 10.1007/s10096-013-1890-0. Epub 2013/05/11. [DOI] [PubMed] [Google Scholar]
- 41.Patel VB, Theron G, Lenders L, et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLoS medicine. 2013;10(10):e1001536. doi: 10.1371/journal.pmed.1001536. Epub 2013/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bahr NC, Tugume L, Rajasingham R, et al. Improved diagnostic sensitivity for tuberculous meningitis with Xpert((R)) MTB/RIF of centrifuged CSF. Int J Tuberc Lung Dis. 2015;19(10):1209–15. doi: 10.5588/ijtld.15.0253. Epub 2015/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sehgal IS, Dhooria S, Aggarwal AN, et al. Diagnostic Performance of Xpert MTB/RIF in Tuberculous Pleural Effusion: Systematic Review and Meta-analysis. J Clin Microbiol. 2016;54(4):1133–6. doi: 10.1128/JCM.03205-15. Epub 2016/01/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lusiba JK, Nakiyingi L, Kirenga BJ, et al. Evaluation of Cepheid’s Xpert MTB/Rif test on pleural fluid in the diagnosis of pleural tuberculosis in a high prevalence HIV/TB setting. PloS one. 2014;9(7):e102702. doi: 10.1371/journal.pone.0102702. Epub 2014/07/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawn SD, Kerkhoff AD, Burton R, et al. Rapid microbiological screening for tuberculosis in HIV-positive patients on the first day of acute hospital admission by systematic testing of urine samples using Xpert MTB/RIF: a prospective cohort in South Africa. BMC medicine. 2015;13:192. doi: 10.1186/s12916-015-0432-2. Epub 2015/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manabe YC, Zawedde-Muyanja S, Burnett SM, et al. Rapid improvement in passive tuberculosis case detection and tuberculosis treatment outcomes after implementation of a bundled laboratory diagnostic and on-site training intervention targeting mid-level providers. Open forum infectious diseases. 2015;2(1):ofv030. doi: 10.1093/ofid/ofv030. Epub 2015/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Darraji HA, Abd Razak H, Ng KP, et al. The diagnostic performance of a single GeneXpert MTB/RIF assay in an intensified tuberculosis case finding survey among HIV-infected prisoners in Malaysia. PloS one. 2013;8(9):e73717. doi: 10.1371/journal.pone.0073717. Epub 2013/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon C, Cattamanchi A, Davis JL, et al. Impact of Xpert MTB/RIF testing on tuberculosis management and outcomes in hospitalized patients in Uganda. PloS one. 2012;7(11):e48599. doi: 10.1371/journal.pone.0048599. Epub 2012/11/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lorent N, Kong C, Kim T, et al. Systematic screening for drug-resistant tuberculosis with Xpert((R)) MTB/RIF in a referral hospital in Cambodia. Int J Tuberc Lung Dis. 2015;19(12):1528–35. doi: 10.5588/ijtld.14.0956. Epub 2015/11/29. [DOI] [PubMed] [Google Scholar]
- 50.van Kampen SC, Tursynbayeva A, Koptleuova A, et al. Effect of Introducing Xpert MTB/RIF to Test and Treat Individuals at Risk of Multidrug-Resistant Tuberculosis in Kazakhstan: A Prospective Cohort Study. PloS one. 2015;10(7):e0132514. doi: 10.1371/journal.pone.0132514. Epub 2015/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *51.Cox HS, Daniels JF, Muller O, et al. Impact of Decentralized Care and the Xpert MTB/RIF Test on Rifampicin-Resistant Tuberculosis Treatment Initiation in Khayelitsha, South Africa. Open forum infectious diseases. 2015;2(1):ofv014. doi: 10.1093/ofid/ofv014. Epub 2015/06/03. Placement of Xpert MTB/RIF impacts on patient care; decentralisation decreases time to treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moyenga I, Roggi A, Sulis G, et al. The impact of Xpert(R) MTB/RIF depends on service coordination: experience in Burkina Faso. Int J Tuberc Lung Dis. 2015;19(3):285–7. doi: 10.5588/ijtld.14.0629. Epub 2015/02/17. [DOI] [PubMed] [Google Scholar]
- 53.Auld SC, Moore BK, Kyle RP, et al. Mixed impact of Xpert((R)) MTB/RIF on tuberculosis diagnosis in Cambodia. Public health action. 2016;6(2):129–35. doi: 10.5588/pha.16.0001. Epub 2016/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *54.Hanrahan CF, Clouse K, Bassett J, et al. The patient impact of point-of-care vs. laboratory placement of Xpert((R)) MTB/RIF. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2015;19(7):811–6. doi: 10.5588/ijtld.15.0013. Epub 2015/06/10 Empiric treatment is common when Xpert MTB/RIF is not available at POC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Den Handel T, Hampton KH, Sanne I, et al. The impact of Xpert((R)) MTB/RIF in sparsely populated rural settings. Int J Tuberc Lung Dis. 2015;19(4):392–8. doi: 10.5588/ijtld.14.0653. Epub 2015/04/11. [DOI] [PubMed] [Google Scholar]
- 56.Balcha TT, Sturegard E, Winqvist N, et al. Intensified tuberculosis case-finding in HIV-positive adults managed at Ethiopian health centers: diagnostic yield of Xpert MTB/RIF compared with smear microscopy and liquid culture. PloS one. 2014;9(1):e85478. doi: 10.1371/journal.pone.0085478. Epub 2014/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cox HS, Mbhele S, Mohess N, et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial. PLoS medicine. 2014;11(11):e1001760. doi: 10.1371/journal.pmed.1001760. Epub 2014/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Durovni B, Saraceni V, van den Hof S, et al. Impact of replacing smear microscopy with Xpert MTB/RIF for diagnosing tuberculosis in Brazil: a stepped-wedge cluster-randomized trial. PLoS medicine. 2014;11(12):e1001766. doi: 10.1371/journal.pmed.1001766. Epub 2014/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theron G, Peter J, Dowdy D, et al. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? The Lancet Infectious diseases. 2014;14(6):527–32. doi: 10.1016/S1473-3099(13)70360-8. Epub 2014/01/21. [DOI] [PubMed] [Google Scholar]
- *60.Mupfumi L, Makamure B, Chirehwa M, et al. Impact of Xpert MTB/RIF on Antiretroviral Therapy-Associated Tuberculosis and Mortality: A Pragmatic Randomized Controlled Trial. Open forum infectious diseases. 2014;1(1):ofu038. doi: 10.1093/ofid/ofu038. Epub 2015/03/04. Centralised Xpert vs FM does not impact on patient mortality and empirical treatment is still common. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *61.Churchyard GJ, Stevens WS, Mametja LD, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. The Lancet Global health. 2015;3(8):e450–7. doi: 10.1016/S2214-109X(15)00100-X. Epub 2015/07/19. Xpert does not decrease patient mortality, indicating that linkage to care must be improved. [DOI] [PubMed] [Google Scholar]
- 62.Hanrahan CF, Selibas K, Deery CB, et al. Time to treatment and patient outcomes among TB suspects screened by a single point-of-care xpert MTB/RIF at a primary care clinic in Johannesburg, South Africa. PloS one. 2013;8(6):e65421. doi: 10.1371/journal.pone.0065421. Epub 2013/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menzies NA, Cohen T, Lin HH, et al. Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: a dynamic simulation and economic evaluation. PLoS medicine. 2012;9(11):e1001347. doi: 10.1371/journal.pmed.1001347. Epub 2012/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **64.Auld AF, Fielding KL, Gupta-Wright A, Lawn SD. Xpert MTB/RIF - why the lack of morbidity and mortality impact in intervention trials? Transactions of the Royal Society of Tropical Medicine and Hygiene. 2016;110(8):432–44. doi: 10.1093/trstmh/trw056. Epub 2016/09/18 The possible reasons for the low impact of Xpert MTB/RIF on mortality and morbidity is discussed, with recommendations for future trials to inform Xpert MTB/RIF use in resource limited settings. [DOI] [PubMed] [Google Scholar]
- 65.Jones M, Chakravorty S, Simmons M, et al., editors. ECCMID 2016. Vol. 2016. Amsterdam, The Netherlands: 2016. Apr 10, MTB-RIF Ultra - design and analytical performance of a second generation GeneXpert assay (Poster 0475) [Google Scholar]
- 66.Qin ZZ, Pai M, Van Gemert W, et al. How is Xpert MTB/RIF being implemented in 22 high tuberculosis burden countries? The European respiratory journal. 2015;45(2):549–54. doi: 10.1183/09031936.00147714. Epub 2014/11/02. [DOI] [PubMed] [Google Scholar]
- 67.Raizada N, Sachdeva KS, Sreenivas A, et al. Catching the missing million: experiences in enhancing TB & DR-TB detection by providing upfront Xpert MTB/RIF testing for people living with HIV in India. PloS one. 2015;10(2):e0116721. doi: 10.1371/journal.pone.0116721. Epub 2015/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schnippel K, Meyer-Rath G, Long L, et al. Diagnosing Xpert MTB/RIF negative TB: impact and cost of alternative algorithms for South Africa. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2013;103(2):101–6. doi: 10.7196/samj.6182. Epub 2013/02/05. [DOI] [PubMed] [Google Scholar]
- 69.South African National AIDS Council. SOUTH AFRICAN HIV AND TB INVESTMENT CASE Phase 1 Reference Report. South Africa: 2016. [Google Scholar]
- 70.Houben RM, Lalli M, Sumner T, et al. TIME Impact - a new user-friendly tuberculosis (TB) model to inform TB policy decisions. BMC medicine. 2016;14:56. doi: 10.1186/s12916-016-0608-4. Epub 2016/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uplekar M. Implementing the End TB Strategy: Well begun will be half done. Indian J Tuberc. 2015;62(2):61–3. doi: 10.1016/j.ijtb.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Scott L, Albert H, Gilpin C, et al. Multicenter feasibility study to assess external quality assessment panels for Xpert MTB/RIF assay in South Africa. J Clin Microbiol. 2014;52(7):2493–9. doi: 10.1128/JCM.03533-13. Epub 2014/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Theron G, Jenkins HE, Cobelens F, et al. Data for action: collection and use of local data to end tuberculosis. Lancet. 2015;386(10010):2324–33. doi: 10.1016/S0140-6736(15)00321-9. Epub 2015/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *74.Andre E, Isaacs C, Affolabi D, et al. Connectivity of diagnostic technologies: improving surveillance and accelerating tuberculosis elimination. Int J Tuberc Lung Dis. 2016;20(8):999–1003. doi: 10.5588/ijtld.16.0015. Epub 2016/07/10 Connectivity of the GeneXpert instruments to a central database allows for national TB surveillance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stevens WS, Cunningham B, Cassim N, et al. Cloud-Based Surveillance, Connectivity, and Distribution of the GeneXpert Analyzers for Diagnosis of Tuberculosis (TB) and Multiple-Drug-Resistant TB in South Africa. In: Persing DH, editor. Molecular Microbiology: Diagnostic Principles and Practice, 3rd Edition. 3. Washington DC, USA: ASM Press; 2016. [Google Scholar]
- 76.World Health Organisation. Molecular line probe assays for rapid screening of patients at risk of multidrup-resistant tuberculosis(MDR-TB) Geneva, Switzerland: World Health Organisation; 2008. [Google Scholar]
- 77.Barnard M, Gey van Pittius NC, van Helden PD, et al. The diagnostic performance of the GenoType MTBDRplus version 2 line probe assay is equivalent to that of the Xpert MTB/RIF assay. J Clin Microbiol. 2012;50(11):3712–6. doi: 10.1128/JCM.01958-12. Epub 2012/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crudu V, Stratan E, Romancenco E, et al. First evaluation of an improved assay for molecular genetic detection of tuberculosis as well as rifampin and isoniazid resistances. J Clin Microbiol. 2012;50(4):1264–9. doi: 10.1128/JCM.05903-11. Epub 2012/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matabane MM, Ismail F, Strydom KA, et al. Performance evaluation of three commercial molecular assays for the detection of Mycobacterium tuberculosis from clinical specimens in a high TB-HIV-burden setting. BMC infectious diseases. 2015;15:508. doi: 10.1186/s12879-015-1229-9. Epub 2015/11/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bai Y, Wang Y, Shao C, et al. GenoType MTBDRplus Assay for Rapid Detection of Multidrug Resistance in Mycobacterium tuberculosis: A Meta-Analysis. PloS one. 2016;11(3):e0150321. doi: 10.1371/journal.pone.0150321. Epub 2016/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *81.Nathavitharana RR, Hillemann D, Schumacher SG, et al. Multicenter Noninferiority Evaluation of Hain GenoType MTBDRplus Version 2 and Nipro NTM+MDRTB Line Probe Assays for Detection of Rifampin and Isoniazid Resistance. J Clin Microbiol. 2016;54(6):1624–30. doi: 10.1128/JCM.00251-16. Epub 2016/04/15 New evidence for the use of LPA for first line resistance testing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.World Health Organisation. WHO Policy on Line Probe Assays 2016. Geneva, Switzerland: World Health Organisation; 2016. (in press) Available from: http://www.who.int/tb/areas-of-work/laboratory/policy_statements/en/ [Google Scholar]
- 83.Hanrahan CF, Dorman SE, Erasmus L, et al. The impact of expanded testing for multidrug resistant tuberculosis using genotype [correction of geontype] MTBDRplus in South Africa: an observational cohort study. PloS one. 2012;7(11):e49898. doi: 10.1371/journal.pone.0049898. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Theron G, Peter J, Richardson M, et al. The diagnostic accuracy of the GenoType((R)) MTBDRsl assay for the detection of resistance to second-line anti-tuberculosis drugs. The Cochrane database of systematic reviews. 2014;(10):CD010705. doi: 10.1002/14651858.CD010705.pub2. Epub 2014/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brossier F, Guindo D, Pham A, et al. Performance of the New Version (v2.0) of the GenoType MTBDRsl Test for Detection of Resistance to Second-Line Drugs in Multidrug-Resistant Mycobacterium tuberculosis Complex Strains. Journal of clinical microbiology. 2016;54(6):1573–80. doi: 10.1128/JCM.00051-16. Epub 2016/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tagliani E, Cabibbe AM, Miotto P, et al. Diagnostic Performance of the New Version (v2.0) of GenoType MTBDRsl Assay for Detection of Resistance to Fluoroquinolones and Second-Line Injectable Drugs: a Multicenter Study. Journal of clinical microbiology. 2015;53(9):2961–9. doi: 10.1128/JCM.01257-15. Epub 2015/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.World Health Organisation. The use of molecular line probe assays for the detection of resistance to second-line anti-tuberculosis drugs. Geneva, Switzerland: World Health Organisation; 2016. [Google Scholar]
- 88.Yuan LY, Li Y, Wang M, et al. Rapid and effective diagnosis of pulmonary tuberculosis with novel and sensitive loop-mediated isothermal amplification (LAMP) assay in clinical samples: a meta-analysis. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2014;20(2):86–92. doi: 10.1016/j.jiac.2013.07.003. Epub 2014/01/28. [DOI] [PubMed] [Google Scholar]
- 89.Nliwasa M, MacPherson P, Chisala P, et al. The Sensitivity and Specificity of Loop-Mediated Isothermal Amplification (LAMP) Assay for Tuberculosis Diagnosis in Adults with Chronic Cough in Malawi. PloS one. 2016;11(5):e0155101. doi: 10.1371/journal.pone.0155101. Epub 2016/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.World Health Organisation. The use of loop-mediated isothermal amplification ( TB-LAMP) for the diagnosis of pulmonary tuberculosis: policy guidance. Geneva, Switzerland: World Health Organisation; 2016. Available from: http://apps.who.int/iris/bitstream/10665/249154/1/9789241511186-eng.pdf. [PubMed] [Google Scholar]
- 91.Gray CM, Katamba A, Narang P, et al. Feasibility and Operational Performance of Tuberculosis Detection by Loop-Mediated Isothermal Amplification Platform in Decentralized Settings: Results from a Multicenter Study. J Clin Microbiol. 2016;54(8):1984–91. doi: 10.1128/JCM.03036-15. Epub 2016/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.World Health Organisation. The use of lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people living with HIV POLICY GUIDANCE. World Health Organisaiton Press; Geneva, Switzerland: 2015. [Google Scholar]
- 93.Lawn SD. Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: a state of the art review. BMC infectious diseases. 2012;12:103. doi: 10.1186/1471-2334-12-103. Epub 2012/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *94.Shah M, Hanrahan C, Wang ZY, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. The Cochrane database of systematic reviews. 2016;5:CD011420. doi: 10.1002/14651858.CD011420.pub2. Epub 2016/05/11 Systematic review regarding the use of LAM in HIV-infected populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **95.Peter JG, Zijenah LS, Chanda D, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet. 2016;387(10024):1187–97. doi: 10.1016/S0140-6736(15)01092-2. Epub 2016/03/14 While LAM has little impact on out-patients with suspected TB, it decreases mortalityat 8 weeks in hospitalised patients with severe immune supression. [DOI] [PubMed] [Google Scholar]
- 96.Chen JH, She KK, Kwong TC, et al. Performance of the new automated Abbott RealTime MTB assay for rapid detection of Mycobacterium tuberculosis complex in respiratory specimens. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2015;34(9):1827–32. doi: 10.1007/s10096-015-2419-5. Epub 2015/06/14. [DOI] [PubMed] [Google Scholar]
- *97.Kostera J, Leckie G, Tang N, et al. Analytical and clinical performance characteristics of the Abbott RealTime MTB RIF/INH Resistance, an assay for the detection of rifampicin and isoniazid resistant Mycobacterium tuberculosis in pulmonary specimens. Tuberculosis. 2016;101:137–43. doi: 10.1016/j.tube.2016.09.006. Epub 15 September 2016 First publication involving the Abbott MTB RIF/INH assay. [DOI] [PubMed] [Google Scholar]
- *98.Hofmann-Thiel S, Molodtsov N, Antonenka U, Hoffmann H. Evaluation of the Abbott RealTime MTB and RealTime MTB INH/RIF assays for direct detection of Mycobacterium tuberculosis complex and resistance markers in respiratory and extra-pulmonary specimens. J Clin Microbiol. 2016 doi: 10.1128/JCM.01144-16. Epub 2016/10/14 First independent publication on the Abbott MTB RIF/INH assay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bates M, Zumla A. The development, evaluation and performance of molecular diagnostics for detection of Mycobacterium tuberculosis. Expert review of molecular diagnostics. 2016;16(3):307–22. doi: 10.1586/14737159.2016.1139457. Epub 2016/01/07. [DOI] [PubMed] [Google Scholar]
- 100.Abubakar I, Lipman M, McHugh TD, Fletcher H. Uniting to end the TB epidemic: advances in disease control from prevention to better diagnosis and treatment. BMC medicine. 2016;14:47. doi: 10.1186/s12916-016-0599-1. Epub 2016/03/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *101.Witney AA, Cosgrove CA, Arnold A, et al. Clinical use of whole genome sequencing for Mycobacterium tuberculosis. BMC medicine. 2016;14:46. doi: 10.1186/s12916-016-0598-2. Epub 2016/03/24 The clinical potential of WGS of TB in the future will impact patient care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haas CT, Roe JK, Pollara G, et al. Diagnostic ‘omics’ for active tuberculosis. BMC medicine. 2016;14:37. doi: 10.1186/s12916-016-0583-9. Epub 2016/03/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pai M, Behr M, Dowdy D, et al. Tuberculosis. Nature Reviews: Disease Primers. 2016:2. doi: 10.1038/nrdp.2016.76. Epub 27 October 2016. [DOI] [PubMed] [Google Scholar]
- *104.Houben RM, Menzies NA, Sumner T, et al. Feasibility of achieving the 2025 WHO global tuberculosis targets in South Africa, China, and India: a combined analysis of 11 mathematical models. The Lancet Global health. 2016 doi: 10.1016/S2214-109X(16)30199-1. Epub 2016/10/11 Scale-up of multiple interventions is necessary to meet the 2025 global TB targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *105.Menzies NA, Gomez GB, Bozzani F, et al. Cost-effectiveness and resource implications of aggressive action on tuberculosis in China, India, and South Africa: a combined analysis of nine models. The Lancet Global health. 2016 doi: 10.1016/S2214-109X(16)30265-0. Epub 2016/10/11. Cost effectiveness strategies and resource requirements must be optimised to each country. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Drain PK, Garrett NJ. The arrival of a true point-of-care molecular assay-ready for global implementation? The Lancet Global health. 2015;3(11):e663–4. doi: 10.1016/S2214-109X(15)00186-2. Epub 2015/10/18. [DOI] [PubMed] [Google Scholar]
- 107.Schumacher SG, Sohn H, Qin ZZ, et al. Impact of Molecular Diagnostics for Tuberculosis on Patient-Important Outcomes: A Systematic Review of Study Methodologies. PloS one. 2016;11(3):e0151073. doi: 10.1371/journal.pone.0151073. Epub 2016/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Furin J, Akugizibwe P, Ditiu L, et al. No one with HIV should die from tuberculosis. Lancet. 2015;386(10010):e48–50. doi: 10.1016/S0140-6736(15)00319-0. Epub 2015/10/31. [DOI] [PubMed] [Google Scholar]